Abstract
One central goal in cognitive neuroscience of learning and memory is to characterize the neural processes that lead to long-lasting episodic memory. In addition to the stronger frontoparietal activity, greater category- or item-specific cortical representation during encoding, as measured by pattern similarity (PS), is also associated with better subsequent episodic memory. Nevertheless, it is unknown whether frontoparietal activity and cortical PS reflect distinct mechanisms. To address this issue, we reanalyzed previous data (Xue G, Dong Q, Chen C, Lu ZL, Mumford JA, Poldrack RA. 2010. Greater neural pattern similarity across repetitions is associated with better memory. Science. 330:97, Experiment 3) using a novel approach based on combined activation-based and information-based analyses. The results showed that across items, stronger frontoparietal activity was associated with greater PS in distributed brain regions, including those where the PS was predictive of better subsequent memory. Nevertheless, the item-specific PS was still associated with later episodic memory after controlling the effect of frontoparietal activity. Our results suggest that one possible mechanism of frontoparietal activity on episodic memory encoding is via enhancing PS, resulting in more unique and consistent input to the medial temporal lobe. In addition, they suggest that PS might index additional processes, such as pattern reinstatement as a result of study-phase retrieval, that contribute to episodic memory encoding.
Keywords: episodic memory, functional MRI, goal-directed process, representation similarity, subsequent memory effect
Introduction
The ability to form long-lasting episodic memories is critical for human survival (Tulving 2002). Although neuropsychological research beginning in the 1950s has emphasized the role of the medial temporal lobe (MTL) in episodic memory (Eichenbaum 2004; Squire et al. 2004), neuroimaging studies have highlighted the prefrontal cortex (PFC) (Ranganath and Knight 2003) and posterior parietal cortex (PPC) (Cabeza et al. 2008; Uncapher and Wagner 2009) in promoting the formation of long-term episodic memories. Using the subsequent memory paradigm (Brewer et al. 1998; Wagner et al. 1998), stronger encoding-related brain activities in the PFC and dorsal PPC have been consistently associated with better later memory performance. (For results of meta-analysis, see Blumenfeld and Ranganath 2007; Uncapher and Wagner 2009; Kim 2011.)
How these brain regions support episodic memory encoding, however, has not been well understood. Neither the PFC nor the PPC is the primary site for memory storage because PPC lesions are not associated with memory deficits (Rossi et al. 2006; Davidson et al. 2008; Haramati et al. 2008) and prefrontal patients can often perform at near-normal levels in structured encoding tasks (Kesner et al. 1994; Alexander et al. 2003). Instead, through the fiber tracts connecting them and the MTL (Goldman-Rakic et al. 1984; Schott et al. 2011), the PFC and the PPC may contribute to certain goal-directed mechanisms that can affect the cortical processing of the material and gate the information encoded by the MTL to form an episodic memory (Brown and Aggleton 2001; Norman and O'Reilly 2003; Squire et al. 2004).
Specifically, in light of the fact that the PFC plays a general role in top-down cognitive control (Miller and Cohen 2001), the PFC has been posited to play a role in controlled processes, such as selection and organization, that aid memory formation (Ranganath and Knight 2003; Blumenfeld and Ranganath 2007). The ventrolateral PFC showing the subsequent memory effect (SME) (Blumenfeld and Ranganath 2007) overlies well with the region that is responsible for interference resolution (Xue et al. 2008) and selection (Thompson-Schill et al. 1997; Badre et al. 2005; Badre and Wagner 2007). When organization was emphasized during encoding, the dorsolateral PFC region involved in manipulation also showed a positive SME (Blumenfeld and Ranganath 2006). Similarly, the PPC has been posited to play a central role in attention, but not in episodic memory per se (Cabeza et al. 2008; Uncapher and Wagner 2009). In particular, the dorsal and ventral PPCs are thought to be involved in goal-directed, top-down attention and stimulus-driven, bottom-up attention, respectively (Corbetta and Shulman 2002).
In addition to the goal-directed mechanisms identified by activation-based analyses, the use of an information-based multivoxel-pattern-analysis (MVPA) approach (Kriegeskorte et al. 2006) has enabled the examination of the specific information encoded by the brain and its contributions to episodic memory. Using this approach, one study found that subsequently recalled items showed greater item-specific pattern similarity (PS) than forgotten items, suggesting that more unique and consistent neural representations during learning benefit memory encoding (Xue, Dong et al. 2010). Another study found that clearer category-specific representations were predictive of better memory formation (Kuhl et al. 2012).
