Abstract
Myocardial ischemia, which results from emotional provocation, occurs in as many as 30–50% of patients with CAD during the discourse of their daily lives. This emotionally provoked or mental stress ischemia is associated with the poor prognosis, with emerging treatment strategies. This chapter will outline the conceptual constructs which support the pathophysiologic underpinnings, and biobehavioral aspects associated with this mental stress ischemia. We will review a biobehavioral model where cognitive stress is transduced in the brain. The response of the brain to psychosocial stress is a highly sophisticated and integrated process by which sensory inputs are evaluated and appraised for its importance in relation to previous experience and current goals. The biologic consequences of such stress transduced in the CNS has its effect upon the cardiovascular flow and function through changes in autonomic balance, which result in various biologic processes that culminate in the perturbation of flow and function of the heart.
Introduction
The stress during the discourse of our daily lives with attendant emotional provocation has been associated with angina and has been a consistent part of the human historical narrative. The impact of the cartesian duality of mind-body has had profound impact on western thought and has served to minimize the impact of cognitive processes upon biologic outcomes.1 The advent of sophisticated neuroimaging technology and modern bio-behavioral constructs have allowed an empiric approach which has guided recent advances reviewed in this chapter. Specifically, we outline testable hypotheses which address mechanisms that support conceptual constructs of how cognition impacts cardiovascular performance.
Epidemiologic studies have shown that acute coronary events can occur in individuals that lack the traditional high-risk profile for coronary artery disease (CAD),2 highlighting the importance of factors such as psychological and biobehavioral factors we all confront during the course of our daily lives.3 Cardiac manifestations of mental stress induced ischemia can include left ventricular dysfunction,4,5 acute coronary events,6 and cardiac arrhythmias.7,8 Studies have shown that as many as 30 to 50% of CAD patients exhibit transient, symptomless myocardial ischemia during mental stress.9,10,11 Furthermore, up to 2/3 of ischemic episodes detected by ambulatory electrocardiographic monitoring in patients with chronic CAD are asymptomatic, noted at very low workloads, and related to factors like mental stress.12,13 Mental stress ischemia (MSI) has been linked to a three-fold increase in adverse clinical outcomes in patients with CAD.14,15,16,17,18,19
These observations, taken in concert with the high frequency of myocardial infarction during catastrophic environmental events,20,21,22 suggests that the identification of patients who are vulnerable to MSI is highly relevant, especially given current global geopolitical concerns.
In this review, we will discuss the current understanding of the pathophysiology of MSI, a phenomenon that is more complex than the demand and supply paradigm observed with exercise stress testing. We will also discuss recent advances that have led to an enhanced understanding of the brain-heart interactions that occur during MSI.
Physiological correlates of mental stress ischemia
Although there are some shared pathophysiologic commonalities, MSI has distinct physiologic correlates compared to demand-induced ischemia as suggested by differences in hemodynamic, vascular, and neuroendocrine responses (Table 1).23,24 For example, exercise produces a substantially greater increase in heart rate and a more modest elevation in systolic blood pressure,25 which parallels the increased metabolic demand and supply gap created by physical activity. In contrast, diastolic blood pressure and systemic vascular resistance rise during mental stress, but are the same or decreased in response to exercise.24,26 Angina is the usual manifestation of myocardial ischemia triggered by physical exertion, but is noticeably absent in most cases of MSI.27 Electrocardiographic changes are also less apparent with MSI when compared to pharmacological stress.
Table 1.
Physiologic Responses to Mental Stress & Exercise
| Mental Stress | Exercise | |
|---|---|---|
| Heart Rate | + | +++ |
| Systolic BP | ++ | +++ |
| Diastolic BP | + | ↔/− |
| SVR | +/++ | ↔/− |
| Angina | +/− | ++ |
The brain-heart interaction
Given that stress and emotional factors are cognitive in nature, we approached this injury by understanding how stress is transduced by the CNS and how this transduction results in ischemic syndromes. We then analyzed if there were specific brain activation patterns associated with emotional provocation and associated physiologic effectors which may perturb the cardiovascular flow/function relationship. Then we compared these brain activation patterns to those associated with ischemic, which results from a traditional demand (exercise) stimulus.
Recent research focusing on the detection of CNS activity accompanying stress and emotional processing has identified multiple areas of the brain that appear to be intimately involved in the awareness of, and response to, stressful circumstances.28 These areas include both cortical and subcortical regions that have been implicated in processing the emotions provoked by external stimuli, and in mediating executive responses to those same emotions and stimuli.
