Abstract
Methamphetamine interacts with sigma (σ) receptors and AC927, a selective σ receptor ligand, protects against methamphetamine-induced dopaminergic neurotoxicity. In the present study, the effects of AC927 on methamphetamine-induced hyperthermia and striatal serotonergic neurotoxicity were evaluated. Male, Swiss Webster mice were injected (i.p.) every two hours, for a total of four times, with one of the following treatments: Saline + Saline; Saline + Methamphetamine (5 mg/kg); AC927 (5, 10, 20 mg/kg) + Methamphetamine (5 mg/kg); or AC927 (5, 10, 20 mg/kg) + Saline. Pretreatment with AC927 (10 mg/kg) significantly attenuated methamphetamine-induced striatal serotonin depletions, striatal serotonin transporter reductions, and hyperthermia. At the doses tested, AC927 itself had no significant effects on serotonin levels, serotonin transporter expression, or body temperature. To evaluate the effects of higher ambient temperature on methamphetamine-induced neurotoxicity, groups of mice were treated at 37°C. Overall, there was an inverse correlation between the body temperature of the animals and striatal serotonin levels. Together, the data suggest that AC927 (10 mg/kg) protects against methamphetamine-induced neurotoxicity. The reduction of methamphetamine-induced hyperthermia by AC927 may contribute to the observed neuroprotection in vivo.
Keywords: Hyperthermia, Methamphetamine, Serotonin, Sigma Receptor
1. Introduction
Methamphetamine is an abused drug which produces neurotoxic effects and psychiatric complications (Cadet et al., 2003; Davidson et al., 2001). In addition to its behavioral and neurotoxic effects on dopaminergic systems (Davidson et al., 2001; McCann and Ricaurte, 2004), methamphetamine also damages serotonergic neurons. Administration of methamphetamine, either at high doses or repeatedly, inhibits the synthesis of serotonin, reduces concentrations of serotonin and its metabolite 5-hydroxyindole acetic acid, and damages transporters responsible for the reuptake of serotonin into nerve terminals (Brunswick et al., 1992; Kovachich et al., 1989; Ricaurte et al., 1980; Seiden et al., 1988). However, the mechanisms by which methamphetamine induces serotonergic neurotoxicity has yet to be fully characterized.
Hyperthermia is often associated with toxic doses of methamphetamine in both rodents and primates (Fukumura et al., 1998; Numachi et al., 2007). Earlier studies have shown that hyperthermia potentiates methamphetamine-induced dopamine and serotonin depletions and exacerbates oxidative stress in the brain (Bowyer et al., 1994; Fukumura et al., 1998; Hirata et al., 1995), whereas hypothermia protects against these effects (Bowyer et al., 1994). Previous clinical reports and animal studies suggest the lethality produced by methamphetamine is closely related to hyperthermia, which may be the primary cause of death (Bowyer et al., 1994; Davidson et al., 2001).
In addition to affecting dopamine systems, serotonin function, and body temperature (Fleckenstein et al., 2000), methamphetamine interacts with sigma (σ) receptors (Itzhak, 1993; Nguyen et al., 2005). σ Receptors are unique proteins which are distinct from other known receptors and consist of at least two subtypes, σ-1 and σ-2 (Guitart et al., 2004; Matsumoto et al., 2003; Su and Hayashi, 2003). They are distributed in the brain and peripheral organs (Itzhak, 1994; Walker et al., 1990). Of the two subtypes, σ-1 receptors are localized intracellularly (Hayashi et al. 2000, 2001) and have been cloned in several species (Mei and Pasternak, 2001; Prasad et al., 1998). σ-1 Receptors have important roles in the modulation of several neurotransmitters by affecting intracellular second messenger systems, particularly calcium mobilization (Hayashi et al. 2000, 2001; Hong et al., 2004). In addition, because of the chaperone like characteristics of σ-1 receptors, they are believed to partake in protein-protein interactions and undergo translocation between various cellular compartments (Hayashi and Su, 2007). σ-2 Receptors, on the other hand, have not been cloned and at 18–22 kDa, are smaller than σ-1 receptors (Hellewell et al., 1994). They are believed to regulate calcium release from stores within the endoplasmic reticulum (Bowen et al., 1996; Vilner and Bowen 2000) and are implicated in the regulation of cell proliferation and cell viability (Vilner and Bowen 1993; Vilner et al., 1995). As with the σ-1 receptor, σ-2 receptors are widely distributed throughout the cell including the mitochondria, endoplasmic reticulum, lysosome, and plasma membrane (Zeng et al., 2007).
Recent evidence has shown that methamphetamine produces some of its physiological and behavioral effects through σ receptors (Nguyen et al., 2005). Specifically, σ receptors may play a role in the hyperthermic effects of methamphetamine (Matsumoto et al., 2008). σ Receptors are found on monoaminergic neurons (Bastianetto et al., 1995; Booth and Baldessarini, 1991; Gundlach et al., 1986 ) and modulate the release of neurotransmitters such as serotontin, which has been linked to changes in body temperature (Salmi and Ahlenius, 1998; Schwartz et al., 1995). In addition, σ receptor ligands can modulate thermoregulation (Rawls et al., 2002), likely through an interaction with thermosensitive neurons in the hypothalamus (Bouchard and Quirion, 1997; Mei and Pasternak, 2001).
The present study investigated whether methamphetamine-induced hyperthermia and serotonin neurotoxicity could be prevented using the σ receptor ligand AC927 (N-phenethylpiperidine oxalate). The effects of AC927 were evaluated under two different ambient conditions (room temperature and 37°C) to determine whether the σ-mediated modulation of methamphetamine on serotonin neurotoxicity involves an interaction with changes in body temperature. Two well known markers of serotonin neurotoxicity were evaluated: serotonin levels and serotonin transporter expression, both of which were measured in the mouse striatum. AC927 was chosen for the present study because it has preferential affinity for σ receptors (Ki = 30 and 138 nM for σ-1 and σ-2 receptors respectively), as compared to its low affinity for other receptors, monoamine transporters, and ion channels (Matsumoto et al., 2008). In addition to its selectivity, AC927 has been shown to reduce apoptosis in tumor cells through σ receptors (Crawford and Bowen, 2002) and prevent methamphetamine-induced dopaminergic neurotoxicity (Matsumoto et al., 2008). Striatal tissue was examined in the present investigation because it contains the terminals of monoaminergic neurons and is the region primarily affected by methamphetamine-induced neurotoxicity (Brunswick et al., 1992; Kovachich et al., 1989; Ricaurte et al., 1980; Seiden et al., 1988).
2. Materials and methods
2.1. Drugs and reagents
Methamphetamine hydrochloride was obtained from Research Biochemicals International (Natick, MA). AC927 was synthesized by converting the free base N-phenethylpiperidine (Sigma-Aldrich, Inc., St. Louis, MO) to the oxalate salt (Maeda et al., 2002). Serotonin Research EIA kits were purchased from Rocky Mountain Diagnostics (Colorado Springs, CO).
