Abstract
Adrenal suppression and lymphocytopenia are commonly monitored pharmacological responses during systemic exposure to exogenously administered corticosteroids. The pharmacodynamics of plasma corticosterone (CS) and blood lymphocytes were investigated in 60 normal rats which received either 50 mg/kg methylprednisolone (MPL) or vehicle intramuscularly. Blood samples were collected between 0.5 and 96 h following treatment. Plasma CS displayed a transient suppression with re-establishment of a normal circadian rhythm 24 h following drug treatment. An indirect response model with suppression of production well captured plasma CS profiles. An early stress-induced rise in CS was also factored into the model. Blood lymphocyte numbers exhibited a sharp decline and then returned to a new circadian rhythm which was half of the original baseline level. An integrated pharmacodynamic (PD) model with inhibition of lymphocyte trafficking from tissue to blood by both MPL and CS and induction of cell apoptosis by MPL reasonably captured this lymphocytopenia. Rats and humans differ in lymphocyte responses with humans showing full recovery of baselines. Modeling provides a valuable tool in quantitative assessment of dual, complex drug responses.
Keywords: pharmacokinetics, pharmacodynamics, hormones, mathematical model, pharmacokinetic/pharmacodynamic models, corticosteroid, lymphocyte, cell trafficking, indirect response model, circadian rhythm
INTRODUCTION
Systemic administration of corticosteroids generates broad responses including desired effects such as anti-inflammatory and immunosuppressive functions as well as undesired disturbances in carbohydrate, lipid and protein metabolism, growth retardation, osteoporosis, and disorders in the cardiovascular and central nervous system.1 Alterations of endogenous glucocorticoid production and lymphocytopenia are typically used as biomarkers of systemic exposure to corticosteroids. While adrenal suppression is generally considered as a challenge to the internal hormone balance maintained by the hypothalamus-pituitary-adrenal axis (HPA), lymphocytopenia is usually viewed as a component of immune system suppression. 2–7 Due to the fact that both biomarkers respond rapidly and significantly to exposure of exogenous corticosteoids and measurements can be conveniently obtained, these two biomarkers are commonly used in the assessment of corticosteroid functions.2
Under normal physiological conditions, endogenous plasma glucocorticoids display circadian rhythms, with opposite patterns between humans and nocturnal animals such as rodents.8 In humans, plasma cortisol reaches its peak in the early morning, then decreases and reaches its nadir by late afternoon.9 The peak of rat CS occurs right after darkness and the nadir occurs in early morning.10
Lymphocytes are constantly trafficking between blood and peripheral lymphatic tissue. The alterations of lymphocyte trafficking and function by corticosteroids are considered as part of their immunosuppressive functions. In humans, a single dose of corticosteroid leads to a transient but marked lymphocytopenia.2,11–13 This is attributed to changes in expression and surface distribution of adhesion molecules which affect the redistribution of lymphocytes between blood and peripheral tissue.14 Mature human lymphocytes are resistant to corticosteroid-induced programmed cell death. However, in other species such as mouse, rat, and rabbit, corticosteroid-induced lymphocytopenia is primarily due to cell apoptosis.13 Thus regulation of both cell trafficking and cell death should be considered in the study of lymphocyte responses to corticosteroids in rats.
Models of both endogenous steroid suppression and lymphocytopenia following corticosteroid administration have been developed.2,11 However, most of these studies were conducted in normal human subjects. This study examines pharmacodynamic responses of these two biomarkers in normal rats. Normal rats received MPL as a large single bolus dose and plasma CS and total blood lymphocytes were measured over time. The PK/ PD models were premised on three major processes. First, MPL effects on lymphocyte trafficking and apoptosis reduce lymphocyte numbers in blood. Second, endogenous CS controls movement of lymphocytes between tissue and blood and their circadian rhythm in blood. Third, MPL treatment causes CS suppression and consequent effects.
MATERIALS AND METHODS
Animals
Sixty normal (150–175 g) male Wistar rats were purchased from Harlan-Sprague-Dawley, Inc. (Indianapolis, IN) and experiments were initiated at body weights of 225–275 g. Animals were housed and allowed to acclimatize in a constant temperature environment (22°C) equipped with a 12 h light/12 h dark cycle for 2 weeks. All rats had free access to rat chow and drinking water. Our research protocol adheres to the “Principles of Laboratory Animal Care” (NIH publication #85-23, revised 1985) and was approved by the University at Buffalo Institutional Animal Care and Use Committee.
Experimental
MPL Treatment
A single dose of 50 mg/kg MPL (Solu-Medrol, Pharmacia-Upjohn Company, Kalamazoo, MI) was given to 54 animals intramuscularly (i.m.) in the left gluteus muscle. Rats were sacrificed by aortic exsanguination at 0.25, 0.5, 0.75, 1, 2, 4, 5, 6, 7, 8, 12, 24, 36, 48, 60, 72, 84, and 96 h after dosing. Three rats were sacrificed at each time point. The sampling time points were selected based on previous studies of receptor dynamics and enzyme induction in muscle and liver.15,16 Six vehicle-treated rats were designated as controls and were sacrificed 12 and 24 h after dosing. The onset of the light cycle on the treatment day was considered as time zero. The i.m. MPL injection was given between 1.5 and 3.5 h after lights on. The treatment and sacrifice times were recorded. Since normal rats were used in the study, minimal animal handling with subtle environmental disturbances were enforced in order to lessen stress. Night vision goggles were used to carry out animal procedures conducted in the dark period. At sacrifice, rats were weighed, anesthetized by ketamine/xylazine, and sacrificed by aortic exsanguination. Blood was drawn from the abdominal aortic artery into syringes using ethylenediami-netetraacetic acid as anticoagulant. A small amount of blood (50 (µL) was used to count blood cell responses. Plasma was harvested from the blood sample and frozen at −20°C until analyzed for glucocorticoids.
CS Circadian Rhythm
Plasma CS concentrations in normal untreated Wistar rats were obtained from a recent study performed in our laboratory under similar conditions.10 In brief, rats were sacrificed at 0.25, 1, 2, 4, 6, 8, 10, 11, 11.75, 12.25, 13, 14, 16, 18, 20, 22, 23, and 23.75 h after 2 weeks acclimatization to 12 h light/12 h dark cycle. Three animals were sacrificed at each time point.
Plasma Glucocorticoid Concentrations
Plasma MPL and CS concentrations were determined by a sensitive normal phase high-performance liquid chromatography method as previously described.17 The limit of quantitation was 5 ng/ mL for both steroids. The inter-day and intra-day coefficients of variation (CV) were less than 10%.
Cell Responses
Complete leukocyte counts and differentials were obtained using an automated hemocytometer (CELLDYN 1700, Abbott Laboratories, Abbott Park, IL). Total lymphocyte counts were obtained by multiplying total number of leukocytes by the proportion of lymphocytes in total leukocytes. The system was monitored with purchased controls from Abbott Laboratories at three levels (high, normal, and low).