The “central autonomic network” (CAN) is a label affixed by some to describe the flexible network of dynamically interconnected areas of the brain that are thought to regulate the physiological effects of mediated emotions through autonomic neurohormonal outputs. The neural pathways involved in these processes modulate the autonomic nervous system in an integrative fashion.29 These regions include the medial prefrontal cortex, amygdala, and anterior cingulate gyrus. The medial prefrontal cortex has a general role in emotional processing and specific roles for emotional/evaluative processing and self regulation. The amygdala has been posited as critical to fear related processing,30 and also corresponds to dispositional affective style. The anterior cingulate has a cognitive dorsal segment mediating response inhibition31 and an affective ventral subdivision involved in processing and integrating emotional information.32
The physiologic consequences of stress initiated CNS activation include increases in heart rate and blood pressure, which define hemodynamic reactivity. The prefrontal cortex-limbic system that controls this response has direct and indirect throughputs to neurohormonal systems which act through norepinephrine and cortisol. Additionally, the amygdala has effectors to the lateral hypothalamus that activate sympathetic and cortisol pathways in the periphery.33 Norepinephrine secreted by the locus coeruleus, the major source of norepinephrine-producing neurons, also has direct pathways to the amygdala and the hippocampus. Below the cortex are several neuronal networks in the brainstem, such as C1 cells in the rostral medulla. These networks send afferent signals to the locus coeruleus, which re-amplifies the cortical process via projection to the hypothalamus.
Significantly, the areas of activation that make up the CAN and the stressors that provoke its activation indicate that the CAN is strongly associated with the limbic system and is involved in the negative feedback circuit between the limbic system and the prefrontal cortex. This circuit appears essential to the regulation of autonomic responses to stressful stimuli. A situational imbalance in the activity between these two, or a fixed inability of the prefrontal cortex to attenuate limbic responses, may be linked with autonomic dysregulation which, during mental stress, can result in a net shift towards increased sympathetic tone and/or withdrawal of parasympathetic tone. If chronic, this imbalance can increase risk for cardiovascular disease by promoting processes that lead to vascular inflammation and intimal perturbation.24,34,35 In addition to the acute responses of hemodynamic reactivity, chronic stress with activation of these autonomic systems leads to changes in the endothelium over time, with an increase in atherosclerotic burden, an increase in risk for arrhythmias, and an increase in platelet aggregation and other factors known to enhance the risk for cardiovascular morbidity and mortality.8
Brain activation patterns during mental stress
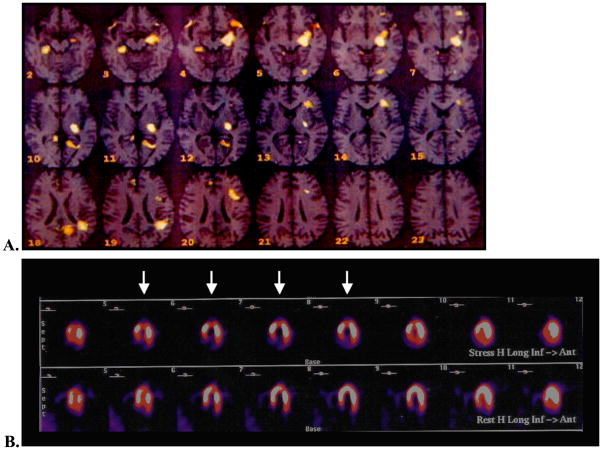
Our group has previously reported the brain activation response to mental stress in subjects with and without CAD utilizing O15 Positron Emission Tomography (PET) imaging of the brain.28 Patients with CAD demonstrated significantly increased blood flow to the left anterior cingulate, the left parietal cortex, and the left parahippocampus, brain regions involved with emotion, memory, attention, and neurohormonal and autonomic regulation. There was also greater limbic to prefrontal activity, suggesting that the limbic regions associated with fear and anxiety possess an enhanced effect on cardiovascular function. In comparison, patients without CAD exhibited brain activation patterns referable to the cognitive effort of the mental task. Among the CAD group, those who became ischemic to mental stress compared to those who did not had even greater activation in the aforementioned sub cortical limbic structures (parahippocampus and related cingulate areas) (Figure 1).
Figure 1.
Top Panel: Brain activation of the Frontal Medial Gyrus and Limbic Hippocampal structures and associated Anterior Cingulate Gyrus.
Bottom Panel: Myocardial Blood flow Image during Mental Stress with reversible ischemia of the apex (arrows).