2.2. Animals
Male, Swiss Webster mice (21–30 g, Harlan, Indianapolis, IN; Frederick, MD) were used in the present experiments. The animals were housed in groups of 4–6 with a 12:12-h light/dark cycle and ad libitum food and water. Each cage was made from polysulfone and provided 84 square inches of floor space (Tecniplast, Philadelphia, PA), which was covered with corn cob bedding and packing material (ULINE, Waukegan, IL). The animals were acclimated for one week before being used in experiments and they were randomly assigned to their treatment groups. All procedures were performed as approved by the Institutional Animal Care and Use Committees at the University of Mississippi and the West Virginia University Health Sciences Center.
2.3. Overview of drug treatments
The mice (N = 115) were assigned to one of the following experimental groups: (1) Saline + Saline; (2) Saline + Methamphetamine (1.25, 2.5, 5 or 10 mg/kg); (3) AC927 (5, 10 or 20 mg/kg) + Methamphetamine (5 mg/kg); or (4) AC927 (5, 10, or 20 mg/kg) + Saline. The mice were injected (i.p.) four times at two hour intervals with their designated treatment. The first compound in each treatment combination (saline or AC927) was administered as a 15 min pretreatment to the second (saline or methamphetamine). All of the mice were treated at room temperature, unless specified otherwise (i.e. high ambient temperature experiments). To minimize the number of animals used in these experiments, core body temperature measurements were taken in all mice, and one week later, their brains were collected for ELISA or immunohistochemistry studies, as described below.
2.4. Body temperature measurements
Core body temperature was measured 1 h following each injection (i.p.) of methamphetamine (or saline) with a Thermalert TH-S monitor (Physitemp Instruments Inc., Clifton, NJ), for a total of four body temperature readings for each mouse. During the temperature measurements, mice were gently held at the base of the tail and a probe (RET-3) inserted approximately 2.5 cm past the rectum into the colon for 8–10 s until a rectal temperature was maintained for 3–4 s.
2.5. Serotonin assays
Serotonin assays were conducted to determine the effects of the following treatments on serotonin levels in the striatum: 1) different doses of methamphetamine alone [Saline + Methamphetamine (0, 1.25, 2.5, 5 or 10 mg/kg), N = 5/group], 2) different doses of AC927 alone [AC927 (0, 5, 10 or 20 mg/kg) + Saline, N = 5/group], and 3) different doses of AC927 in combination with methamphetamine [AC927 (0, 5, 10 or 20 mg/kg) + Methamphetamine (5 mg/kg), N = 5/group]. As indicated above, each mouse received the same designated treatment four times over an 8 h period, with 2 h intervening between each injection.
The animals were sacrificed by decapitation and their brains were removed one week after treatment to allow ample time for degeneration of serotonin nerve terminals to occur (Cappon et al., 2000). In addition, tissues from naïve mice (N = 5) were collected as an additional control. The striatum was dissected from each of the mice and frozen in liquid nitrogen. The tissues were stored at −80°C for later analysis of serotonin content.
Brain striatal serotonin concentrations were quantified using Serotonin Research ELISA kits and protocols supplied by the manufacturer (Rocky Mountain Diagnostics, Colorado Springs, CO). Briefly, brain tissues were homogenized in 0.2 M perchloric acid, followed by centrifugation at 3,000 rpm for 10 min at 4°C. Supernatants so obtained were used for the serotonin measurements. Serotonin was first quantitatively derivatised into N-acylserotonin using the acylation buffer provided with the kit. Acylated serotonin from the tissue samples was then incubated with solid phase bound serotonin and serotonin antiserum to compete for a fixed number of antiserum binding sites. Free antigen and free antigen-antiserum complexes were removed by washing. The antibody bound to the solid phase serotonin was detected using an anti-rabbit IgG-peroxidase conjugate with TMB as the substrate. The amount of antibody bound to the solid phase serotonin was measured by monitoring the reaction at 450 nm. The solid phase serotonin so measured was inversely proportional to the serotonin concentration of the tissue sample and was quantified relative to a standard curve of known concentrations.
2.6. Immunohistochemistry
To support the interpretation that serotonin depletions were reflective of neurotoxic damage, striatal sections were evaluated for serotonin transporter expression. Mice were randomly assigned to one of the following treatment groups: (1) Saline + Saline (N = 4); (2) Saline + Methamphetamine (5 mg/kg) (N = 4); (3) AC927 (10 mg/kg) + Methamphetamine (5 mg/kg) (N = 3); (4) AC927 (10 mg/kg) + Saline (N = 4). Each combination of treatments was given i.p. at 2 h intervals, a total of four times, over an 8 h period.
One week later, the mice were perfused transcardially with 0.1 M phosphate buffered saline (pH 7.4), followed by 4% paraformaldehyde. The brains were fixed overnight in 4% paraformaldehyde. Coronal sections (50 μm) of the fixed tissue were made through the rostral-caudal extent of the striatum using a cryostat, and processed in a free-floating state in 0.1 M Tris–HCl buffered saline (TBS, pH 7.5). The sections were treated with 0.3% H2O2 in TBS for 30 min at room temperature. The sections were then treated with TBS containing 0.2% Triton X-100 and 1.5% normal goat serum for 30 min at room temperature. Incubation of the sections with anti-mouse SERT antibody (Millipore, Temecula, CA; AB9726, dilution 1:5,000) in TBS was performed overnight at 4°C. The labeled sections were then washed three times in TBS and incubated with secondary anti-rabbit IgG-peroxidase (Sigma Aldrich, A0545; diluted 1:400) in TBS for 60 min. The labeled sections were then washed three times in TBS and processed using Histostain-Plus kits (DAB, Broad Spectrum) (Invitrogen, Camarillo, CA). Briefly, the labeled sections were incubated in a combined 100 μL solution of concentrated substrate buffer (20x), concentrated chromagen solution, DAB (20x), and 0.6% H2O2 for 4 min. The sections were then washed in 5 mL of distilled water.
The stained sections were mounted onto gelatin-coated slides and dried. The sections were then dehydrated, cleared, and coverslipped. The images were captured digitally using a Carl Zeiss Microimaging LLC Invertoskop 40C (Leica Microsystems, Bannockburn, IL) and optical density readings were quantified in anterior regions of the striatum using ImageJ software (National Institutes of Health, Bethesda, MD). To obtain the data point for a given animal, at least two sections per mouse brain were processed and the optical density readings from both the striatal regions were averaged.
2.7. Ambient temperature studies
To produce core body temperatures in naïve mice equivalent to those treated with methamphetamine (5 mg/kg, i.p.) at room temperature (22°C), animals (N = 5/group) were exposed to ambient temperatures that varied within the range 22–41°C. Core body temperature was measured in each animal after it had been maintained at a given ambient temperature for 30 min. Core body temperature measurements and brain collections were performed as described for earlier experiments conducted at room temperature.