Pharmacokinetic/Pharmacodynamic Models
MPL Kinetics
The kinetics of plasma MPL (CMPL) versus time (t) following i.m. injection was described by a biexponential equation with first-order absorption (k a).
| (1) |
where Ci and λi are the intercept and slope coefficients. The pharmacokinetic (PK) parameters were estimated first and then fixed in further modeling.
CS Suppression
The plasma concentrations of CS (CCS) in the 24-h circadian rhythm study were modeled by harmonic functions.10
| (2) |
where ai and bi are Fourier coefficients and i from 1 to n represent the frequency of the harmonic functions. The circadian rhythm period T was 24 h. A two-harmonics function was sufficient to capture the apparent circadian rhythm.10 Parameters reported previously were used for simulations of CS kinetics10 (Tab. 1).
Table 1.
Pharmacokinetics of Methylprednisolone and CS Circadian Rhythm Parameters
| Parameter (Units) | Definition | Value | ||
|---|---|---|---|---|
| MPL pharmacokinetics | ||||
| C1 (µg/mL) | Intercept coefficient | 1709 | ||
| C2 (µg/mL) | Intercept coefficient | 179 | ||
| λ1 (h−1) | Slope coefficient | 0.813 | ||
| λ2 (h−1) | Slope coefficient | 0.242 | ||
| ka (h−1) | Absorption rate constant | 0.747 | ||
| CS circadian rhythm | ||||
| T (h) | Biorhythmic period | 24 | ||
| n | Number of Harmonics | 0 | 1 | 2 |
| αn | Fourier coefficient | 116 | −121 | 38.8 |
| bn | Fourier coefficient | — | −41.1 | 1.58 |
The plasma concentrations of CS with no drug treatment can be described using a changing production rate/volume (R(t)) and first-order elimination rate (kout_CS) by:
| (3) |
The production rate/volume of CS (R(t)) is derived from Eqs. (2) and (3):
| (4) |
Administration of MPL causes suppression of CS production. It is also well known that physical stress such as animal handing and pain stimulate secretion of CS. The effect of MPL on plasma CS is complicated by both of these factors. Figure 1 shows the PD model for MPL effects on plasma CS. The equations describing both effects are:
| (5) |
| (6) |
where IC50 is the concentration of plasma MPL causing 50% of maximum inhibition of CS production, kin_H is the induced CS production rate/volume by physical stress, SH is the maximum stress-induced CS production rate/volume, kH is the disappearance rate constant for stress-induced CS production rate/volume, and Tinj is the injection time of each animal. It is assumed that CS production can be completely suppressed at high concentrations of MPL. Eq. (3) was used to model baseline CS while Eqs. (5) and (6) were applied to fit plasma CS concentrations following MPL injection. The initial condition of plasma CS was fixed at the measured value of 33.59 ng/mL.
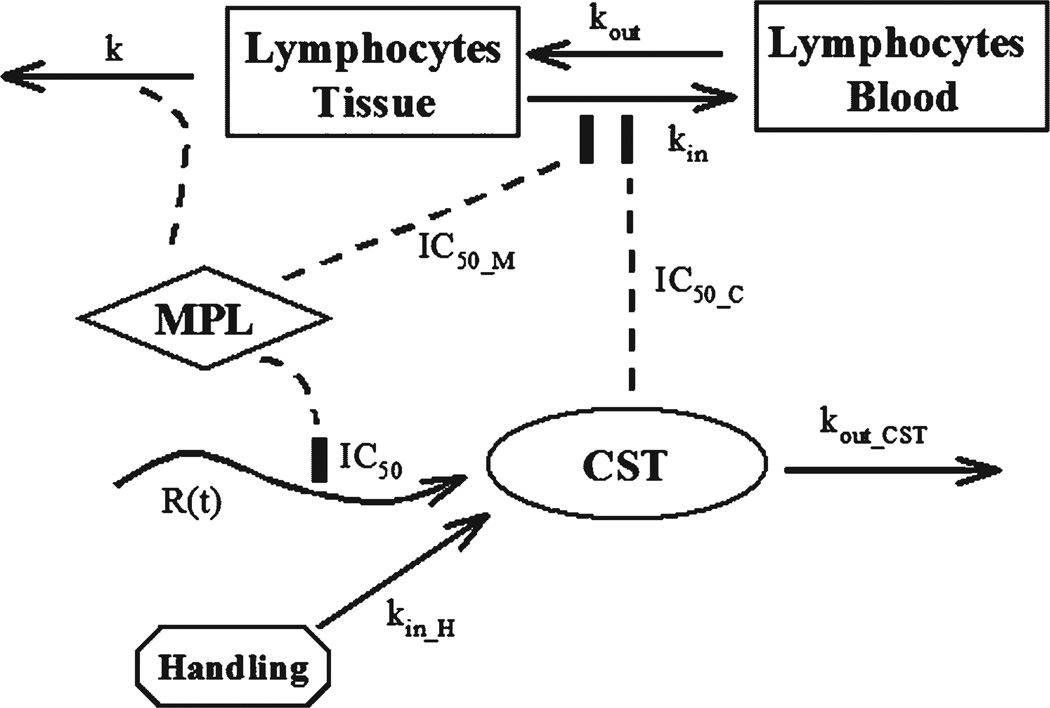
Figure 1.
Pharmacodynamic model of CS response and lymphocytopenia following MPL dosing. Plasma CS is described by an indirect response model with a changing production rate/volume (R(t)) and first-order elimination (kout_CST). Animal handing induced stress leads to increased CS production rate/volume (kin_H) which diminishes with time. Lymphocytes are continuously trafficking between blood and tissue compartments. Both MPL and CS inhibit the movement of lymphocytes from tissue to blood (kin). MPL also induces lymphocyte apoptosis in the tissue compartment (k).
Lymphocytopenia
Lymphocyte trafficking between blood and tissue is regulated by glucocorticoids, including both synthetic glucocorticoids such as MPL and naturally produced steroids such as CS. Both human and rat blood lymphocytes exhibit daily fluctuations related to endogenous glucocorticoid circadian rhythms.2,11 Thus both plasma MPL and CS serve as factors driving the blood lymphocyte changes. Additionally in rodents, exogenous glucocorticoids produce apoptosis of lymphocytes.12,18 The proposed PD model is shown in Figure 1. The following equations were applied in the modeling of trafficking and apoptosis of lymphocytes under the influence of both steroids.
| (7) |
| (8) |
| (9) |
where CTE represents total effective steroid concentration, and IC50_M and IC50_C are concentrations of MPL and CS causing 50% of maximum inhibition of cell trafficking. Movement of lymphocytes between blood (LYMB) and tissues (LYMT) are governed by first-order rate constants kin (from tissue to blood) and kout (from blood to tissue). The second-order rate constant, k, represents the apoptosis process induced by MPL. 19 It is assumed that only exogenous glucocorticoids cause apoptosis of lymphocytes. The kinetics of MPL and CS were fixed in analysis of lymphocyte suppression. The lymphocyte baselines in blood (LYMB (0)) and tissues (LYMT (0)) were fixed as 7.8 and 400 K/µL in the modeling.