Questions remain regarding whether and how frontoparietal goal-directed processing might affect information coding and memory formation. Many studies have shown that top-down processing such as selective attention can modulate brain responses in the lower cortical areas, such as excluding external noise (Lu et al. 2011), amplifying behaviorally relevant signals (Fries et al. 2001), and enhancing neural representation of attended objects (Zhang et al. 2011) or features (Jehee et al. 2011). Presumably, increased frontoparietal activity could lead to stronger cortical representations of particular features and therefore greater PS across instances, which in turn could lead to better memory encoding. To examine this hypothesis, we reanalyzed the data from Experiment 3 of our previous study (Xue, Dong et al. 2010). This experiment used a slow event-related design (Fig. 1A), which allowed us to accurately estimate brain responses to individual items and to examine within-subject relationship between frontoparietal activity and item-specific cortical representations. By combining activation-based and information-based analyses, we found that frontoparietal activity was correlated with PS in distributed brain regions. Nevertheless, PS made additional contributions to memory encoding after controlling for frontoparietal activity.
Figure 1.
Schema of experimental design and data analysis. (A) Experimental design. A slow event-related design (each trial lasting 12 s) was used. Each trial started with a 1 s fixation point, followed by a Chinese word (e.g. “Computer”) that was presented on the screen for 3 s (or until a response was made, whichever came first). Subjects were asked to make a semantic judgment on each word (i.e. living vs. nonliving) by pressing 1 of the 2 buttons. Three seconds after the onset of the word, subjects were asked to perform a perceptual judgment task for 8 s. A self-paced procedure was used to make this task engaging. In each run, 20 words were studied, each repeated 3 times with an inter-repetition interval ranging between 4 and 8 trials. (B) PS analysis using the searchlight method. For each 5 × 5 × 5 cubic, the activation patterns for the 3 repetitions (P1–P3) of a single item were extracted. Pearson's correlation was used to calculate their similarity. The mean PS for each single item was then assigned to the center of the cubic. Meanwhile, the mean activation (ACT) in that cubic across 3 repetitions was also calculated by averaging. (C) The frontoparietal regions showing SME (recalled > forgotten) in activation levels. (D) Cross-trial analysis of the relationship between PS, activation level, frontoparietal activity, and memory performance (R: recalled; F: forgotten; N: nuisance items, which were recognized with high confidence but not recalled).
Materials and Methods
Detailed information regarding the participants, experimental design, behavioral, and functional magnetic resonance imaging (MRI) data preprocessing can be found in Supplementary Material of our previously published paper (Xue, Dong et al. 2010). Briefly, 22 subjects (11 males, mean age = 19.56 ± 1.76 years, ranging from 17 to 25 years) participated in this study. The full data set will be made openly available via the OpenFMRI web site (http://www.openfmri.org).
We used a slow event-related design (12 s for each trial) in this study (Fig. 1A). Subjects studied 60 words in the scanner over 3 scanning runs. Each item was repeated 3 times. The trial started with a 1 s fixation point, followed by a Chinese word that was presented on the screen for 3 s (or until a response was made, whichever came first). Subjects were asked to make a semantic judgment (i.e. living or nonliving) on the word by pressing 1 of the 2 buttons with their left or right thumb. Three seconds after the onset of the word, subjects were asked to perform a perceptual judgment task for 8 s. A self-paced procedure was used to make this task very engaging. Each time a Gabor image tilting 45° to the left or the right of vertical was randomly selected and presented on the screen, and subjects were asked to identify the orientation of the Gabor by pressing 1 of the 2 buttons. Subjects were asked to respond as quickly and accurately as possible. The next trial started 100 ms after subjects' response. Subjects on average finished about 13 trials of the visual orientation judgment task in each 8 s.
Thirty minutes after the scan, subjects were asked to perform 2 surprise memory tests; they were not warned of these memory tests in advance. In the first test (i.e. the free recall test), subjects were asked to write down the words they had studied in the scanner, regardless of the order of presentation. After the free recall test, they were given a recognition test on a 6-point scale, with 1 indicating definitely new and 6 indicating definitely old. Trials were categorized as either recalled (R) (recalled in the free recall test) or forgotten (F) items (forgotten during recall and scored ≤5 in the recognition task). To balance the number of trials in each category, items recognized with high confidence (scored 6) but not recalled were treated as nuisance trials (N). Our previous analysis showed that there was no difference between these trials and forgotten trials (Xue, Dong et al. 2010).