To evaluate the effects of higher ambient temperature on serotonin levels, animals were placed in an environment with an ambient temperature of 37°C. This ambient temperature produces core body temperatures which are equal to methamphetamine (5 mg/kg, i.p.)-induced hyperthermia at room temperature. Thirty minutes later, mice (N = 5/group) were pretreated with either saline or AC927 (10 mg/kg). Saline or methamphetamine (5 mg/kg) was administered 15 min after the pretreatment. Each combination of treatments was given i.p. at 2 h intervals, a total of four times while the mice were maintained at an ambient temperature of 37°C. At the same time, two other groups of control animals (Saline + Saline, and Saline + Methamphetamine, N = 5/group) were treated i.p. at room temperature (22°C).
2.6. Statistical analyses
The data from the serotonin level, serotonin transporter expression, and body temperature measurements were evaluated using analysis of variance (ANOVA). Post-hoc analyses were conducted using Tukey’s tests for multiple comparisons. Correlations were performed to determine if there were significant relationships between: 1) ambient and core body temperature, and 2) core body temperature and brain serotonin levels. The proportion of animals experiencing lethality following various experimental manipulations was evaluated using Fisher’s exact tests. For all of the statistical analyses, P < 0.05 was considered statistically significant.
3. Results
3.1 Hyperthermia
Figure 1 shows the dose response of methamphetamine (0–10 mg/kg, i.p.) and AC927 (0–20 mg/kg, i.p.) on body temperature. A one-way ANOVA revealed that methamphetamine increased body temperature in a dose dependent manner, with the effects at the higher doses of methamphetamine (5 and 10 mg/kg, i.p.) being statistically significant (after 1st injection, F[4,25] = 19.58; P < 0.0001, 2nd injection, F[4,25] = 12.85; P < 0.0001, 3rd injection, F[4,25] = 12.35; P < 0.0001, 4th injection, F[4,25] = 12.05; P < 0.0001) (Figure 1A). Moreover, a one-way ANOVA determined that AC927 in the absence of methamphetamine did not significantly alter core body temperature at the tested doses (F[3,12] = 3.25; n.s.) (Figure 1B). However, pretreatment with AC927 (5, 10 or 20 mg/kg, i.p.) significantly attenuated the ability of methamphetamine to produce hyperthermic effects (after 1st injection, F[4,20] = 5.06; P < 0.01, 2nd injection, F[4,20] = 19.74; P < 0.001, 3rd injection, F[4,20] = 20.99; P < 0.001 and 4th injection, F[4,20] = 14.44; P < 0.001), one-way ANOVA, post-hoc Tukey’s (Figure 2).
Figure 1.
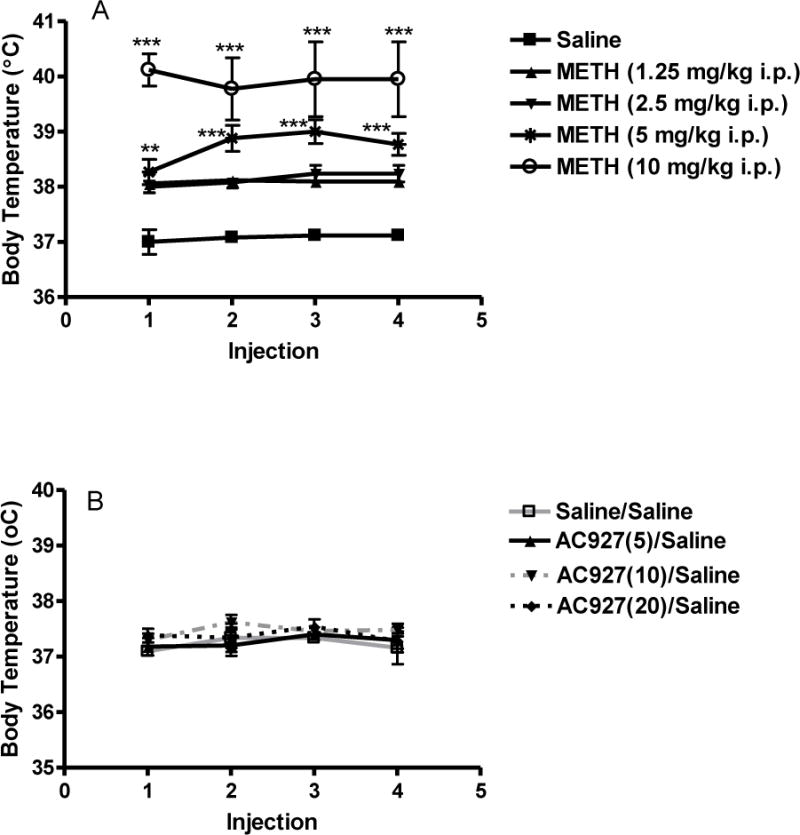
Effects of methamphetamine and AC927 on body temperature. (A) Methamphetamine-induced hyperthermia. Male, Swiss Webster mice (N = 5 per group) were injected with methamphetamine (METH, 1.25–10 mg/kg, i.p.) or saline at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each methamphetamine injection and data were reported as mean ± S.E.M. ***P < 0.001 vs. saline. (B) Dose-related effects of AC927 on body temperature. Male, Swiss Webster mice (N = 5 per group) were injected with AC927 (5–20 mg/kg, i.p.) or saline at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each methamphetamine injection. No significant change in body temperature was noted after administration of AC927.
Figure 2.
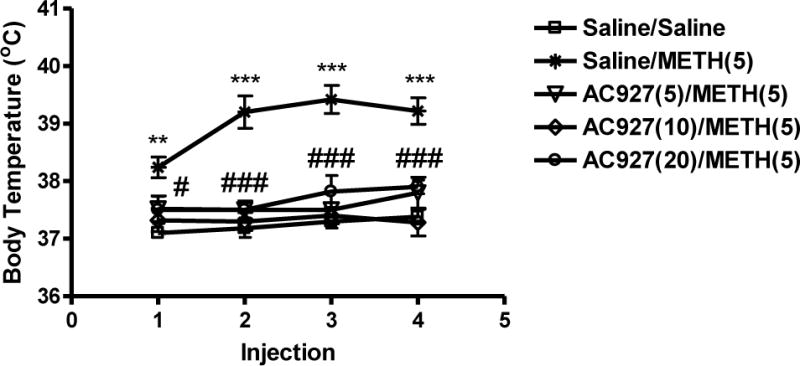
Effects of AC927 (0–20 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced hyperthermia. Male, Swiss Webster mice (N = 5 per group) were pretreated with saline or AC927 (5, 10, 20 mg/kg, i.p.). The mice were then challenged 15 min later with saline or methamphetamine (METH, 5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each methamphetamine injection and data were reported as mean ± S.E.M. *P < 0.05, AC927 (10 mg/kg) + Methamphetamine vs. Saline + Methamphetamine; ***P < 0.001, AC927 (10 and 20 mg/kg) + Methamphetamine vs. Saline + Methamphetamine, Tukey’s post-hoc comparisons.