Lymphocyte data from vehicle-treated animals were modeled simultaneously with data from MPL treated animals. Due to different baseline values between the two groups, the baselines of LYMB (LYMB_C (0)) and LYMT (LYMT_C (0)) in vehicle-treated animals were expressed as:
| (10) |
| (11) |
where BaselineC is the ratio of lymphocyte baselines in control to treated animals.
Data Analysis
The kinetic parameters for MPL were estimated using nonlinear regression analysis. Assuming that the errors from the observed and predicted responses are normally distributed, the ADAPT II program20 with the maximum likelihood method was applied for the fitting. The variance model was:
| (12) |
where Vi is the variance of the response at the ith time point, ti is the actual time at the ith time point, θ represents the systemic parameter vector for the PK model, σ is defined as the vector of variance parameters for the model, Y(θ, ti) is the predicted response value at time ti from the model. Variance parameters σ1 and σ2 were estimated along with model parameters during fittings. All data were used in fitting the models. The goodness-of-fit criteria included: visual inspection of the fitted curves, estimator criterion value, sum of squared residuals, Akaike information criterion, Schwartz criterion, and CV of the estimated parameters.
Plasma CS and blood lymphocyte data were analyzed by a nonlinear mixed-effect model using NONMEM (NONMEM version V Level 1.1, NONMEM project Group, GloboMax, Hanover, MD) to evaluate the parameters and residual random effects. Since only one observation was obtained from one individual and our sample size is small, inter-subject random effects were not estimated. Calculations were performed on a Pentium IV 1.6 GHz computer with a Microsoft Windows XP operating system using the compiler digital Visual Fortran V6.6. Subroutine Advan6 and the first-order conditional estimation (FOCE) method were used. For residual errors, a series of error models was tested including additive error model, constant coefficient variance (CCV) model, and combined additive plus CCV model. The best residual error model was selected based on the objective function value (OFV) and goodness-of-fit (GOF) plots.
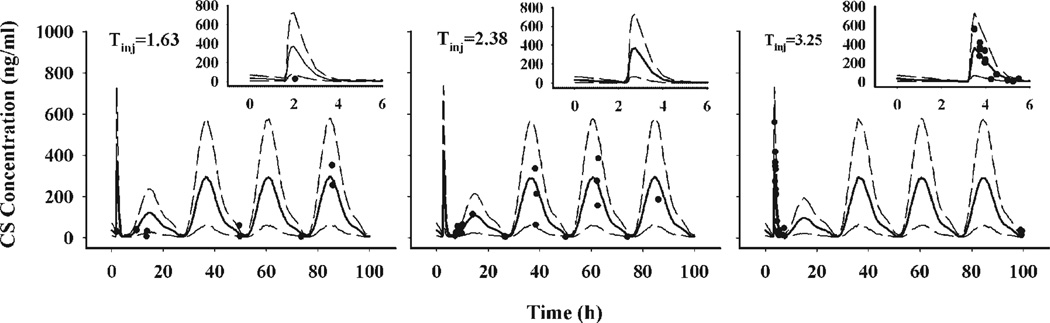
Visual predictive plots were generated by simulating 10,000 individual profiles using the estimated structural parameters and random error estimates. The 90% predictive interval was constructed to assess the bias and predictive performance of modeling. Animals dosed at 1.5 and 1.75 h were simulated with an injection time (Tinj) of 1.63 h. Animals dosed at 2, 2.25, 2.5, and 2.75 h were simulated with Tinj = 2.38 h. The Tinj of 3.25 h was used for animals dosed at 3, 3.25, and 3.5 h.
Simulations of CS suppression following MPL dosing at different times were performed. The 50 mg/kg MPL i.m. profile was used to simulate the kinetics. The treatment times were selected at 2, 6, 10, 14, 18, and 22 h after the onset of the light period. The CS responses from 0 to 100 h were simulated and compared with the basal circadian rhythm.
RESULTS
MPL Kinetics
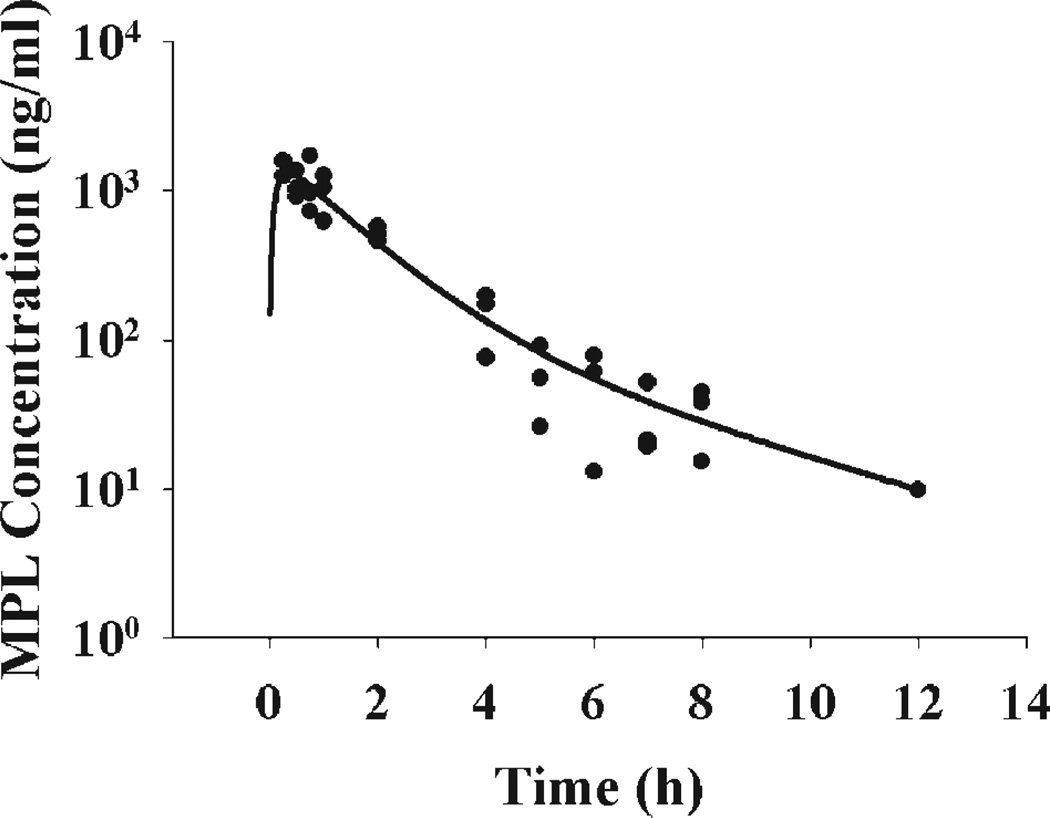
The pharmacokinetics of MPL following 50 mg/kg is shown in Figure 2 and was well captured by biexponential kinetics with first-order absorption. The absorption of MPL from the muscle injection site was fast with the peak occurring at 0.25 h, the first time point in our study. The estimated slope and intercept coefficients and absorption rate constant are listed in Table 1.
Figure 2.