Image preprocessing and statistical analyses were carried out using FEAT (FMRI Expert Analysis Tool) version 5.98, part of the FSL (FMRIB software library, version 4.1, www.fmrib.ox.ac.uk/fsl). The first 3 volumes before the task were automatically discarded by the scanner to allow for T1 equilibrium. The remaining images were then realigned, spatially smoothed [using a 5 mm full-width-half-maximum (FWHM) Gaussian kernel], and temporally filtered (using a nonlinear highpass filter with a 60 s cut-off). The EPI images were first registered to the magnetization-prepared rapid gradient echo structural image and then into the standard Montreal Neurology Institute (MNI) space, using affine transformations (Jenkinson and Smith 2001). Registration from structural images to the standard space was further refined using FNIRT nonlinear registration (Andersson et al. 2007a, 2007b). Statistical analyses were performed in the native image space, with the statistical maps normalized to the standard space prior to higher-level analysis.
The SME in Activation Level
The general linear model within the FILM module of FSL was used to model the data. As described earlier, the recalled and forgotten words were separately modeled. The recognized but not recalled trials were treated as a nuisance variable. The error trials in the orientation task were coded as an additional nuisance variable, whereas the correct orientation trials were not coded and thus were treated as an implicit baseline. The major contrast for the SME was recalled versus forgotten. A higher-level analysis created cross-run contrasts for each subject for a set of contrast images using a fixed-effects model. These were then input into a random-effects model for group analysis, using FLAME (FMRIB's Local Analysis of Mixed Effect) stage 1 only with automatic outlier detection (Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008). Unless otherwise noted, group images were thresholded using cluster detection statistics, with a height threshold of z> 2.3 and a cluster probability of P< 0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory.
Ridge Regression for Single Trial Response Estimation
To accurately estimate the blood oxygen level-dependent (BOLD) responses associated with each single trial, we created a new model that modeled each trial as a separate regressor (Rissman et al. 2004; Mumford et al. 2012). The error trials in the orientation task were encoded as one nuisance variable, whereas the correct orientation trials were not coded and thus treated as implicit baseline. Thus, there were 61 predictors per run, 1 for each of the 60 words plus the incorrect orientation regressor. The hemodynamic delay was modeled by convolving delta functions with the canonical double gamma hemodynamic response function. The regression analysis was carried out in a voxel-wise fashion and was estimated using ridge regression (Hoerl and Kennard 1970). Details of the ridge regression can be found from Supplementary Material of Xue, Dong et al. (2010).
Representation Similarity Analysis
Instead of calculating the PS within anatomically defined regions of interest (ROIs) as in Xue, Dong et al. (2010), a searchlight method was used to achieve whole-brain coverage at a higher spatial resolution (Fig. 1B). At each voxel, a cubic ROI containing 125 voxels (5*5*5) centered on that voxel was generated. Pearson's correlations on the activation patterns within that ROI across the 3 repetitions of a given item were calculated and then averaged to represent the PS of that item, which was assigned to the center of that ROI. We also calculated the between-item PS, separately for recalled and forgotten items. Special attention was paid to make sure that the interval of the between-trial pairs matched that of the within-trial pairs (Gilbert et al. 2012). The searchlight analysis was conducted at subjects’ native space, separately for each run. The results were then transformed into standard space and concatenated across runs.
The SME in PS
The PS for recalled and forgotten items was separately averaged, and their differences were input into a random-effects model for group analysis. Since no first-level variance was available, an ordinary least square (OLS) model was used.
The Relationship Between Frontoparietal Activity, Activation Level, and PS
Using the searchlight method, we also calculated the mean activation (ACT) of each trial for each searchlight, which was simply the averaged activation within that ROI across 3 repetitions (Fig. 1B). Meanwhile, frontoparietal activation for each trial was quantified by calculating the mean activation level (across 3 repetitions) in the left prefrontal gyrus (LPFC) and the left inferior parietal lobule (LIPL) that showed SME in the activation level (Fig. 1C). Together with the PS for each trial, we could examine the relationship among frontoparietal activation (LPFC and LIPL), activation level (ACT), and PS in other brain regions (Fig. 1D). For each correlation analysis, Pearson's correlation was used and the resulting correlation coefficients were transformed to Fisher's z-score. The group-averaged correlation map was then generated by averaging the correlation map across 22 subjects.
A partial correlation analysis, which included the ACT in each voxel as covariate, was conducted to separate out the effect of frontoparietal activity on PS that was not achieved by increasing the activation level. The resulting correlation coefficients were transformed into Fisher's z-scores and then input into a random-effects model for group analysis, using OLSs.