We have also shown that the patterns of brain activation differ significantly between patients with MSI versus those with demand-related ischemia.36 58 patients with CAD underwent simultaneous O15 PET brain activation imaging and evaluation for myocardial ischemia during a laboratory arithmetic mental stress and infusion of dobutamine, a surrogate for demand stimulus ischemia. 8 patients had ischemia to both mental and dobutamine stress, while 13 had myocardial ischemia to mental stress alone. In comparison to patients with dobutamine-induced ischemia, patients with MSI again demonstrated hyperactivation in frontolimbic circuits associated with neurohormonal and autonomic regulation, emotion, memory, fear, and anxiety. These findings suggest that MSI has a distinct cerebral activation pattern referable to the cognitive nature of mental stress in comparison to demand-induced ischemia where activations occurred in the peripheral sensorimotor areas (Figure 2).
Figure 2.
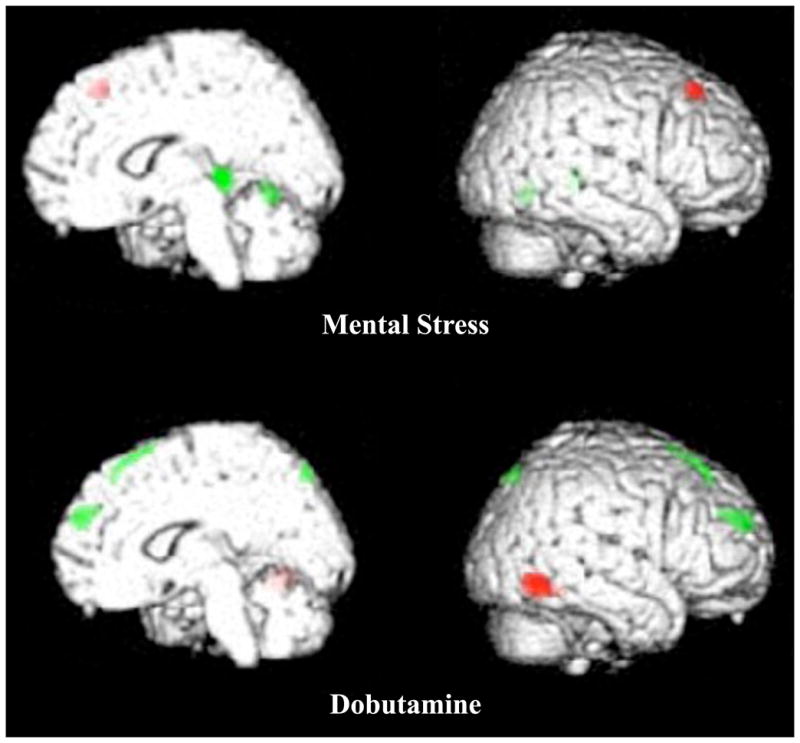
Top panel is the brain activation during mental stress and associated myocardial ischemia. Bottom panel is the brain map of the same subject ischemic to a demand surrogate for exercise with dobutamine infusion. Grey (Green for online version) is activation and black (Red for online version) is deactivation.
Brain activations during mental stress occurs in subcortical areas associated with emotion, memory and Neurohormonal sympathetic regulation. Deactivation occurs in the frontal evaluative areas, such as the dorsal lateral prefrontal cortex association with parasympathetic tone. During dobutaine the activations are associated with somatosensory areas in the periphery of the cortex
Gender Interaction and the Brain during Mental Stress
Our group also demonstrated gender differences in the patterns of brain activation during mental stress.37 Women with CAD have greater bilateral and medial temporal activation (amygdala and hippocampus) during mental stress than do men with CAD. Women with CAD also have greater activation in the prefrontal cortex (middle frontal gyrus and inferior frontal gyrus) than women without CAD, suggesting a greater evaluative component to their response to mental stress. There was no difference in brain activation patterns between men and women without CAD during mental stress.
In summary, when one is ischemic to emotional stress, brain activation occurs in areas that initiate physiologic effectors which accentuate heightened sympathetic response, decrease in parasympathetic tone with associated release of neurohormonal factors which promote augmented systemic/coronary vasculature resistance, and sympathovagal imbalance. Next we will discuss how this brain activation pattern which results in autonomic dysregulation promotes the generation of pro-inflammatory cytokines which render patients vulnerable to myocardial ischemia and/or ventricular arrhythmias.38,39,40
The inflammatory reflex
The inflammatory reflex is fundamental to understanding the pathophysiology of MSI, and describes a cholinergic anti-inflammatory pathway. CNS efferent activity in the vagus nerve leads to acetylcholine (ACh) release in organs of the reticuloendothelial system, including the liver, heart, spleen and gastrointestinal tract. ACh interacts with a-bungarotoxin-sensitive nicotinic receptors (ACh receptor) on tissue macrophages, which inhibit the release of TNF, IL-1, HMGB1 and other cytokines.41,42,43 Thus the observed withdrawal of parasympathetic activity during mental stress results in the converse i.e. the release of inflammatory cytokines and attendant effects on vasomotor tone and function.