Time course of plasma MPL following 50 mg/kg intramuscular injection in normal rats. Solid dots are individual data and the line represents the model fitted curve (Eq. (1)).
CS Suppression
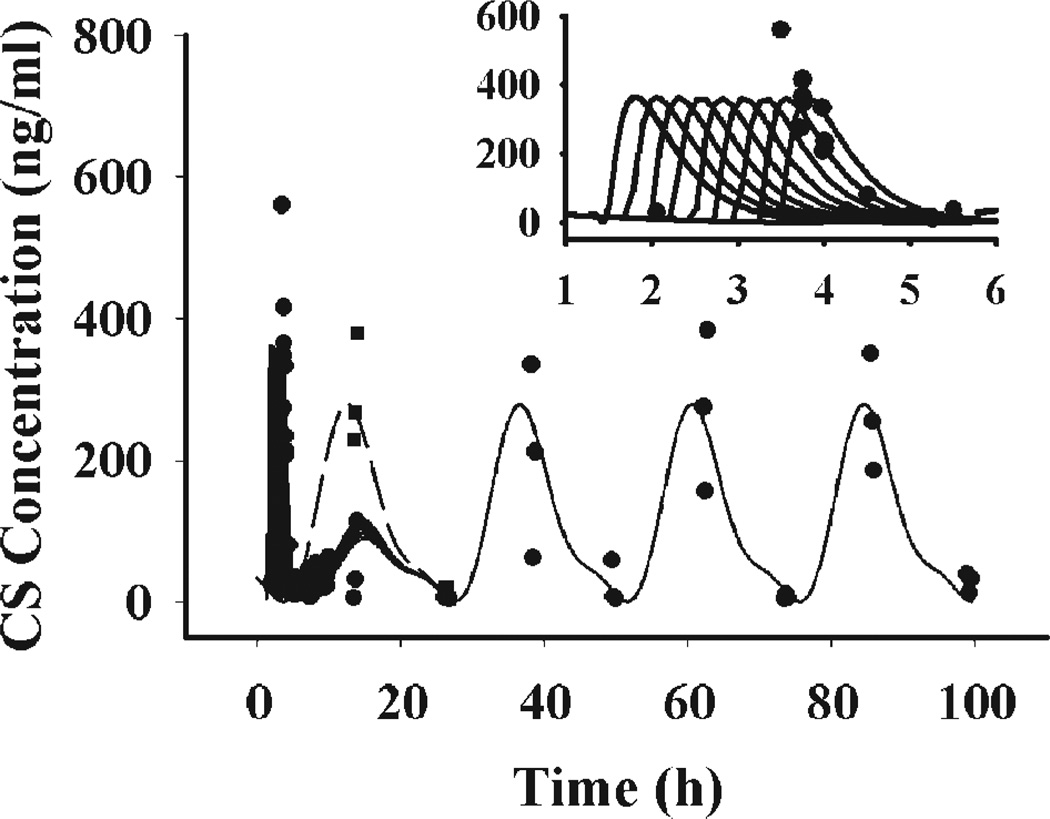
Normal rat plasma CS exhibited a circadian rhythm fluctuating between below quantitation limit during the early morning to about 300 ng/mL at the onset of darkness (Fig. 3). Following MPL treatment, plasma CS first showed a striking increase from almost zero to about 300–600 ng/ mL. However, this increase of plasma CS did not last long and quickly dropped within 2 h. Superimposing the CS data after MPL dosing with the normal CS circadian rhythm curve showed that plasma CS was suppressed after drug treatment. The CS peak at the onset of darkness after dosing was significantly lower than expected. Additionally, the suppressive effect of MPL only lasted for 24 h. Although higher variability was shown for data after 24 h, it could be seen that the plasma CS returned to its normal circadian rhythm thereafter.
Figure 3.
Time course of plasma CS following 50 mg/ kg MPL in normal rats. Solid dots (●) and solid squares (■) represent individual data of MPL and vehicle-treated animals. Solid lines represent the model fitted curves at treatment times of 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25, and 3.5 h. The dashed line is plasma CS circadian rhythm under no drug treatment. The right upper panel shows the data with fitted curves during the early period.
The proposed model was able to capture all features of the dynamics, including initial stimulation, later suppression, and the late return to the normal circadian rhythm. The fitted curves are shown in Figure 3. In this study, animals were treated at 9 different times between 1.5 and 3.5 h, therefore 9 predicted curves are shown, which overlap after 24 h. The right upper corner shows the data and predictions at early periods. Most animals sacrificed at early time points (0.5–4 h) were dosed between 3.25 and 3.5 h. Thus the predicted curves with dosing times of 3.25 and 3.5 h better characterized the data in the early period, which can be clearly seen from the visual predictive plots.
The estimated parameters are listed in Table 2. The maximum stress-induced CS production rate/ volume is estimated at 3230 ng/mL/h, suggesting the production and release of CS is very sensitive to physical stress. The kH representing the disappearance rate of the stress-induced production rate/volume was 2.19 h−1suggesting that this effect vanishes very fast. The concentration of MPL producing 50% of maximum inhibition (IC50) is 23.4 ng/mL and is higher than the corresponding value (0.52–1.87 ng/mL) in humans, suggesting less sensitivity of endogenous steroid production to MPL inhibition.2
Table 2.
Pharmacodynamic Parameters for CS Suppression by MPL
| Parameter (Units) | Definition | Estimate | %SEM |
|---|---|---|---|
| Structural | |||
| kout_CS (h−1) | CS elimination rate constant | 4.56 | 31.6 |
| IC50 (ng/mL) | Concentration at 50% maximum inhibition | 23.4 | 26.6 |
| SH (ng/mL/h) | Maximum CS production rate/volume by stress | 3230 | 21.5 |
| kH (h−1) | Diminishing rate of stress-induced CS production rate/volume | 2.19 | 20.2 |
| Residual variability | |||
| Proportional (%) | Proportional random error | 43.8 | 29.4 |
| Additive (ng/mL) | Additive random error | 18.1 | 33.7 |
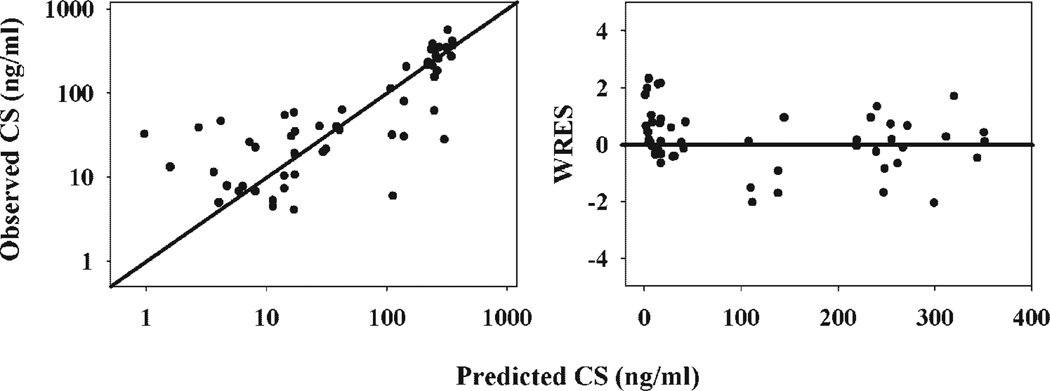
In the modeling of CS suppression, the combined CCV plus additive model was selected to estimate the random error. The GOF plots are provided in Figure 4. The proportional random error was 43.8% and the additive error was 18.1 ng/mL. Relatively high values of random errors are due to the fact that inter-subject variability could not be estimated. Since there is no prior knowledge of this variability, we assumed all variability comes from random error.
