Abstract
The involvement of matrix metalloproteinase (MMP) 9 in methamphetamine-induced neurotoxicity was evaluated. Injection of mice with stimulant or toxic doses of methamphetamine up regulated MMP9 gene expression in the brain within 5 min. By 24 h, MMP9 gene expression returned to control levels in the stimulant-treated mice, but remained elevated in animals exposed to toxic doses of methamphetamine. Reductions in striatal dopamine levels, a marker of methamphetamine neurotoxicity, developed 1–7 days following methamphetamine exposure, but were not accompanied by concomitant changes in MMP9 gene expression. In MMP9 knock out mice, methamphetamine retained its ability to elicit neurotoxicity. The data suggest that MMP9 expression does not contribute to methamphetamine-induced neurotoxicity, and may instead be involved in remodeling of the nervous system.
Keywords: MMP9, methamphetamine, neurotoxicity, sensitization
Introduction
Matrix metalloproteinase (MMP) 9 is up regulated following various types of insults to the brain, and has been suggested as a marker of neurodegeneration [1–5]. However, recent studies have also implicated MMP9 in neural remodeling [4, 6–12], making it unclear whether increased expression of MMP9 contributes to damage or recovery from damage in the central nervous system. The psychomotor stimulant methamphetamine up regulates MMP9, and recent studies suggest that this contributes to neural remodeling [13–15]. However, the involvement of MMP9 in methamphetamine-induced neurotoxicity remains unclear and was thus evaluated in the present study.
Methods
Subjects: Male, Swiss Webster (Harlan, Indianapolis, IN), FVB and MMP9−/− (Jackson Laboratory, Bar Harbor, ME) mice were used. Animal procedures were conducted as approved by the Institutional Animal Care and Use Committee at each participating university.
Drugs
Methamphetamine hydrochloride (Research Biochemicals International, Natick, MA) was administered to the mice as either a large bolus (40 mg/kg) or using a repeated dosing schedule to achieve the same cumulative dose (10 mg/kg, 4×, 2 h intervals) [16]. For the time course studies, bolus injections were given to facilitate determining the progression of the gene expression changes. However, since more deaths resulted following bolus injections of the high methamphetamine dose, as compared to repeated administrations to the same cumulative dose, for the studies involving MMP9 knockout mice, the repeated dosing schedule was used.
Time course
Swiss Webster mice were injected with methamphetamine (1, 10, or 40 mg/kg, i.p.) and their brains collected at designated time points. For earlier time points (5 min to 24 h) which precede the development of neurotoxicity, MMP9 gene expression was determined in whole brain. For the later time points during which neurotoxicity develops (Days 1, 2, 3, 7), MMP9 gene expression was measured in half brain and also striatum, a brain region profoundly affected by methamphetamine neurotoxicity [17–19].
Real time
PCR: MMP9 gene expression was quantified using real time PCR. Total RNA was extracted from each sample using Trizol reagents, followed by first strand cDNA synthesis using Superscipt II RNase H Reverse Transcriptase (Gibco BRL, Life Technologies, Rockville, MD) and random decamers (Ambion, Austin, TX). Primer Express software (Applied Biosystems, Foster City, CA) was used to design upper (CACCTTCACCCGCGTGTAC) and lower (TGCTCCGCGACACCAAA) primers, and a stock solution prepared by diluting the oligos to100 pmol/μl in sterile water. Each PCR reaction was comprised of 2 μl cDNA template, 12.5 μl master mix, 0.125 μl of each primer (upper and lower), and 10.25 μl sterile water. Thermal cycling, using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), was initiated at 50° C for 2 min, followed by a first denaturing step at 95° C for 10 min, and then 40 cycles at 95° C for 15 s, and at 60° C for 1 min. Threshold cycle (Ct) was determined using SDS software (Applied Biosystems, Foster City, CA) and relative gene expression levels evaluated by the ΔΔCt method. 28s rRNA was used as a reference.
MMP9 knock out mice: MMP9−/− and wild type FVB control mice were injected with methamphetamine (0–10 mg/kg, 4×, 2 h intervals), and body temperature measured one hour after each injection. One week after the treatments, the brains were collected and striatal dopamine levels measured using a Dopamine Research Enzyme Immunoassay kit and protocols supplied by the manufacturer (Rocky Mountain Diagnostics, Colorado Springs, CO). A separate group of mice were injected with a stimulant dose of methamphetamine (1 mg/kg, i.p.) once a day for nine consecutive days and locomotor activity measured for 90 min using an automated activity monitoring system (San Diego Instruments, San Diego, CA) to quantify the development of behavioral sensitization.