The involvement of specific brain regions, including the medial prefrontal cortex, has been reported in the vagal component of heart rate regulation during self-generated emotions.44 We have also found these regions associated with parasympathetic withdrawal during mental stress.
Vagal efferents are distributed widely throughout the reticuloendothelial system and peripheral organs. Brain derived output through vagal efferents is therefore rapid and more precise in location than humorally mediated pathways. The heart is well enervated by vagal efferents, and low parasympathetic tone is a well-described predictor of early post-MI mortality.45 Pyridostigmine, a reversible cholinesterase inhibitor, was recently shown to attenuate the LV systolic and diastolic dysfunction caused by mental stress in patients with CAD, thus highlighting the importance of the parasympathetic system in MSI.46 Parasympathetic withdrawal, which has been observed during mental stress,47 results in the generation of various inflammatory cytokines such as TNF-α. Macrophages that reside in the myocardial vasculature are a main source of TNF-α48,49 and the release of TNF-α is a significant contributor to cardiac dysfunction and cardiomyocyte death in such diverse conditions as sepsis, chronic heart failure, ischemic reperfusion injury, and transplant rejection.48 The rapidity with which the inflammatory reflex operates, and the wide distribution of macrophages in diseased coronary vessels, implicates this reflex as a mechanism through which mental stress acts on coronary microvascular beds. Thus, the parasympathetic withdrawal seen during mental stress causes the synthesis and release of TNF-α as well as other inflammatory cytokines by macrophages. This, in turn, results in the subsequent stimulation and release of macrophage-derived ET-1, which augments the effects of endothelial ET-1.
As direct evidence of the role inflammation plays in MSI, our group reported a positive correlation between C reactive protein (CRP) and MSI. Each unit (1mg/ml) increase in CRP level was associated with a 20% higher risk of MSI (OR 1.2, 95%CI 1.01–1.29, p=0.04).35 These results correlate well with the more recent findings of Kop et al50 who reported that mental arithmetic and anger recall both cause transiently increased levels of the inflammatory markers CRP and interleukin-6 in patients with CAD. Baseline inflammatory markers were higher in CAD patients than controls as expected. Kop et al further demonstrated that patients with high catecholamine responses to mental stress also had exaggerated increases of these inflammatory markers. They hypothesized that the individuals who reacted to mental stress with higher levels of epinephrine and norepinephrine may be at increased risk of CAD progression and atherosclerotic plaque rupture due to higher circulating levels of CRP and pro-inflammatory cytokines.
We have discussed how cognitive mental stress is transduced in the brain and results in hyperactivation of sympathetic output with concomitant decrement in parasympathetic tone with the generation of inflammatory vascular factors. We next turn our attention to how this pathophysiology impacts myocardial blood flow in response to mental stress.
Myocardial blood flow and endothelial function
Mental stress induces heterogeneity of myocardial perfusion in patients with CAD. In the earliest demonstration of this effect, Deanfield et al51 used PET to demonstrate that 12 of 16 patients with CAD and positive exercise stress tests developed regional myocardial perfusion abnormalities during mental stress. Although the rate-pressure product was lower with MSI compared to exercise stress, the degree of the perfusion defects was comparable. Others have demonstrated similar effects using different imaging modalities.52
In a PET study of patients with CAD, our group compared the coronary flow reserve (CFR) response during mental stress to the CFR during persantine infusion.53 Using this technique, we measured absolute blood flow. We noted that mental stress often resulted in impaired myocardial blood flow in coronary distributions served by epicardial vessels with subcritical epicardial blockages. Furthermore, the regions with lower myocardial blood flow reserve during mental stress showed increased coronary microvascular resistance compared to vascular beds with normal CFR. These findings have been shown by others using different techniques,54 which suggests that impairment of the vasomotor response in the coronary microvascular bed is an essential feature of MSI. Coronary angiography studies have similarly shown that mental stress promotes epicardial coronary vasoconstriction in vascular beds with subcritical disease.55,56 In addition, this mental stress-induced vasoconstriction occurs in the same coronary segments that demonstrate a paradoxical constrictor response to intracoronary ACh infusion.55 In total, these findings implicate the coronary endothelium and, in particular, endothelial dysfunction in the pathophysiology of MSI.
Role of the peripheral adrenergic system and ET-1
In patients with CAD, mental stress produces an increase in sympathetic tone evidenced by elevations of epinephrine and norepinephrine.57 Norepinephrine has an important role in vasomotor tone during mental stress, causing vasoconstriction via its effects on vascular smooth muscle.