Figure 4.
Goodness-of-fit plots of modeling CS responses following 50 mg/kg MPL in normal rats. Solid dots are individual data.
Figure 5 shows visual predictive plots of CS suppression following MPL dosing. Only three injection times were used in the simulation in order to condense the data. The solid curves represent median CS responses while the dashed lines represent the 90% predictive interval. The insert panels show the predictions at early periods. Most data are within the 90% predictive interval, which suggests that the model well characterized CS response to MPL treatment.
Figure 5.
Visual predictive plots of modeling CS responses following 50 mg/kg MPL in normal rats. Animals dosed at 1.5 and 1.75 h were simulated with Tinj = 1.63 h. Those dosed at 2, 2.25, 2.5, and 2.75 h were simulated with Tinj = 2.38 h, and animals dosed at 3, 3.25, and 3.5 h had Tinj = 3.25 h. Solid dots are individual data and the solid lines represent median CS responses. The dashed lines represent the 90% percentiles. The insert panels show predictions during the early period.
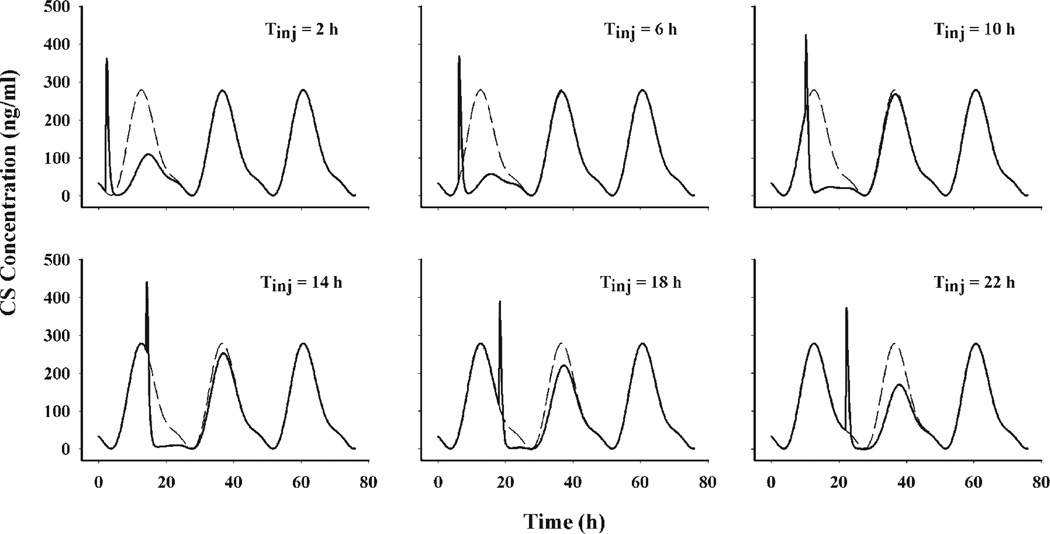
The circadian baseline of CS leads to diverse extents of disturbance at different dosing times. Simulations were performed to illustrate the influence of treatment time on extent of CS suppression after 50 mg/kg i.m. MPL. Figure 6 shows the simulations of six treatment times during a 24-h period. Treatment at 6 h leads to the maximum extent while treatment at 18 h leads to a minimum extent of CS suppression.
Figure 6.
Simulations of plasma CS concentrations following 50 mg/kg MPL at injection times of 2, 6, 10, 14, 18, and 22 h. The solid lines represent simulated profiles following MPL treatment while the dashed lines depict plasma CS circadian baselines.
Lymphocytopenia
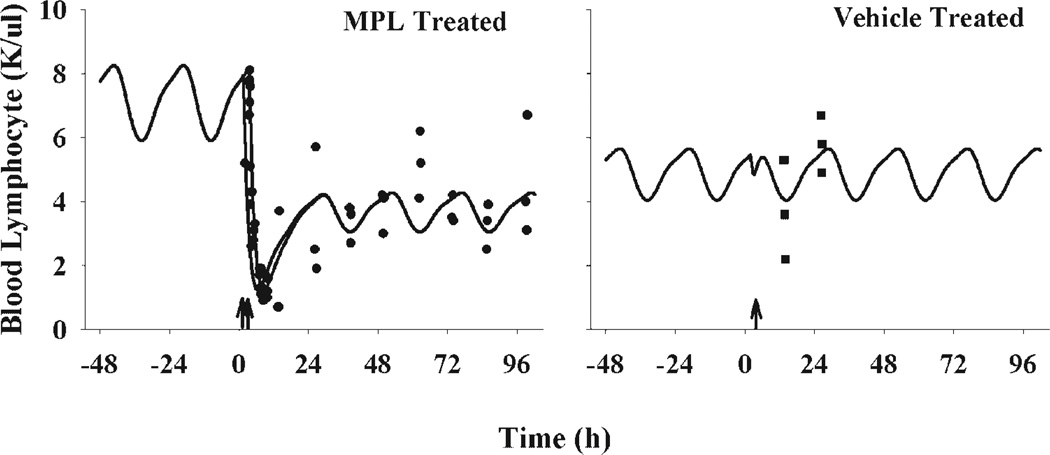
Following MPL, lymphocyte numbers decreased from 8 K/µL to 1 K/µL within 8 h. Lymphocytes started to increase 12 h after dosing and reached a new steady-state. Since it is known that both exogenous MPL and endogenous CS drive the movement of lymphocytes between tissues and blood, blood lymphocyte numbers exhibit a circadian rhythm before and after drug treatment. Although the high variability in the later phase concealed the circadian changes, the model was developed based on the assumption that both steroids serve as driving forces. Figure 7 displays the data following MPL and vehicle treatment along with fitted curves. Two fitted curves are shown for the treatment group, representing dosing times of 1.5 and 3.5 h. In order to demonstrate the circadian rhythm before drug was given, the predicted blood lymphocyte profile 48 h before dosing is also shown. The proposed model well captured the data. The predicted curve suggests that after 50 mg/kg MPL dosing, lymphocytes return to half the original numbers until 96 h. Lymphocyte numbers at 12 and 24 h after vehicle treatment are also captured by the model, with a lower baseline than the MPL group. The dent in the control profile was caused by stimulation of CS production by the animal handling procedure. The fitted curve with a treatment time of 2 h is shown for the vehicle group.
Figure 7.