3.2 Neurotoxicity Studies
3.2.1 Serotonin Assays
In naïve animals, the average basal serotonin level in the striatum was 0.42 ± 0.06 μg/g wet tissue. These concentrations are consistent with the range reported using high pressure liquid chromatography (HPLC) methods (Fumagalli et al., 1998; Ke et al., 2008).
Figure 3 shows the effects of methamphetamine (0–10 mg/kg, i.p.) on serotonin levels in the striatum. A one-way ANOVA revealed that methamphetamine produced reductions in serotonin in the striatum (F[4,19] = 12.24; P < 0.0001) in a dose-dependent manner. Post-hoc Tukey’s multiple comparison tests revealed that the following doses of methamphetamine produced depletions in serotonin levels that differed significantly from saline controls: 5 mg/kg (q = 5.88; P < 0.01), 10 mg/kg (q = 8.83; P < 0.001). A methamphetamine dose of 5 mg/kg was used for subsequent studies due to the increased incidence of lethality with higher doses of methamphetamine (10 mg/kg).
Figure 3.
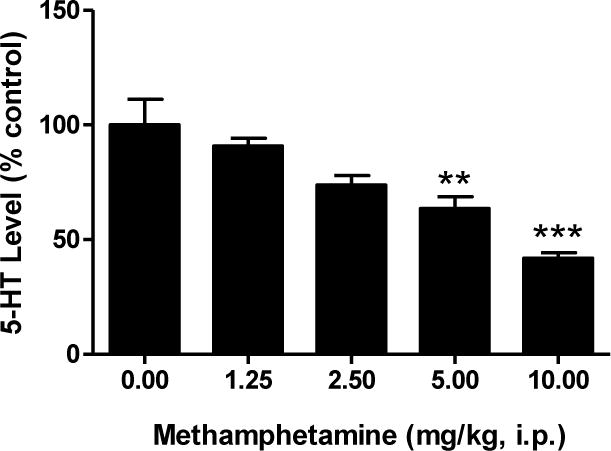
Dose response effects of methamphetamine on serotonin levels in the striatum. Male, Swiss Webster mice (N = 5 per group) were injected (i.p.) with methamphetamine (1.25–10 mg/kg) or saline (0 mg/kg) at 2 h intervals, a total of four times. Striatal serotonin levels were measured one week later. The data were reported as mean ± S.E.M. after normalization to saline controls (100%). **P < 0.01, ***P < 0.001 vs. saline, Tukey’s post-hoc comparisons.
Figure 4 shows the effects of the σ receptor ligand AC927 (0–20 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced serotonin depletions in the striatum of mice. To determine the effects of AC927 against methamphetamine induced serotonin depletions, a one-way ANOVA followed by post-hoc Tukey’s multiple comparisons were conducted. Pretreatment with AC927 (5, 10 or 20 mg/kg, i.p.) significantly attenuated the ability of methamphetamine to produce neurotoxic effects in the striatum (F[7,32] = 4.49; P < 0.005). Post-hoc Tukey’s multiple comparisons tests confirmed that pretreatment with the 10 mg/kg dose of AC927 significantly attenuated the striatal (q = 6.17; P < 0.01) serotonin depletions caused by methamphetamine (5 mg/kg, i.p.). Moreover, the serotonin levels of mice treated with AC927 (10 mg/kg) along with methamphetamine did not differ significantly from the saline control group (q = 0.77; n.s.). To determine the effects of AC927 alone, a one-way ANOVA was conducted, indicating no significant effects on serotonin levels at any of the tested doses (F[3,16] = 0.72; n.s.) (Figure 4).
Figure 4.
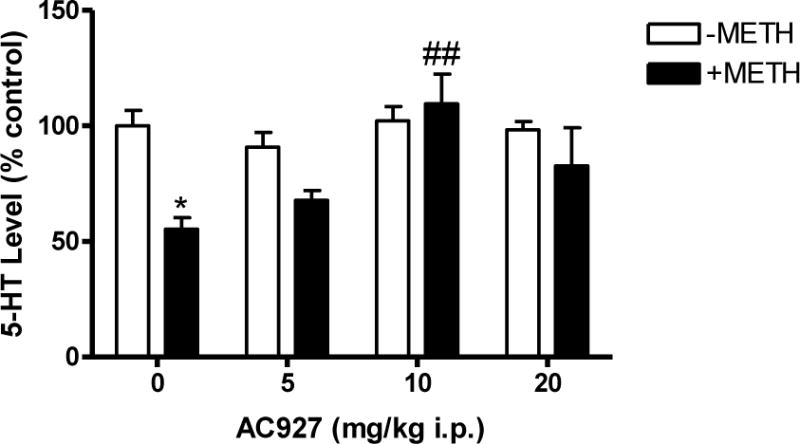
Effects of AC927 (0–20 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced alterations of serotonin levels in the striatum. Male, Swiss Webster mice (N = 5 per group) were pretreated (i.p.) with saline (0 mg/kg) or AC927 (5, 10, 20 mg/kg). The mice were then challenged 15 min later with saline (−METH; 0 mg/kg, i.p.) or methamphetamine (+METH; 5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. Striatal serotonin levels were measured one week later. The data were reported as mean ± S.E.M. after normalization to saline controls (100%). *P < 0.05, Saline + Methamphetamine vs. Saline + Saline; ##P < 0.01, AC927 + Methamphetamine vs. Saline + Methamphetamine, Tukey’s post-hoc comparisons.
3.2.2 SERT Immunohistochemistry
Analysis of serotonin transporter expression using immunohistochemistry studies revealed methamphetamine-induced decreases (Figure 5). Significant differences between the treatment groups (F[4,40] = 62.75; P < 0.001) were shown using a one-way ANOVA. Post-hoc Tukey’s multiple comparisons tests confirmed that methamphetamine (5 mg/kg) caused a significant reduction in SERT immunoreactivity relative to treatment with saline alone (q = 15.90; P < 0.001). Pretreatment with AC927 (10 mg/kg) significantly attenuated methamphetamine (5 mg/kg)-induced neurotoxicity (q = 12.98; P < 0.001), whereas treatment with AC927 alone had no significant effects on SERT expression compared to saline (q = 0.87; n.s.) (Figure 5A).
Figure 5.