There is increasing evidence that local endothelial factors, like endothelin-1 (ET-1), play an important role in mediating this vasoconstriction response to norepinephrine. For example, at concentrations that exert only a minimal direct pressor effect, ET-1 was found to nearly double the degree of vasoconstriction that was observed during an infusion of norepinephrine in diseased coronary artery segments.58 During a laboratory mental stress, the blockade of ET-1 by the specific endothelin-A receptor blocker BQ-123 significantly attenuated the degree of vasoconstriction observed during norepinephrine infusion.59 These findings suggest that sympathetic activation may act through ET-1 to produce arterial vasoconstriction, particularly in the microvascular bed. Furthermore, we have observed in preliminary data a rise of ET-1 in CAD subjects during a mental stress provocation.
Mental stress, autonomic tone and inflammation
The vasoconstrictive effects of ET-1 may last for hours. In contrast, MSI is a transient condition that resolves in most cases when the stress is terminated. Therefore, additional factors are required to explain the vascular findings in MSI studies. Autonomic dysregulation in response to mental stress promotes the aforementioned Inflammatory processes which promotes further factors as well as accentuate the biologic effect of such factors upon the coronary vasculature. For example as previously mentioned, the “inflammatory reflex”, states that the reduction in parasympathetic tone and ACh, serves to generate macrophage release of TNF-α. In turn, TNF-α, a potent pro-inflammatory cytokine, can provoke the release of ET-1 from macrophages60,61 and has been observed in combination with ET-1 to promote vasoconstriction of the microvascular bed.62 TNF-α significantly impairs endothelial function, and is thought to be involved in atherosclerotic plaque rupture, coronary artery vasospasm, and ischemic injury.63
The hyperactivation of the sympathetic nervous system during mental stress also plays an important pro-inflammatory role, working through α-2 receptors to increase production of TNF-α and comparable agents and substantially augment the vascular effects of ET-1.
Thus the mental stress response is initiated in the CNS which results in the divergence of the autonomic nervous system with an increase in sympathetic and decrease in parasympathetic tone (Figure 3). This divergence serves to promote the generation of inflammatory vascular factors, and synergistically promotes vascular endothelial dysfunction and vasoconstriction which results in myocardial ischemia.
Figure 3.
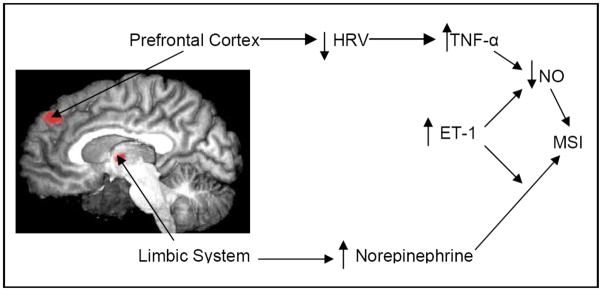
Mental Stress Provocation results in activation of visceral effectors from the CNS that promote parasympathetic withdrawal (HRV) and accentuation of sympathetic tone (Norepinephrine). This imbalance promotes inflammatory vascular factors which serve to promote vascular dysfunction and results in Mental Stress Ischemia (MSI)
Mental stress and genetics
There may be individual, inheritable differences in the degree of adrenergic stimulation triggered by MSI. Hassan et al64 recently reported that individual responses to mental stress may arise in part from genetic polymorphisms of the beta-1 adrenergic receptor (ADRB1). They demonstrated that patients homozygous for the Ser49 allele of the ADRB1 gene (codon 49) were 3 times more likely to have MSI on myocardial perfusion imaging than carriers of an alternative allele. Much work remains to be done to identify other genetic determinants underlying the individual variation in the neurohormonal activation of MSI.
Prognostic implications and clinical application
In patients with known CAD, MSI has consistently been shown to be associated with an increased risk of adverse cardiovascular outcomes and mortality.15,16,65 The PIMI investigators have reported on the largest population studied in the laboratory regarding the prognostic significance of MSI in their multi-center study. In this study of 196 patients who had undergone mental stress testing in the laboratory, patients were followed for an average of 5.2 years. During the follow-up period there were 17 deaths. New wall motion abnormalities during laboratory mental stress were seen in 40% of those who subsequently died, but only 17% of those who survived (rate ratio=3.0; p<0.04). Other indicators of ischemia during MS testing, including LV ejection fraction and/or ECG changes did not predict death, a finding with important implications for future research. This is the only controlled study to show an effect of mental stress on death.