Results
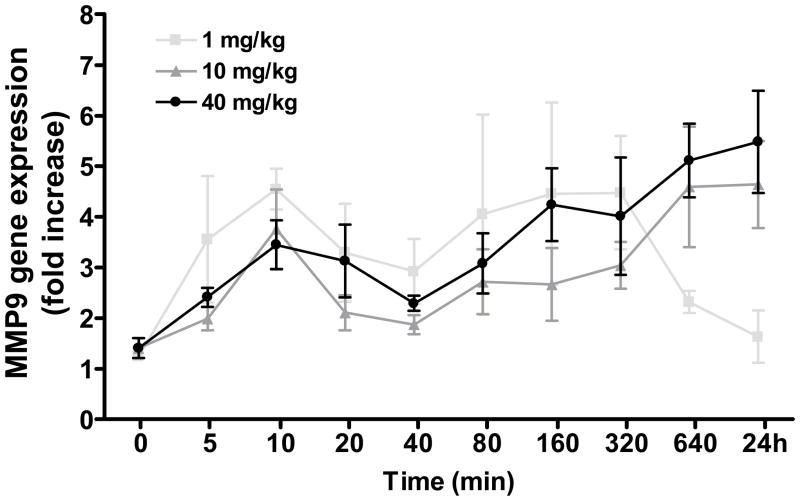
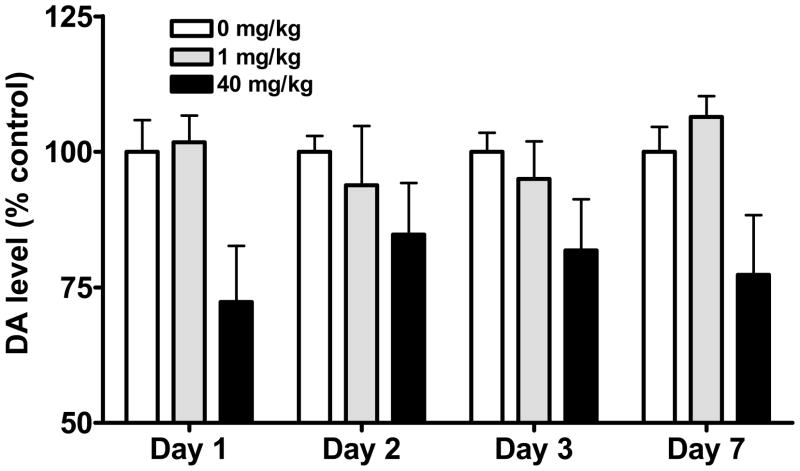
Time course of MMP9 gene expression: Analysis of variance revealed a significant effect of time (F[9,105]=3.87, P<0.0005). As shown in Figure 1, treatment with methamphetamine up regulated MMP9 gene expression within 5 min. At 24 h, MMP9 gene expression returned to control levels in the stimulant (1 mg/kg)-treated mice, but remained elevated in animals exposed to higher (10 and 40 mg/kg) doses of methamphetamine (post-hoc tests 0 vs. 24 h, P<0.01). Neurotoxicity was evident 1–7 days following methamphetamine exposure as depletions in striatal dopamine levels (Figure 2). Two way analysis of variance confirmed a significant effect of methamphetamine dose (F[2,47]=10.25, P<0.0005). However, in mice treated with the neurotoxic 40 mg/kg dose of methamphetamine, there was a lack of correlation between striatal dopamine levels, a well established marker of methamphetamine neurotoxicity, and MMP9 gene expression in the half brain (r2=0.18, n.s.) or striatum (r2=0.53, n.s.).
Figure 1.
Time course of MMP9 gene expression in mouse whole brain following administration of methamphetamine. Methamphetamine produced a rapid up regulation of MMP9 gene expression which was sustained after 24 h at higher (10 and 40 mg/kg) doses.
Figure 2.
Dopamine levels in mouse striatum following administration of methamphetamine. Exposure to a neurotoxic (40 mg/kg) dose of methamphetamine elicited a significant reduction in striatal dopamine levels.