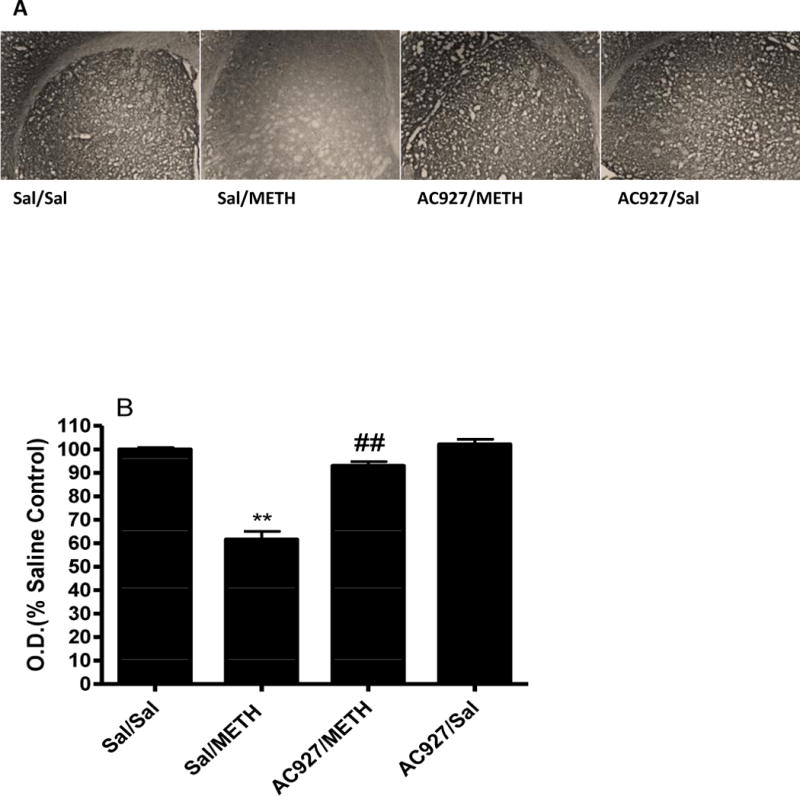
Effects of methamphetamine and AC927 on serotonin transporter (SERT) immunoreactivity in the mouse striatum. Male, Swiss Webster mice were pretreated with either saline or AC927 (10 mg/kg, i.p.) and then challenged 15 min later with saline or methamphetamine (5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals for a total of four times. One week later, the brains were removed and processed for SERT immunoreactivity. A representative section from each treatment group is shown (Figure 5A) in addition to the average optical density readings (mean ± S.E.M) (Figure 5B). There was a significant decrease in SERT immunoreactivity in the methamphetamine treated group (**P < 0.001), which was attenuated by the pretreatment of AC927 (##P < .001), Tukeys post-hoc comparison.
3.3 High Ambient Temperature Studies
Figure 6 illustrates the association between increases in ambient temperature and increases in core body temperature (r2 = 0.70; P < 0.0001). Higher ambient temperatures increased core body temperatures (F[5,24] = 13.60; P < 0.0001), and the core body temperature equivalent to that produced by methamphetamine (5 mg/kg, i.p.) was observed at an ambient temperature of 37°C.
Figure 6.
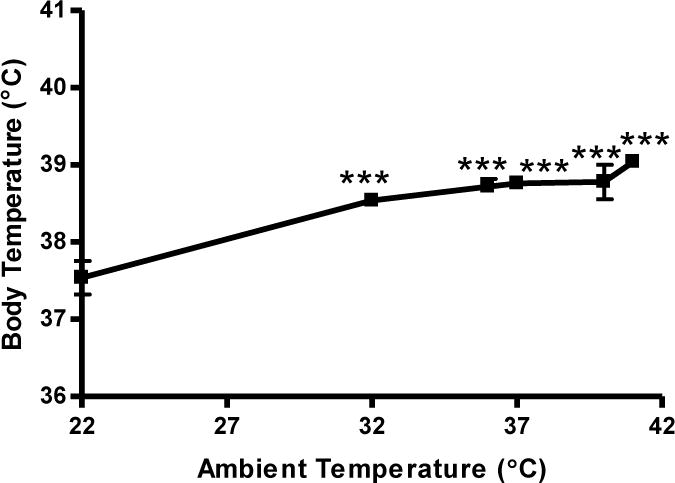
Effects of ambient temperature on core body temperature. Male, Swiss Webster mice (N = 5 per group) were placed in varying ambient temperatures. Core body temperature was measured and data was reported as mean ± S.E.M. ***P < 0.001 vs. room temperature (22°C), Tukey’s post-hoc tests.
Figure 7 shows the effects of the σ receptor ligand AC927 (10 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced changes in serotonin levels in the striatum of mice exposed to the higher (37°C) ambient temperature. Compared to saline, a one-way ANOVA determined that administration of AC927 in the absence of methamphetamine at 37°C had no significant effects on serotonin levels in the striatum (q = 1.19; n.s.). Unlike at room temperature (Figure 4), pretreatment of mice with AC927 (10 mg/kg, i.p.) at 37°C was unable to maintain serotonin levels equivalent to saline-treated mice at 37°C (q = 11.21; P < 0.001) (Figure 7).
Figure 7.
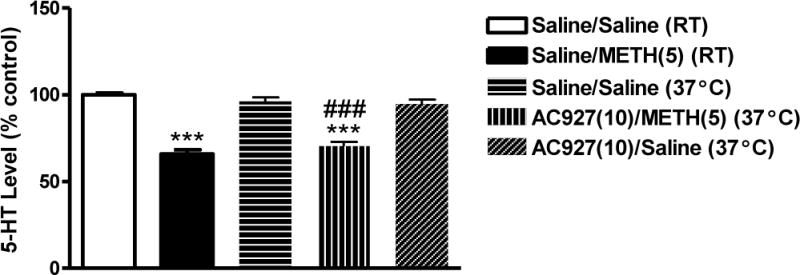
Effects of AC927 (10 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced changes on striatal serotonin levels at different ambient temperatures. Male, Swiss Webster mice (N = 5 per group) were pretreated with saline or AC927 (10 mg/kg, i.p.) at 37°C. The mice were then challenged 15 min later with saline (0 mg/kg, i.p.) or methamphetamine (5 mg/kg, i.p.). For comparison, some mice were treated with Saline + Saline, or Saline + Methamphetamine (5 mg/kg) at room temperature (RT). The assigned schedule of treatment was repeated at 2 h intervals, a total of four times. Striatal serotonin levels were measured one week later and data were reported as mean ± S.E.M. after normalization to saline controls (100%). ***P < 0.001 vs. Saline + Saline at room temperature (RT, 22°C), ###P < 0.001 vs. Saline + Saline at 37°C, Tukey’s post-hoc comparisons.
Meanwhile, all of the methamphetamine-treated mice exposed to higher environmental temperature (37°C) died within 1 h after the first injection (Table 1), representing significant mortality compared to saline-treated mice at 37°C (P < 0.005) or methamphetamine-treated mice at room temperature (P < 0.005). In contrast, there was no significant difference in mortality in mice treated with AC927 + Methamphetamine at 37°C, compared to saline-treated mice at the same temperature (n.s.; Table 1) (Fisher’s exact test).
Table 1.