The identification of specific psychologic factors that contribute to the risk of MSI may help determine when mental stress testing is likely to provide important prognostic information. For example, patients with easily provokable anger and hostility have twice the incidence of CAD with a 5-fold increased risk of recurrent MI.66 Hostility has also been associated with the accelerated progression of atherosclerosis67 and a higher rate of post-angioplasty restenosis.68 Data from our group demonstrates that patients identified by interview as having a hostile, angry, emotionally arousable psychological profile have an increased risk of MSI,9 suggesting that this type of clinical assessment may be useful in identifying patients for whom mental stress testing may provide prognostic cardiovascular information. Stress can be modified through numerous approaches including stress management, meditation, and cognitive behavioral therapy, and treatment strategies specifically tailored to decrease mental stress have been shown to decrease the incidence of cardiovascular events.69,70
Conclusions
Much work has been done to illuminate the pathophysiology of mental stress ischemia, but much more work remains to be completed before mental stress testing enters clinical practice as a viable diagnostic strategy to benefit patients at risk. The further identification of risk factors to help stratify patients into low, intermediate, and high risk probabilities for mental stress ischemia, similar to the Framingham Risk score, would be a major advance in this direction. Published reports using noninvasive techniques, which outline a “mental stress test”, are emerging.71 A national consensus statement defining guidelines for mental stress testing, similar to those existing for exercise stress testing, would promote the standardization of future research in the field. The increasing demands of a competitive modern global society require that we broaden our understanding of the impact of these cognitive adjustments on our biology.
Contributor Information
Robert Soufer, Professor of Medicine, Chief of Cardiology VACT Healthcare System, Yale University School of Medicine, VA-CT Healthcare System, 950 Campbell Avenue, 111B, West Haven, CT 06516
Hitender Jain, Instructor of Medicine, VACT Healthcare System, Yale University School of Medicine, VA-CT Healthcare System, 950 Campbell Avenue, 111B, West Haven, CT 06516
Andrew J. Yoon, Cardiovascular Medicine Fellow, VACT Healthcare System, Yale University School of Medicine, VA-CT Healthcare System, 950 Campbell Avenue, 111B, West Haven, CT 06516
References
- 1.Damasio A. Descartes’ Error: Emotion, Reason, and the Human Brain. Avon Books; New York: 1994. [Google Scholar]
- 2.Ruberman W, Weinblatt E, Goldberg JD, Chaudhary BS. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1984;311:552–559. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- 3.Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki N, Kinugawa T, Yamawaki M, Furuse Y, Shimoyama M, Ogino K, Igawa O, Hisatome I, Shigemasa C. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ J. 2004;68:1081–1083. doi: 10.1253/circj.68.1081. [DOI] [PubMed] [Google Scholar]
- 5.Wittstein IS, Thiemann DR, Lima JA. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 6.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 8.Lampert R, Jain D, Burg MM, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101:158–164. doi: 10.1161/01.cir.101.2.158. [DOI] [PubMed] [Google Scholar]
- 9.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Card. 1993;22:440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 10.Barry J, Selwyn AP, Nabel EG, Rocco MB, Mead K, Campbell S, Rebecca G. Frequency of ST-segment depression produced by mental stress in stable angina pectoris from coronary artery disease. Am J Cardiol. 1998;61:989–993. doi: 10.1016/0002-9149(88)90112-9. [DOI] [PubMed] [Google Scholar]
- 11.Specchia G, Falcone C, Traversi E, La Rovere MT, Guasti L, De Micheli G, Ardissino D, De Servi S. Mental stress as a provocative test in patients with various clinical syndromes of coronary heart disease. Circulation. 1991;83:II-108–II-114. [PubMed] [Google Scholar]
- 12.Stern S, Tzivoni D. Early detection of silent ischemic heart disease by twenty four hours electrocardiographic of active subject. Br Heart J. 1974;36:481–486. doi: 10.1136/hrt.36.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schang SJ, Peppine C. Transient asymptomatic depression during daily activity. Am J Cardiol. 1977;39:396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- 14.Powell LH, Thoresen CE. Behavioral and physiologic determinants of long term prognosis after myocardial infarction. J Chronic Dis. 1985;38:253–263. doi: 10.1016/0021-9681(85)90068-2. [DOI] [PubMed] [Google Scholar]
- 15.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 16.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O’Connor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 18.Ruberman W, Weinblatt E, Goldberg JD, Chaudhary BS. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1984;311:552–559. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- 19.Rosengren A, Tibblin G, Wilhelmsen L. Self-perceived psychological stress and incidence of coronary artery disease in middleaged men. Am J Cardiol. 1991;68:1171–1175. doi: 10.1016/0002-9149(91)90189-r. [DOI] [PubMed] [Google Scholar]