Time course of blood lymphocytes following 50 mg/kg MPL or vehicle intramuscular injection in normal rats. Solid dots are individual data. The solid lines represent model fitted curves at treatment times of 1.5 and 3.5 h for the MPL group and treatment time of 2 h for the vehicle group. The arrows on the time-axis are selected Tinj.
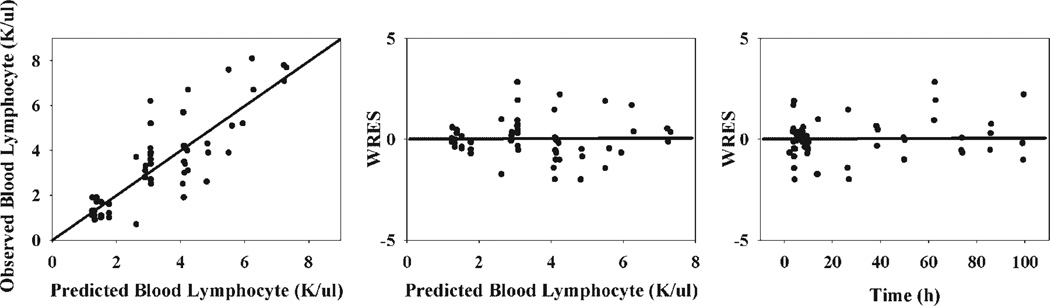
All parameters were estimated with reasonable precision and are listed in Table 3. The kout of 0.548 h−1 suggests that the half-life of blood lymphocyte movement from blood to tissues is about 1.3 h. The value of kin is much lower than kout primarily due to the very large peripheral pool compared to blood. The IC50_M was estimated at 29.5 ng/mL. Due to overparameterization, IC50_C was fixed as 20-fold of IC50_M, resulting in a value of 590 ng/mL. The control group baseline is about 68.5% of the treated group. An additive model was selected to estimate the random variability in the modeling of blood lymphocyte numbers based on OFV and GOF plots (Fig. 8). The random error was 1.11 K/µL.
Table 3.
Pharmacodynamic Parameters for Lymphocytopenia Induced by MPL
| Parameter (Unit) | Definition | Estimate | %SEM |
|---|---|---|---|
| Structural | |||
| kout (h−1) | Blood to tissue trafficking rate constant | 0.548 | 16.5 |
| IC50_M (ng/mL) | MPL concentration at 50% maximum inhibition | 29.5 | 30.5 |
| LYMB (0) (K/µL) | Blood compartment lymphocyte baseline | 7.8 | Fixed |
| LYMT (0) (K/µL) | Tissue compartment lymphocyte baseline | 400 | Fixed |
| BaselineC (%) | Baseline ratio between control and treated animals | 68.5 | 10.9 |
| kin (h−1) | Tissue to blood trafficking rate constant | 0.0115 | 20.2 |
| k (1/(ng/mL/h)) | Second-order cell loss rate constant | 0.000258 | 16.8 |
| IC50_C (ng/mL) | CS concentration at 50% maximum inhibition | 590 | NAa |
| Residual variability | |||
| Additive (K/µL) | Additive random error | 1.11 | 19.9 |
Secondary parameter.
Figure 8.
Goodness-of-fit plots of modeling lymphocytopenia following 50 mg/kg MPL in normal rats. Solid dots are individual data.
DISCUSSION
CS Suppression
Indirect response models have been successfully used to characterize diverse effects of corticosteroids including adrenal suppression by exogenous corticosteroids in humans.2,9,11 In the present study, the model well captured the plasma CS response to the single dose of MPL in rats. Besides regulation by MPL, the model included the CS response to physical stress. Endogenous glucocorticoids are involved in animal adaptation to stress. In response to external challenge or threats, glucocorticoids can increase many-fold.21 This phenomenon was observed in the present study. Although we tried to limit disturbance of the animals, a marked rise in plasma CS was seen within 2 h of animal handling. We applied an empirical function to describe this process. When plotted versus time after dosing, plasma CS peaked at the time of handling and then disappeared with time (data not shown). A transient production rate which diminished at a first-order rate was added into the model. We assumed that this handling-induced CS production was independent of its regular circadian production. The model was able to capture the sharp increase and retreat of CS in the early period. However, this empirical model may not be appropriate to apply in other situations. A similar model was used by Sallstrom et al.22 to describe the body temperature increase following animal surgery.
The extent of drug response is dependent on the baseline level for indirect response models.23 A higher baseline and thus higher production rate brings about more marked drug responses than a lower baseline. Since MPL exerts its suppressive effect on the production of plasma CS and the production rate shifts with time of day, it is expected that different dosing times can produce different extents of suppression. Our simulations with various injection times were consistent with these expectations. As illustrated in Figure 6, dosing MPL when the CS baseline is increasing results in greater suppression while dosing during the decreasing phase only causes a minimal disturbance. Since the handling effect is independent of the CS circadian production rate in the model, its induced CS enhancement does not differ much with various dosing times.
Animals were dosed at nine different times relative to the beginning of the light period. Different extents of CS responses necessitate the use of individual dosing times instead of an averaged time. Additionally, the rapid CS rise after drug injection demands an accurate dosing time during data analysis. In the present study, each animal’s exact Tinj was utilized in the modeling in order to better characterize the early changes of drug responses.
Lymphocytopenia
Lymphocytes are part of the body’s defense system that detects and fights intrusion by microbial organisms and other foreign matter. This component of the immune system is unique in that cells continuously circulate between blood and the lymphatics in an attempt to increase the chance of detecting microbial organisms and deliver lymphocytes expressing specific receptors to the site of inflammation. Blood contains only 2% of the total lymphocytes in the body, while the remaining cells reside in lymphoid tissue.24 In many situations, the alteration of blood lymphocyte numbers mainly reflects changes in movement of the cells between blood and lymphatic tissues. Due to the large pool of lymphocytes in peripheral tissues, small changes of cell entry and exit may cause marked fluctuations in blood. On the other hand, alterations of the peripheral lymphatic system might be reflected in blood as decreased or increased lymphocyte numbers. Lymphocytopenia following MPL might be due to increased cell sequestration in peripheral tissues or reduced total lymphocyte numbers in peripheral lymphatic tissues or both.