Effects of ambient temperature on animal survival
| Treatment group | % Animal survival | ||
|---|---|---|---|
|
|
|
||
| RT | 37°C | ||
|
|
|||
| Saline/Saline | 6/6 (100%) | 6/6 (100%) | |
| Saline/Meth(5) | 6/6 (100%) | 0/6 (0%) | |
| AC927(10)/Meth(5) | 6/6 (100%) | 4/6 (67%) | |
|
| |||
| Saline/Saline vs Saline/Meth | P value | n.s. | < 0.001 |
| Saline/Saline vs AC927/Meth | P value | n.s. | n.s. |
|
| |||
The percent of animal survival under varying ambient temperatures was determined using three different treatment combinations. The table summarizes the percent survival for each of the groups, with statistical results from Fisher’s exact tests shown below. RT = room temperature.
Figure 8 shows the effects of the σ receptor ligand AC927 (10 mg/kg, i.p.) at higher ambient temperature (37°C) on methamphetamine (5 mg/kg, i.p.)-induced changes in core body temperature. In contrast to room temperature experiments (Figure 4), AC927 (10 mg/kg, i.p.) at 37°C was unable to attenuate the methamphetamine (5 mg/kg, i.p.)-induced hyperthermia to saline-like levels (Figure 8). Moreover, a one-way ANOVA followed by a post hoc Tukeys test revealed the hyperthermic effects produced in AC927 (10 mg/kg, i.p.) + Methamphetamine (5 mg/kg, i.p.) treated mice at 37°C were even higher than mice treated with methamphetamine (5 mg/kg, i.p.) at room temperature (22°C) following the first three injections (after 1st injection, F[4,21] = 23.91; P < 0.0001, q = 10.11; P < 0.001, 2nd injection, F[4,21] = 68.04; P < 0.0001, q = 15.33; P < 0.001, 3rd injection, F[4,19] = 22.33; P < 0.0001, q = 6.68; P < 0.01, 4th injection, F[4,19] = 23.32; P < 0.0001, q = 3.93 n.s.).
Figure 8.
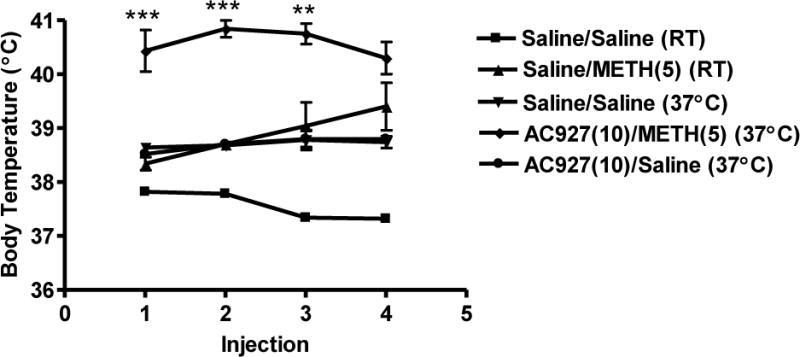
Effects of higher ambient temperature (37°C) on core body temperature of animals treated with AC927 (10 mg/kg, i.p.) in the presence or absence of methamphetamine (5 mg/kg, i.p.). Male, Swiss Webster mice (N = 5–6 per group) were pretreated with saline or AC927 (10 mg/kg, i.p.) at 37°C. The mice were then challenged 15 min later with saline or methamphetamine (5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. For comparison, some animals were treated with Saline + Saline, or Saline + Methamphetamine (5 mg/kg) at room temperature. Core body temperature was measured 1 h after each methamphetamine injection and data were reported as mean ± S.E.M. **P < 0.01, ***P < 0.001 vs. methamphetamine at room temperature (RT, 22°C).
Table 2 shows that when the data from the animals in all of the experimental groups reported herein were combined, there was a significant correlation between core body temperature at each of the four time points and brain serotonin levels measured one week later (P < 0.05 to P < 0.0001).
Table 2.
Relationship between core body temperature and serotonin levels in striatum.
| Treatment group | 5-HT (μg/g wet tissue) | Core Body Temperature (°C) | |||
|---|---|---|---|---|---|
|
| |||||
| 1st Injection | 2nd Injection | 3rd Injection | 4th Injection | ||
| Saline/Saline (RT) | 0.322 ± 0.003 | 37.82 ± 0.04 | 37.78 ± 0.05 | 37.34 ± 0.09 | 37.32 ± 0.04 |
| Saline/METH(5) (RT) | 0.213 ± 0.008 | 38.34 ± 0.12 | 38.70 ± 0.09 | 39.04 ± 0.43 | 39.40 ± 0.44 |
| Saline/Saline (37°C) | 0.310 ± 0.008 | 38.64 ± 0.07 | 38.68 ± 0.05 | 38.78 ± 0.18 | 38.74 ± 0.11 |
| AC927(10)/METH(5) (37°C) | 0.227 ± 0.007 | 40.43 ± 0.38 | 40.88 ± 0.26 | 40.75 ± 0.19 | 40.30 ± 0.18 |
| AC927(10)/Saline (37°C) | 0.305 ± 0.008 | 38.52 ± 0.06 | 38.70 ± 0.07 | 38.80 ± 0.15 | 38.80 ± 0.05 |
|
| |||||
| r2 | 0.22 | 0.35 | 0.41 | 0.53 | |
| P | < 0.05 | < 0.005 | < 0.001 | < 0.001 | |
The values from individual mice were used to calculate the correlation between core body temperature at each of the four time points and striatal serotonin levels measured one week later. The table summarizes the mean ± S.E.M. for each of the groups, with the statistical results shown at the bottom. The numbers in the parentheses of the treatment group column represent doses (in mg/kg, i.p.) of AC927 or methamphetamine (METH). RT = room temperature.
4. Discussion
σ Receptors have an important role in methamphetamine-induced neurotoxicity, and AC927 has previously been shown to attenuate methamphetamine-induced dopamine damage and hyperthermia (Matsumoto et al., 2008). In the present study, we evaluated the effects of AC927 on methamphetamine-induced serotonin damage, reductions in striatal serotonin transporter levels, and hyperthermia in mice. Consistent with earlier reported observations of methamphetamine-induced serotonin neurotoxicity (Broening et al., 2005; Ricaurte et al., 1980; Seiden et al., 1988), methamphetamine produced a significant dose dependent reduction in serotonin levels in the striatum of mice in addition to a significant decrease in serotonin transporter expression. The striatal tissue used in the present study contains not only the terminals of monoaminergic neurons that are damaged following methamphetamine exposure (Brunswick et al., 1992; Kovachich et al., 1989; Ricaurte et al., 1980; Seiden et al., 1988), but also a relatively high concentration of sigma receptors (Gundlach et al., 1986; Walker et al., 1990). Under the conditions used in the present study, AC927 alone did not alter serotonin levels or serotonin transporter expression. However, when mice were pretreated with AC927 (10 mg/kg), it significantly attenuated methamphetamine-induced serotonin depletions and reductions in serotonin transporters at room temperature.