- 20.Chi JS, Poole WK, Kandefer SC, Kloner RA. Cardiovascular mortality in New York City after September 11, 2001. Am J Cardiol. 2003;92(7):857–61. doi: 10.1016/s0002-9149(03)00901-9. [DOI] [PubMed] [Google Scholar]
- 21.Gurfinkel EP, Bozovich GE, Dabbous O, Mautner B, Anderson F. Socio economic crisis and mortality. Epidemiological testimony of the financial collapse of Argentina. Thromb J. 2005;3:22. doi: 10.1186/1477-9560-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakaeen FG, Huh J, Chu D, Coselli JS, LeMaire SA, Mattox KL, Wall MJ, Jr, Wang XL, Shenaq SA, Atluri PV, Awad SS, Berger DH. Hurricane Katrina impact on cardiac surgery case volume and outcomes. Tex Heart Inst J. 2008;35(3):273–278. [PMC free article] [PubMed] [Google Scholar]
- 23.Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone PH, Forman S, Knatterud G, Sheps DS, Kaufmann PG. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation. 1996;94:2768–2777. doi: 10.1161/01.cir.94.11.2768. [DOI] [PubMed] [Google Scholar]
- 24.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–1322. doi: 10.1016/s0735-1097(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 25.Burg MM, Vashist A, Soufer R. Mental stress ischemia: present status and future goals. J Nucl Cardiol. 2005;12:523–529. doi: 10.1016/j.nuclcard.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 26.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1998;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 27.Schang SJ, Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- 28.Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, Goldman-Rakic P. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci USA. 1998;95(11):6454–6459. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thayer J, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 30.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 32.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahern DK, Gorkin L, Anderson JL, Tierney C, Hallstrom A, Ewart C, Capone RJ, Schron E, Kornfeld D, Herd JA, et al. Biobehavioral variables and mortality or cardiac arrest in the Cardiac Arrhythmia Pilot Study (CAPS) Am J Cardiol. 1990;66:59–62. doi: 10.1016/0002-9149(90)90736-k. [DOI] [PubMed] [Google Scholar]
- 34.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22:440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 35.Shah R, Burg MM, Vashist A, Collins D, Liu J, Jadbabaie F, Graeber B, Earley C, Lampert R, Soufer R. C-reactive protein and vulnerability to mental stress-induced myocardial ischemia. Mol Med. 2006;12(11–12):269–274. doi: 10.2119/2006-00077.Shah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashist A, Burg MM, Arrighi JA, Jadbabaie F, Blumenfeld H, Lampert R, Graeber B, Soufer R. Central nervous system correlates of myocardial ischemia: neurocardiac distinctions between mental stress and dobutamine provocation. Psychosom Med. 2005;67:A8–9. [Google Scholar]
- 37.Soufer R, Bremner JD, Burg MM. Gender differences among normal and coronary disease subjects during mental stress: a positron emission tomography study. Presented at: American Psychosomatic Society 63rd Annual Meeting; March 2–5, 2005; Vancouver, BC, Canada. [Google Scholar]
- 38.Soufer R. Neurocardiac interaction during stress-induced myocardial ischemia: how does the brain cope? Circulation. 2004;110(13):1710–1713. doi: 10.1161/01.CIR.0000144841.84987.50. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Lampert, et al. Anger-induced T-wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Am Coll Card. doi: 10.1016/j.jacc.2008.10.053. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Inv. 2007;117(2):289–96. doi: 10.1172/JCI30555. This review article nicely describes the mechanisms underlying the neurological modulation of systemic inflammatory response, i.e. inhibition of cytokine release by stimulation of vagus nerve, also known as ‘cholinergic anti-inflammatory response’. Parasympathetic withdrawal may be responsible for heightened inflammatory response noted during mental stress via this mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 43.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 44.Lane RD, Reiman EM, Ahern GL, Thayer JF. Activity in Medial Prefrontal Cortex Correlates with Vagal Component of Heart Rate Variability during Emotion. Rothman Institute Abstracts. 2001:97–100. [Google Scholar]
- 45.Honzikova N, Semrad B, Fiser B, Labrova R. Baroreflex sensitivity determined by spectral method and heart rate variability, and two-years mortality in patients after myocardial infarction. Physiol Res. 2000;49:643–650. [PubMed] [Google Scholar]
- 46.Nobrega AC, Loures DL, Pontes PV, Sant’anna ID, Mesquita ET. Cholinergic stimulation with pyridostigmine prevents the impairment in ventricular function during mental stress in coronary artery disease patients. Int J Cardiol. 2008;125(3):418–421. doi: 10.1016/j.ijcard.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 47.Fukudo S, Lane JD, Anderson NB, Kuhn CM, Schanberg SM, McCown N, Muranaka M, Suzuki J, Williams RB., Jr Accentuated vagal antagonism of beta-adrenergic effects on ventricular repolarization. Evidence of weaker antagonism in hostile type A men. Circulation. 1992;85:2045–2053. doi: 10.1161/01.cir.85.6.2045. [DOI] [PubMed] [Google Scholar]