Besides their regulation of cell trafficking, high concentrations of corticosteroids produce large reductions in the mass of lymphoid tissues including thymus, spleen and lymph nodes.21 A single dose of 5 mg/kg dexamethasone reduces the rat thymus gland from 969 to 145 g.18 The loss of lymphoid tissue is primarily due to apoptosis of lymphocytes rather than destruction of stromal cells or supporting structures.21 In this study, thymocyte numbers decreased from 109 to 3 × 107.18 Although immature lymphocytes are more sensitive to this effect, certain fully differentiated subtypes such as natural killer cells, T cytotoxic, and T helper cells exhibit DNA fragmentation and cell death in the presence of corticosteroids. 25–27 Additionally, the adrenocortical response is critical to the recovery of rats from experimental allergic encephalomyelitis (EAE), an animal model characterized by T-cell infiltration and inflammation of the central nervous system.28 Adrenalectomized rats, which do not produce endogenous CS, develop rapid and fatal EAE with only 1.9–3.8% of T cell apoptosis upon transfer of myelin basic protein-sensitized T cells. However, the same procedure in normal rats is associated with increased circulating CS, 32% T cell apoptosis, and disease remission after 7 days.28 The cytolytic effect of glucocorticoids is reflected as decreased circulating lymphocytes.21
Several studies were performed in humans to assess the response of blood lymphocytes to systemic administration of various corticosteroids including dexamethasone, MPL, and prednisolone.2,11,29,30 In those studies, lymphopenia induced by corticosteroids was mainly accounted for by cell trafficking instead of cell loss. Examination of the data revealed that human blood lymphocytes returned to the baseline circadian rhythm in 24 h. However in the present study, blood lymphocyte numbers attained about half of original values 96 h after MPL dosing. This discrepancy can be attributed to two reasons. First, human lymphocytes are more resistant to the destructive functions induced by corticosteroids.13 Second, the doses used in these human studies were relatively low compared to the present dose. The proposed model differs from those previous models in that it incorporates both lymphocyte trafficking between blood and tissue and cell apoptosis in tissue. This model not only evaluates the effect of steroids on lymphocytes in blood, but also reflects the cell changes in peripheral tissues. The tissue compartment was ignored in those previous models, obviating their usefulness when lymphocytes in tissue are also affected by drug.
We utilized a cell killing model with the loss rate dependent on both MPL concentration and lymphocyte number. We assumed that only exogenous corticosteroids are capable of inducing cell apoptosis. It has been found that even endogenous steroid may induce lymphocyte apoptosis in pathological conditions or under stress. Long-term stress induced high levels of endogenous glucocorticoids are associated with involution of the thymus and a decrease of mass of all lymphoid tissues.21 However in our study, CS concentrations were maintained within the normal physiological range during most of our study. Thus apoptosis by endogenous CS was not included in our modeling.
In modeling of lymphocytopenia, effects from both exogenous and endogenous steroids need to be incorporated. It has been demonstrated that the circadian rhythm of blood lymphocytes is a regular and highly reproducible phenomenon.24,31 A circadian rhythm was not apparent in our study, perhaps due to relatively high inter-individual variability. In our study, each data point was obtained from an individual animal. Additionally, blood lymphocyte number varies with animal growth.32,33 Although we tried to maintain sampling times within 5 days, age differences may produce a noticeable alteration for young rats at the age of 8 weeks.
The present model does not include production and removal of lymphocytes from the lymphoid system. We recognize the fact that lymphocytes are continuously being produced in various anatomical sites, primarily in bone marrow. However, the complexity of the model and available data do not allow accurate estimation of these two processes. We assumed that during our study period, the production and elimination of lymphocytes are balanced and do not contribution to the observed drug response.
The blood compartment lymphocyte baseline (LYMB (0)) was fixed to 7.8 K/µL. Simulations suggest that the initial condition of the baseline value does not affect model estimates. The tissue compartment lymphocyte baseline (LYMT (0)) is correlated with kin. However, blood lymphocytes comprise 2% of total lymphocytes in the body and thus LYMT (0) was fixed as 400 K/µL.24 This value should be interpreted as lymphocyte number per µL if the volume of tissue compartment is assumed the same as the blood compartment.
The estimated MPL concentration leading to 50% maximum inhibition of total lymphocyte trafficking (IC50_M) in rats is comparable to the corresponding values in humans, which is 9.93 ng/mL for T helper cells and 58.0 ng/mL for T suppressor cells. The CS concentration producing 50% maximum inhibition (IC50_C) is higher than that reported in humans, suggesting lower sensitivity of cell trafficking to endogenous steroid in rats.2,10 It has been demonstrated that although plasma CS circadian rhythm in rats has a higher magnitude of fluctuation (0– 300 ng/mL) than cortisol in humans (10–80 ng/ mL), total lymphocyte numbers oscillate to a similar degree in both species.2,10,31,34 This is consistent with the difference in IC50 values between rats and humans.
This study was limited by the absence of lymphocyte subtype information as total blood lymphocytes were used as the biomarker. Different subpopulations of lymphocytes respond differently to exogenous corticosteroids. For cell trafficking, T-helper cells and B cells are more sensitive to both endogenous and exogenous steroids than T-suppressor cells.2,11,35–37 Certain subtypes of lymphocytes such as natural killer cells, cytotoxic T cells and T-helper cells undergo apoptosis in response to corticosteroids, while mature B cells are generally insensitive.12 Thus the present model and parameters reflect the pooled responses of several types of lymphocytes.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Nancy Pyszczynski and Ms. Suzette Mis for technical assistance and Dr. Donald E. Mager and Dr. Jurgen Bulitta for the help in data analysis. This study was supported by Grant No. GM 24211 from the National Institutes of Health and by a research grant from NASA.
Abbreviations
- CS
corticosterone
- MPL
methylprednisolone
- HPA
hypothalamus-pituitary-adrenal axis
- PK
pharmacokinetic
- PD
pharmacodynamic
- CV
coefficient of variation
- FOCE
first-order conditional estimation
- CCV
constant coefficient variance
- OFV
objective function value
- GOF
goodness of fit
REFERENCES
- 1.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 2.Stark JG, Werner S, Homrighausen S, Tang Y, Krieg M, Derendorf H, Moellmann H, Hochhaus G. Pharmacokinetic/pharmacodynamic modeling of total lymphocytes and selected subtypes after oral budesonide. J Pharmacokinet Pharmacodyn. 2006;33:441–459. doi: 10.1007/s10928-006-9013-5. [DOI] [PubMed] [Google Scholar]