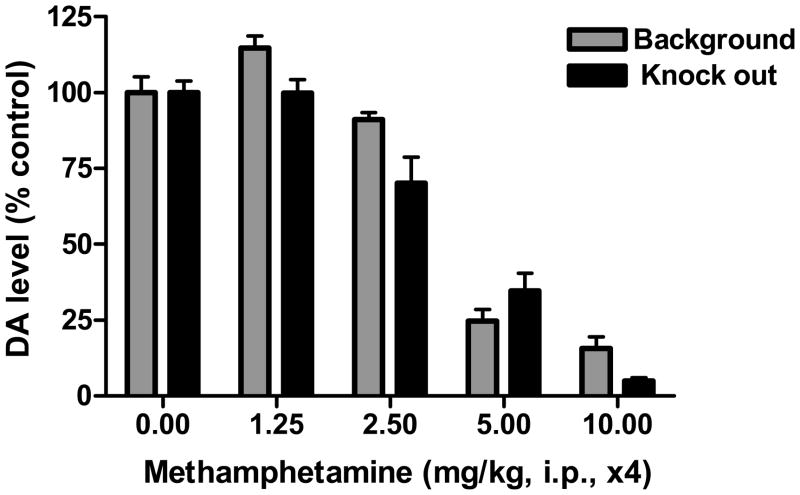
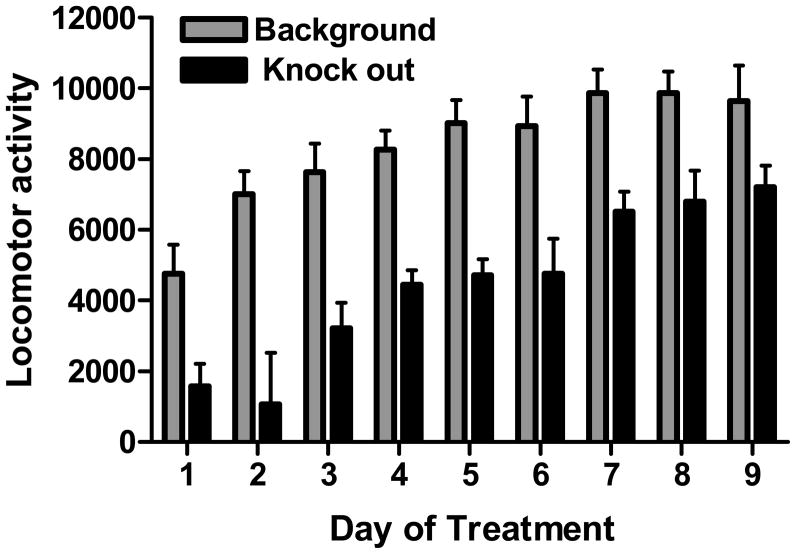
Effects of methamphetamine in MMP9−/− mice: In MMP9 knock out mice, neurotoxic dosing with methamphetamine produced increases in body temperature (F[4,20]=5.88 to 14.97, P<0.005 to 0.0001) and depletions in striatal dopamine levels (F[4,19]=56.21, P<0.0001) that were comparable to wild type mice (Figure 3). Although the absence of MMP9 did not affect methamphetamine neurotoxicity, the knock out mice exhibited the expected decrease in behavioral sensitization following repeated administration of stimulant doses of methamphetamine (Figure 4).
Figure 3.
Effect of neurotoxic dosing with methamphetamine in MMP9−/− mice. MMP9 knock out mice exhibited methamphetamine-induced neurotoxicity, as measured as depletions in striatal dopamine levels, which were comparable to background mice.
Figure 4.
Effect of repeated stimulant dosing with methamphetamine in MMP9−/− mice. MMP9 knock out mice exhibited a reduction in behavioral sensitization to methamphetamine as compared to background mice.
Discussion
Treatment with methamphetamine resulted in a rapid up regulation of MMP9 gene expression, which was measurable within minutes. By 24 h, MMP9 gene expression returned to control levels in stimulant treated mice, but remained elevated in animals exposed to higher doses of methamphetamine. To determine if the elevated levels of MMP9 gene expression were associated with the development of neurotoxicity, striatal dopamine levels and MMP9 gene expression were monitored 1–7 days following methamphetamine. During this time frame in which methamphetamine neurotoxicity develops [20, 21], there was the expected depletion in striatal dopamine levels in mice exposed to toxic doses of methamphetamine. However, this well established marker of methamphetamine neurotoxicity was not accompanied by concomitant changes in MMP9 gene expression, suggesting that MMP9 gene expression does not serve as a marker for neurodegeneration in methamphetamine-induced neurotoxicity, as it appears to following some types of stroke [5, 11, 22, 23]. To determine if MMP9 protein expression has consequences for methamphetamine neurotoxicity, MMP9 knock out mice were treated with methamphetamine. Mice lacking MMP9 still developed hyperthermia and neurotoxicity to methamphetamine. However, similar to an earlier study [13–15], MMP9 knockout mice exhibited a reduced response to the locomotor sensitizing effects of methamphetamine, an effect which is thought to involve remodeling of the nervous system.
Conclusion
Together, the data suggest that changes in MMP9 expression do not affect methamphetamine-induced neurotoxicity, and may instead contribute to remodeling of the nervous system.
Acknowledgments
Sources of support: National Institute on Drug Abuse (R01 DA11979) and National Center for Research Resources (P20 RR016476)
References
- 1.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and disease of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 2.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SR, Tsuji K, Lee SR, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24:671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;39 (Suppl 2):748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 6.Vaillant C, Didier-Bazès M, Hutter A, Belin MF, Thomasset N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves TM, Prins ML, Zhu J, Povlishock JT, Phillips LL. Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J Neurosci. 2003;23:10182–10189. doi: 10.1523/JNEUROSCI.23-32-10182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J. The role of matrix metalloproteinases in the morphogenesis of the cerebellar cortex. Cerebellum. 2005;4:239–245. doi: 10.1080/14734220500247646. [DOI] [PubMed] [Google Scholar]
- 10.Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 12.Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, et al. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J Neurochem. 2007;100:1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- 15.Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]
- 16.Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- 17.Sabol KE, Roach JT, Broom SL, Ferreira C, Preau MM. Long-term effects of a high-dose methamphetamine regimen on subsequent methamphetamine-induced dopamine release in vivo. Brain Res. 2001;892:122–129. doi: 10.1016/s0006-8993(00)03244-3. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 19.Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann N Y Acad Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- 20.Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees C. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998;806:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- 21.Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosell A, Ortega-Aznar A, Alvarez_Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]