- 48.Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–1493. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- 49.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 50.Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Glaes SB, Krantz DS, Gottdiener JS, Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008;101(6):767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2(8410):1001–1005. doi: 10.1016/s0140-6736(84)91106-1. [DOI] [PubMed] [Google Scholar]
- 52.Giubbini R, Galli M, Campini R, Bosimini E, Bencivelli W, Tavazzi L. Effects of mental stress on myocardial perfusion in patients with ischemic heart disease. Circulation. 1991;83:II100–107. [PubMed] [Google Scholar]
- 53.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL, Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356 (9226):310–311. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 54.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically medicated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 55.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 56.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M, Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: Relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359–1366. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 57.Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340–342. [PubMed] [Google Scholar]
- 58.Yang ZH, Richard V, von Segesser L, Bauer E, Stulz P, Turina M, Lüscher TF. Threshold concentrations of endothelin-1 potentiate contractions to norepinephrine and serotonin in human arteries. A new mechanism of vasospasm? Circulation. 1990;82:188–195. doi: 10.1161/01.cir.82.1.188. [DOI] [PubMed] [Google Scholar]
- 59.Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Lüscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105(24):2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 60.Kahaleh MB, Fan PS. Effect of cytokines on the production of endothelin by endothelial cells. Clin Exp Rheumatol. 1997;15:163–167. [PubMed] [Google Scholar]
- 61.Woods M, Mitchell JA, Wood EG, Barker S, Walcot NR, Rees GM, Warner TD. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999 May;55:902–909. [PubMed] [Google Scholar]
- 62.Hohlfeld T, Klemm P, Thiemermann C, Warner TD, Schror K, Vane JR. The contribution of tumour necrosis factor-alpha and endothelin-1 to the increase of coronary resistance in hearts from rats treated with endotoxin. Br J Pharmacol. 1995;116:3309–3315. doi: 10.1111/j.1476-5381.1995.tb15140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizia-Stec K, Gasior Z, Zahorska-Markiewicz B, Janowska J, Szulc A, Jastrzebska-Maj E, Kobielusz-Gembala I. Serum tumour necrosis factor-alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron Artery Dis. 2003;14:431–438. doi: 10.1097/00019501-200309000-00003. [DOI] [PubMed] [Google Scholar]
- *64.Hassan M, York KM, Li H, Li Q, Gong Y, Langaee TY, Fillingim RB, Johnson JA, Sheps DS. Association of beta1-adrenergic receptor genetic polymorphism with mental stress-induced myocardial ischemia in patients with coronary artery disease. Arch Intern Med. 2008;168(7):763–770. doi: 10.1001/archinte.168.7.763. For the first time showing a possible genetic link to explain individual variations in response to mental stress ischemia. Showed a three fold higher likelihood of inducing mental stress ischemia in patients who were homozygous for the Ser/Ser 49 allele (beta 1-adrenergic receptor gene) [DOI] [PubMed] [Google Scholar]
- 65.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozansk A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 66.Rosenman RH, Brand RJ, Jenkins D, Friedman M, Straus R, Wurm M. Coronary heart disease in Western Collaborative Group Study: Final follow-up experience of 8 1/2 years. JAMA. 1975;233:872–877. [PubMed] [Google Scholar]
- 67.Julkunen J, Salonen R, Kaplan GA, Chesney MA, Salonen JT. Hostility and the progression of carotid atherosclerosis. Psychosom Med. 1994;56:519–525. doi: 10.1097/00006842-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Goodman M, Quigley J, Moran G, Meilman H, Sherman M. Hostility predicts restenosis after percutaneous transluminal coronary angioplasty. Mayo Clin Proc. 1996;71:729–734. doi: 10.1016/S0025-6196(11)64836-2. [DOI] [PubMed] [Google Scholar]
- 69.Blumenthal JA, Babyak M, Wei J, O’Connor C, Waugh R, Eisenstein E, Mark D, Sherwood A, Woodley PS, Irwin RJ, Reed G. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in man. Am J Cardiol. 2002;89:164–168. doi: 10.1016/s0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- 70.Blumenthal JA, Jiang W, Babyak MA, Krantz DS, Frid DJ, Coleman RE, Waugh R, Hanson M, Appelbaum M, O’Connor C, Morris JJ. Stress management and exercise training in cardiac patients with myocardial ischemia. Effects on prognosis and evaluation of mechanisms. Arch Intern Med. 1997;157:2213–2223. [PubMed] [Google Scholar]
- 71.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R, Soufer R. Noninvasive Detection of Risk for Emotion Provoked Myocardial Ischemia. Psychosom Med. 2008 doi: 10.1097/PSY.0b013e318187c035. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]