- 3.Oneda K. Dexamethasone-induced apoptosis in peripheral T lymphocytes from patients with asthma. Arerugi. 1999;48:13–22. [PubMed] [Google Scholar]
- 4.Fokkens WJ, van de Merwe JP, Braat JP, Over-beek SE, Hooijkaas H. The effect of intranasal and inhaled corticosteroids in healthy volunteers on the number of circulating lymphocytes and lymphocyte subsets. Allergy. 1999;54:158–164. doi: 10.1034/j.1398-9995.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Gossum A, Schmit A, Peny MO. Oral budesonide for lymphocytic colitis. Am J Gastroenterol. 1998;93:270. doi: 10.1111/j.1572-0241.1998.270_1.x. [DOI] [PubMed] [Google Scholar]
- 6.Milgrom H. Asthma—Something old, something new. Postgrad Med J. 1991;67:S13–S19. [PubMed] [Google Scholar]
- 7.Majori M, Piccoli ML, Bertacco S, Cuomo A, Cantini L, Pesci A. Inhaled beclomethasone dipropionate downregulates CD4 and CD8 T-lymphocyte activation in peripheral blood of patients with asthma. J Allergy Clin Immunol. 1997;100:379–382. doi: 10.1016/s0091-6749(97)70252-5. [DOI] [PubMed] [Google Scholar]
- 8.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology. 2003;28:207–227. doi: 10.1016/s0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty A, Krzyzanski W, Jusko WJ. Mathematical modeling of circadian cortisol concentrations using indirect response models: Comparison of several methods. J Pharmacokinet Biopharm. 1999;27:23–43. doi: 10.1023/a:1020678628317. [DOI] [PubMed] [Google Scholar]
- 10.Yao Z, DuBois DC, Almon RR, Jusko WJ. Modeling circadian rhythms of glucocorticoid receptor and glutamine synthetase expression in rat skeletal muscle. Pharm Res. 2006;23:670–679. doi: 10.1007/s11095-005-9608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 12.Schwartzman RA, Cidlowski JA. Glucocorticoid-induced apoptosis of lymphoid cells. Int Arch Allergy Immunol. 1994;105:347–354. doi: 10.1159/000236781. [DOI] [PubMed] [Google Scholar]
- 13.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann Intern Med. 1993;119:1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun YN, McKay LI, DuBois DC, Jusko WJ, Almon RR. Pharmacokinetic/Pharmacodynamic models for corticosteroid receptor down-regulation and glutamine synthetase induction in rat skeletal muscle by a receptor/gene-mediated mechanism. J Pharmacol Exper Ther. 1999;288:720–728. [PubMed] [Google Scholar]
- 16.Ramakrishnan R, DuBois DC, Almon RR, Pyszczynski NA, Jusko WJ. Fifth-generation model for corticosteroid pharmacodynamics: Application to steady-state receptor down-regulation and enzyme induction patterns during seven-day continuous infusion of methylprednisolone in rats. J Pharmacokinet Pharmacodyn. 2002;29:1–24. doi: 10.1023/a:1015765201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haughey DB, Jusko WJ. Analysis of methylprednisolone, methylprednisone and corticosterone for assessment of methylprednisolone disposition in the rat. J Chromatogr. 1988;430:241–248. doi: 10.1016/s0378-4347(00)83159-x. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg NB, Evans-Storms RB, Cidlowski JA. In vivo resistance to glucocorticoid-induced apoptosis in rat thymocytes with normal steroid receptor function in vitro. Endocrinology. 1997;138:810–818. doi: 10.1210/endo.138.2.4912. [DOI] [PubMed] [Google Scholar]
- 19.Jusko WJ. Pharmacodynamics of chemotherapeutic effects: Dose-time-response relationships for phase-nonspecific agents. J Pharm Sci. 1971;60:892–895. doi: 10.1002/jps.2600600618. [DOI] [PubMed] [Google Scholar]
- 20.D’Argenio DZ, Schumitzky A. In: ADAPT II user’s guide: Pharmacokinetic/pharmacodynamic systems analysis software. Version V, editor. Los Angeles: Biomedical Simulation Resource; 1997. [Google Scholar]
- 21.Johnson LR. 1st edition. New York: Raven Press, Ltd; 1992. Essential medical physiology. [Google Scholar]
- 22.Sallstrom B, Visser SA, Forsberg T, Peletier LA, Ericson AC, Gabrielsson J. A pharmacodynamic turnover model capturing asymmetric circadian baselines of body temperature, heart rate and blood pressure in rats: Challenges in terms of tolerance and animal-handling effects. J Pharmacokinet Pharmacodyn. 2005;32:835–859. doi: 10.1007/s10928-005-0087-2. [DOI] [PubMed] [Google Scholar]
- 23.Sun YN, Jusko WJ. Role of baseline parameters in determining indirect pharmacodynamic responses. J Pharm Sci. 1999;88:987–990. doi: 10.1021/js9901155. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell CW, Lacombe F. Evaluation of peripheral blood lymphocytosis. 1st edition. Santa Cruz, CA: Academic Information Systems, Inc; 2000. [Google Scholar]
- 25.Migliorati G, Nicoletti I, D’Adamio F, Spreca A, Pagliacci C, Riccardi C. Dexamethasone induces apoptosis in mouse natural killer cells and cytotoxic T lymphocytes. Immunology. 1994;81:21–26. [PMC free article] [PubMed] [Google Scholar]
- 26.Zubiaga AM, Munoz E, Huber BT. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992;149:107–112. [PubMed] [Google Scholar]
- 27.Nieto MA, Lopez-Rivas A. Glucocorticoids activate a suicide program in mature T lymphocytes: Protective action of interleukin-2. Ann N Y Acad Sci. 1992;650:115–120. doi: 10.1111/j.1749-6632.1992.tb49106.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith T, Schmied M, Hewson AK, Lassmann H, Cuzner ML. Apoptosis of T cells and macrophages in the central nervous system of intact and adrenalectomized Lewis rats during experimental allergic encephalomyelitis. J Autoimmun. 1996;9:167–174. doi: 10.1006/jaut.1996.0020. [DOI] [PubMed] [Google Scholar]
- 29.Xu ZX, Lee MJ, Blum RA, Jusko WJ. Pharmacodynamic modeling of prednisolone effects on natural killer cell trafficking. Pharm Res. 1995;12:459–463. doi: 10.1023/a:1016229307923. [DOI] [PubMed] [Google Scholar]
- 30.Imani S, Jusko WJ, Steiner R. Diltiazem retards the metabolism of oral prednisone with effects on T-cell markers. Pediatr Transplant. 1999;3:126–130. doi: 10.1034/j.1399-3046.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 31.Pelegri C, Vilaplana J, Castellote C, Rabanal M, Franch A, Castell M. Circadian rhythms in surface molecules of rat blood lymphocytes. Am J Physiol Cell Physiol. 2003;284:C67–C76. doi: 10.1152/ajpcell.00084.2002. [DOI] [PubMed] [Google Scholar]
- 32.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 33.Robel GL, Lochmiller RL, McMurry ST, Qualls CW., Jr Environmental, age, and sex effects on cotton rat (Sigmodon hispidus) hematology. J Wildl Dis. 1996;32:390–394. doi: 10.7589/0090-3558-32.2.390. [DOI] [PubMed] [Google Scholar]
- 34.Committee on Military Nutrition Research IoM. Military Strategies for Sustainment of Nutrition and Immune Function in the Field. The National Academies Press; 1999. Accessible online (July, 2007) at http://www.nap.edu/openbook.php?record_id=6450 & page=444. [PubMed] [Google Scholar]
- 35.Cupps TR, Edgar LC, Thomas CA, Fauci AS. Multiple mechanisms of B cell immunoregulation in man after administration of in vivo corticosteroids. J Immunol. 1984;132:170–175. [PubMed] [Google Scholar]
- 36.Walzer PD, LaBine M, Redington TJ, Cushion MT. Lymphocyte changes during chronic administration of and withdrawal from corticosteroids: Relation to Pneumocystis carinii pneumonia. J Immunol. 1984;133:2502–2508. [PubMed] [Google Scholar]
- 37.Ozbek N, Yetgin S, Tuncer AM. Effect of high-dose methylprednisolone and G-CSF treatments on lymphocyte subtypes in neutropenic children with acute lymphoblastic leukemia: A pilot study. Pediatr Hematol Oncol. 1998;15:539–544. doi: 10.3109/08880019809018316. [DOI] [PubMed] [Google Scholar]