The SME in PS After Controlling for Frontoparietal Activity
For each individual subject, 3 models were constructed using the general linear model within the FILM module of FSL. In these models, the PS was the dependent measure. The recalled and forgotten words were separately modeled, with the LPFC activation, the LIPL activation, or both as covariates of no interest. The contrast between recalled and forgotten words thus represented the SME in PS after controlling the top-down processing effect. Again, a random-effects model was used for group analysis, using OLSs.
ROI Analysis
For the frontoparietal regions showing SME on the activation level, 2 ROIs, naming the LPFC and the LIPL, were defined by including all the voxels in each cluster showing suprathreshold activation for the contrast of recalled versus forgotten items. The mean activations for each trial (across repetitions) were then extracted and correlated with other measures, including the PS and mean activation level in other regions.
Four regions showing an SME on PS were also defined, namely the left middle frontal gyrus (LMFG), left inferior frontal gyrus (LIFG), the left angular gyrus (LAnG), and left middle temporal gyrus (LMTG), by growing a 4 mm radial sphere centering the local maxima within each region. Focussed on these regions, we examined (1) whether there was item-specific PS (within-item> cross-item PS), especially for recalled items; (2) whether the LPFC and LIPL activation correlated with the PS in these regions, even after controlling for the activation level; and (3) whether the SME in PS was still significant after controlling for LPFC and LIPL activity.
Results
The SME in Activation Level
To examine the encoding-related processes that are associated with successful episodic memory encoding, we did an activation-based analysis of the SME. This analysis revealed stronger activations in the LPFC [MNI coordinates (x, y, z): −50, 14, 34, z = 4.17] and LIPL (MNI: −40, −66, 46, z = 3.48) for recalled than for forgotten items (Fig. 1C). The location of the LIPL falls within the region showing positive SME, but is dorsal and posterior to the ventral PPC area showing negative SME as revealed by a meta-analysis of 37 studies (Uncapher and Wagner 2009). Based on the literature, we consider activations in these frontoparietal regions mainly as reflections of the involvement of goal-directed processes, such as selection and attention, which contribute to memory encoding.
The SME in PS
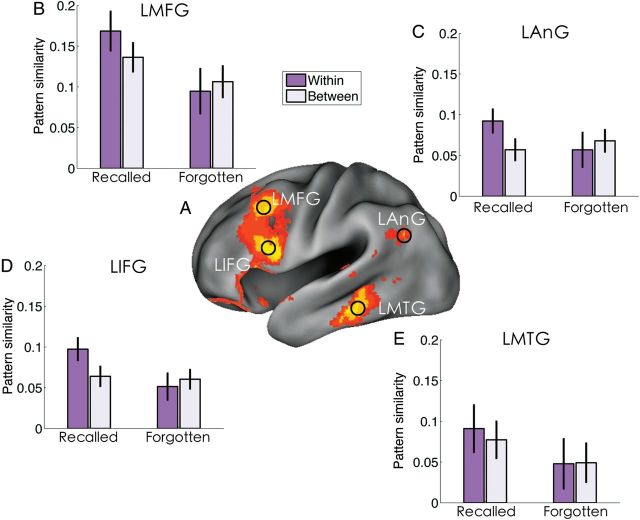
In contrast to our previous ROI-based approach, the present study used a searchlight method (Kriegeskorte et al. 2006) to calculate the PS of each item (across 3 repetitions) over the whole brain (Fig. 1B). There was significantly greater PS for subsequently recalled than for forgotten items in the LMFG (MNI: −48, 20, 40, z = 3.85), LIFG (MNI: −46, 16, 14, z = 4.06), LAnG (MNI: −44, −54, 28, z = 3.63), and LMTG (MNI: −62, −34, −18, z = 3.72) (Fig. 2A).
Figure 2.
SME in PS. (A) Stronger PS for recalled than for forgotten items was found in the LMFG, LIFG, LAnG, and LMTG. The differences, thresholded at z > 2.3 (whole-brain-corrected), are rendered onto a population-averaged surface atlas using multi-fiducial mapping (Van Essen 2005). (B–E) Item-specific PS. Only recalled item showed stronger within-item than between-item similarity in the above 4 ROIs, which were defined by growing a 4 mm sphere around the local maximum of each region showing significant SME in PS (recalled > forgotten). The between-item PS was calculated by averaging the correlation coefficients of all possible cross-item combinations, separately for recalled and forgotten items. Error bars represent within-subject errors.