The protective properties of AC927 most likely occur through several different mechanisms involving cellular homeostasis, as well as temperature regulation. Cellular mechanisms related to the generation of reactive oxygen species, and processes dependent on calcium and protein kinase C appear particularly important. An early action of methamphetamine is to enhance serotonin release into the cytoplasm of serotonin nerve terminals. A subsequent autooxidation of serotonin enhances reactive oxygen species generation (De Vito and Wagner, 1989; Hirata et al., 1995), which can stimulate numerous cellular death cascades that contribute to methamphetamine-induced neurotoxicity. Since σ-2 receptor agonists have been shown to produce reactive oxygen species (Ostenfeld et al., 2005), methamphetamine may act in part as a σ-2 receptor agonist with AC927 blocking its access to these sites.
AC927 may also convey neuroprotective effects by modulating calcium-dependent processes. One of the main functions of mitochondria in cells is to reduce excess cytosolic calcium and regulate calcium-dependent signaling in the cytosol (Duchen, 2000). Under physiological conditions, calcium released from the endoplasmic reticulum generates a microdomain of high calcium at the mitochondria-associated endoplasmic reticulum membrane (Vance, 1990). The σ-1 receptor, which has been implicated in neuroprotection and neuroplasticity, is a calcium-sensitive and ligand-operated receptor chaperone at the mitochondria-associated endoplasmic reticulum membrane (Hayashi and Su, 2007). Under conditions of endoplasmic reticulum stress, as may result following methamphetamine exposure, calcium mobilizes from the endoplasmic reticulum, leading to prolonged calcium signaling into the mitochondria. Since increasing σ-1 receptors in cells counteracts the endoplasmic reticulum stress response and provides neuroprotection (Hayashi and Su, 2007), it is plausible that the σ-1 receptor ligand AC927 protects against methamphetamine-induced neurotoxicity by blocking the mobilization of calcium. This would imply that AC927 may have agonist actions at σ-1 receptors, and antagonist actions at σ-2 receptors.
Another location in the cell where σ receptors may modulate calcium signaling is at lipid rafts. σ-2 Receptors are located in lipid rafts and agonists can stimulate calcium signaling via sphingolipid products (Gebreselassie and Bowen, 2004). In presynaptic neurons, serotonin transporters are also found in lipid rafts where they may be subject to modulation by AC927 via interactions with σ-2 receptors.
Activation of protein kinase C also modulates vesicular serotonin release and its uptake via serotonin transporters (Qian et al., 1997). The prolonged activation of protein kinase C further leads to calcium-dependent serotonergic neurotoxicity (Kramer et al., 1998). Therefore, modulation of serotonin release and this pathway via protein kinase C and calcium-dependent processes using σ-1 and σ-2 ligands (Derbez et al., 2002; Takahashi et al., 2001) may explain part of the neuroprotective effects of AC927 against methamphetamine-induced serotonergic neurotoxicity.
Temperature is an important factor in methamphetamine-induced neurotoxicity and serotonin has a definitive role in thermoregulation. Increases in serotonin are linked to increases in brain and core body temperatures (Salmi and Ahlenius, 1998; Schwartz et al., 1995). Following entry into nerve terminals, methamphetamine facilitates serotonin release (Ago et al., 2006; Kuczenski et al., 1995) and resulting hyperthermia (Fukumura et al., 1998; Numachi et al., 2007). The experiments reported here demonstrate that pretreatment of mice with AC927 significantly attenuates methamphetamine-induced hyperthermia which may be due, in part, to modulation of thermoregulatory factors within the hypothalamus. Thermosensitive neurons and σ receptors in the hypothalamus appear closely associated (Bouchard and Quirion, 1997; Mei and Pasternak, 2001). Since σ receptor ligands modulate thermoregulation (Rawls et al., 2002), AC927 may affect thermoregulatory factors (e.g., core cold receptors and/or core warm receptors) in the hypothalamus (Boulant, 2000; Cooper, 2002), which control core body temperature.
To evaluate whether the σ-mediated modulation of methamphetamine on serotonin neurotoxicity involves an interaction with changes in body temperature, the mice were exposed to the various treatments at high (37°C) ambient temperature. In contrast to the room temperature experiments where pretreatment with AC927(10 mg/kg) protected against both methamphetamine-induced hyperthermia and serotonergic neurotoxicity, at high ambient temperature, pretreatment with AC927 before methamphetamine resulted in increases in body temperature and serotonin loss significantly above saline. This suggests that body temperature can influence the extent of neurotoxicity under in vivo conditions. It is important to note that brain cells are temperature sensitive, and increased body temperature (e.g. 40°C ) produces irreversible changes in the structure and function of cells (Sharma and Hoops, 2003). Since many physicochemical processes modulating neural activity depend on temperature (Kiyatkin, 2005), hyperthermia could strongly influence the magnitude of neurotoxic effects.
The data provided in this experiment at higher environmental temperature demonstrate a significant correlation between the body temperature of the animals and the level of brain serotonin depletion subsequently measured which suggests that the hyperthermic effects of methamphetamine may contribute to the subsequent neurotoxicity observed. However, it is not possible to conclude that hyperthermia is solely responsible for the methamphetamine-induced neurotoxicity because previous studies with reserpine, a compound which lowers body temperature, showed that hyperthermia is not essential for methamphetamine-induced toxicity (Albers and Sonsalla, 1995).
Finally, it should be noted that at high ambient temperature, direct comparisons of striatal serotonin levels and body temperature were not possible between AC927 + Methamphetamine vs. Saline + Methamphetamine animals because the latter group of mice had high lethality rates (100%), necessitating statistical comparisons to the Saline + Methamphetamine room temperature control group. Although at 37°C, AC927 pretreatment was unable to maintain striatal serotonin levels similar to Saline + Saline controls, there appears to be a protective effect because 67% of the mice treated with AC927 + Methamphetamine survived, in contrast to the 0% survival rate of mice treated with Saline + Methamphetamine at high ambient temperature.
In summary, the results, together with observations previously reported, suggest that σ ligands can modulate methamphetamine-induced hyperthermia and serotonergic neurotoxicity. Further studies are needed to fully delineate the mechanisms through which AC927 mitigates the actions of methamphetamine as well as the therapeutic potential of AC927.
Research highlights.
AC927 alone has no significant effects on striatal serotonin levels, striatal serotonin transporter expression, or body temperature.
AC927 attenuates methamphetamine-induced hyperthermia at ambient, room temperature.
AC927 attenuates methamphetamine-induced neurotoxicity as measured as reductions in striatal serotonin levels and serotonin transporter expression.
There is a significant correlation between body temperature and striatal serotonin levels.
At high ambient temperature, AC927 attenuates methamphetamine-induced lethality, but does not reduce body temperature to saline-like levels.
Acknowledgments
We appreciate the technical assistance provided by Bahar Noorbakhsh and Alisa Elliott during some of the immunohistochemistry studies. This work was supported by the National Institute on Drug Abuse (DA013978). Andrew Coop is the recipient of an Independent Scientist Award from the National Institute on Drug Abuse (K01 DA019634). The funding source was not involved in the conduct of the research; preparation of the article; study design; collection, analysis and interpretation of the data; writing of the report; or decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ago Y, Nakamura S, Uda M, Kajji Y, Abe M, Baba A, et al. Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology. 2006;51:914–22. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacologica profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–14. [PubMed] [Google Scholar]
- Bastianetto S, Rouquier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between sigma sites and nigro-striatal dopaminergic pathways. Neuropharmacology. 1995;34:281–287. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- Booth RG, Baldessarini RJ. (+)-6,7-Benzomorphan sigma ligands stimulate dopamine synthesis in rat corpus striatum tissue. Brain Res. 1991;557:349–352. doi: 10.1016/0006-8993(91)90159-s. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–77. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–61. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Bowen WD, Vilner BJ, Bandarage UK, Kuehne ME. Ibogaine and ibogamine modulate intracellular calcium levels via interaction with sigma-2 receptors. Soc Neurosci Abstr. 1996;22(7875) [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, et al. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–80. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with D-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse. 2005;56:84–93. doi: 10.1002/syn.20130. [DOI] [PubMed] [Google Scholar]
- Brunswick DJ, Benmansour S, Tejani-Butt SM, Hauptmann M. Effects of high-dose methamphetamine on monoamine uptake sites in rat brain measured by quantitative autoradiography. Synapse. 1992;11:287–93. doi: 10.1002/syn.890110404. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–88. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single dose treatment. Brain Res. 2000;863:106–11. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cooper KE. Some historical perspectives on thermoregulation. J Appl Physiol. 2002;92:1717–24. doi: 10.1152/japplphysiol.01051.2001. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–22. [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Derbez AE, Mody RM, Werling LL. Sigma(2)-receptor regulation of dopamine transporter via activation of protein kinase C. J Pharmacol Exp Ther. 2002;301:306–14. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–50. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signaling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Broening HW, Vorhees CV. Methamphetamine-induced dopamine and serotonin reductions in neostriatum are not gender specific in rats with comparable hyperthermic responses. Neurotoxicol Teratol. 1998;20:441–8. doi: 10.1016/s0892-0362(97)00094-9. [DOI] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. Sigma-2 receptors are specially localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–19. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+) 3H-3-(3-hydroxyphenyl)-N-(1-propyl) piperdine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su T-P. Ca2+ signaling via σ1 receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther. 2000;293:788–98. [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci (USA) 2001;98:491–6. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma-1 and sigma-2 receptors. Characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL. Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res. 1995;677:345–7. doi: 10.1016/0006-8993(95)00218-f. [DOI] [PubMed] [Google Scholar]
- Hong W, Nuwayhid SJ, Werling LL. Modulation of bradykinin-induced calcium changes in SH-SY5Y cells by neurosteroids and sigma receptor ligands via a shared mechanism. Synapse. 2004;54:102–10. doi: 10.1002/syn.20069. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Repeated methamphetamine-treatment alters brain σ receptors. Eur J Pharmacol. 1993;230:243–4. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Sigma Receptors. San Diego: Academic Press; 1994. [Google Scholar]
- Ke J-J, Chen H-I, Jen CJ, Kuo Y-M, Cherng CG, Tsai Y-P, et al. Mutual enhancement of central neurotoxicity induced by ketamine followed by methamphetamine. Toxicol Appl Pharmacol. 2008;227:239–47. doi: 10.1016/j.taap.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain hyperthermia as physiological and pathologica phenomena. Brain Res Rev. 2005;50:27–56. doi: 10.1016/j.brainresrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effects of high-dose methamphetamine administration on serotonin uptake sites in rat brain measured using [3H]cyanoimipramine autoradiography. Brain Res. 1989;505:123–9. doi: 10.1016/0006-8993(89)90122-4. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Poblete JC, Azmitia EC. Characterization of the translocation of protein kinase C (PKC) by 3,4-methylenedioxymethamphetamine (MDMA/ecstasy) in synaptosomes: evidence for a presynaptic localization involving the serotonin transporter (SERT) Neuropsychopharmacology. 1998;19:265–77. doi: 10.1016/S0893-133X(98)00027-X. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–17. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda DY, Williams W, Kim WE, Thatcher LN, Bowen WD. N-Arylalkylpiperidines as high affinity sigma-1 and sigma-2 receptor ligands: phenylpropylamines as potential leads for selective sigma-2 agents. Bioorg Med Chem Lett. 2002;12:497–500. doi: 10.1016/s0960-894x(01)00788-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anticocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam LL, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (σ) receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–81. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev. 2004;27:821–6. doi: 10.1016/j.neubiorev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem Pharmacol. 2001;62:349–55. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–45. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, et al. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur J Pharmacol. 2007;572:120–8. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005;65:8975–83. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Li HW, Fei Y-J, Ganapathy ME, Fujita T, Plumley LH, et al. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J Neurochem. 1998;70:443–51. doi: 10.1046/j.1471-4159.1998.70020443.x. [DOI] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Geller EB, Adler MW. Sigma sites mediate DTG-evoked hypothermia in rats. Pharmacol Biochem Behav. 2002;73:779–86. doi: 10.1016/s0091-3057(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methamphetamine administration on dopamine and serotonin neurons in the rat brain: regional study. Brain Res. 1980;193:153–63. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol Toxicol. 1998;82:122–7. doi: 10.1111/j.1600-0773.1998.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Wehr TA, Rosenthal NE, Bartko JJ, Oren DA, Luetke C, et al. Serotonin and thermoregulation. Physiologic and pharmacologic aspects of control revealed by intravenous m-CPP in normal human subjects. Neuropsychopharmacology. 1995;13:105–15. doi: 10.1016/0893-133X(95)00026-A. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Commins DL, Vosmer G, Axt K, Marek G. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann N Y Acad Sci. 1988;537:161–72. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Hoopes PJ. Hyperthermia-induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19:325–54. doi: 10.1080/0265673021000054621. [DOI] [PubMed] [Google Scholar]
- Su T-P, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–80. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Horikomi K, Kato T. MS-377, a novel selective sigma (1) receptor ligand, reverses phencyclidine-induced release of dopamine and serotonin in rat brain. Eur J Pharmacol. 2001;427:211–9. doi: 10.1016/s0014-2999(01)01254-7. [DOI] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–56. [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J. Pharmacol Exp Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- Vilner BJ, de Costa BR, Bowen WD. Cytoxic effects of sigma ligands; sigma receptor-medicated alterations in ceullar morphology and vaibility. J Neurosci. 1995;15:117–134. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. Sigma receptor-active neuroleptics are cytotoxic to C6 glioma cells in culture. Eur J Pharmacol Mol Pharmacol Sect. 1993;244:199–201. doi: 10.1016/0922-4106(93)90029-9. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, de Costa BR, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Zeng C, Vangveravong S, Xu J, Chang KC, Hotchkiss S, Wheeler KT, Shen D, Zhuang ZP, Kung HF, Mach RH. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2000;67:6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]