To examine whether the PS for recalled items reflected item-specific representations, or the engagement of neural processes common to all recalled items, we also calculated the between-item PS, separately for recalled and forgotten items. Repeated-measures analysis of variance revealed significant (marginally significant to significant after Bonferroni correction across 4 ROIs, effective P-value of 0.0125) interactions between subsequent memory (recalled vs. forgotten) and item specificity (within vs. between items) in the LMFG (F1,21 = 6.91, P = 0.015), LIFG (F1,21 = 12.31, P = 0.002), and LAnG (F1,21 = 10.47, P = 0.004), but not the LMTG (P = 0.36) (Fig. 2B–E). Specifically, recalled items showed stronger within- than between-item PS in LMFG [t(21) = 2.45, P = 0.02], LIFG [t(21) = 2.86, P = 0.01], LAnG [t(21) = 2.43, P = 0.02], but not in LMTG [t(21) = 1.18, P = 0.25]. No such differences were found for forgotten items (P> 0.16), suggesting that the PS for recalled items reflected item-specific representations and the failure to reproduce item-specific patterns was associated with forgetting.
Since the regions showing SME in the activation level and that in PS were only partially overlapping, we did an additional ROI analysis to examine whether there were subtle differences in activity levels between recalled and forgotten items in these regions, showing that SME in PS that did not survive whole-brain correction. This analysis indeed revealed stronger activations for recalled than for forgotten items in these regions (P< 0.01) (Fig. 3).
Figure 3.
Regions showing SME in PS also showed subtle differences in the activation level. The bar graphs represent the mean difference in BOLD response (percent signal change) between subsequently recalled and forgotten items in the 4 regions showing SME in PS (as shown in Fig. 2A), including (A) LIFG, (B) LMFG, (C) LMTG, and (D) LAnG, separately for recalled and forgotten items as well as for each repetition. Error bars represent the within-subject error.
We also found strong differences between subjects in mean PS (averaged across all items, recalled and forgotten), which were positively correlated with individual differences in memory performance. The significant positive correlation was found in the posterior cingulate cortex (PCC) that extended to the precuneus (Supplementary Fig. S1). Memory performance was not correlated with the activation level in this region (r= 0.35, P = 0.15); after controlling for the activation level, the correlation between PS and memory performance was still significant (r = 0.46, P = 0.038), suggesting that the correlation was not driven by the overall activation level.
Frontoparietal Activity Correlated with PS in Distributed Brain Regions
The above analyses replicated and refined our previous results by showing that 2 neural mechanisms are associated with successful episodic memory encoding. We then turned to the core question of the present study: does the frontoparietal activity reflecting goal-directed processing affect PS? To address this issue, we extracted the activations for each item (averaged across 3 repetitions) in the LPFC and LIPL, the 2 regions that exhibited SME in activation levels, and correlated them with PS on a voxel-by-voxel basis (which actually reflects a searchlight-by-searchlight basis since the value on each voxel represented the PS for the searchlight centered on that voxel) (Fig. 1C,D). This analysis revealed moderate positive correlations in widespread brain regions, and the correlation patterns were largely similar for LPFC and LIPL (Supplementary Fig. S2 and Table S1). The maximum group-averaged Fisher's z-score was 0.6 (equivalent to r of 0.54), with the strongest correlation located in the bilateral middle/inferior frontal gyrus, the bilateral posterior inferior parietal lobule (pIPL) and adjacent region, the bilateral middle/inferior temporal gyrus, the PCC, the precuneus, and the paracingulate cortex.
Frontoparietal Activity Increased PS via Enhanced Activation
Previous studies suggest that top-down processes such as attention can increase the activation level and also enhance task-related feature encoding in lower cortical areas (Jehee et al. 2011). Consistent with this, we found that LPFC and LIPL activations were strongly positively correlated with the activation level in many brain regions (Supplementary Fig. S3A,B and Table S2), and a strong activation level was associated with greater PS (Supplementary Fig. S3C and Table S3; see also Supplementary Figs S4 and S5 for ROI results on the 4 regions showing the SME in PS), suggesting that frontoparietal activity could increase PS by enhancing the brain activity associated with task processing.
Frontoparietal Activity Increased PS via Enhanced Feature Encoding
To examine whether frontoparietal activity could also enhance task-related feature encoding that is independent of the increase in the activation level, we calculated partial correlations between LPFC and LIPL activities and PS in each cube while controlling its univariate activation level, which still revealed positive correlation in widespread brain regions, including bilateral IFG/precentral gyrus, superior parietal lobule, IPL, PCC, precuneus, MTG, among others (Supplementary Fig. S6 and Table S4).
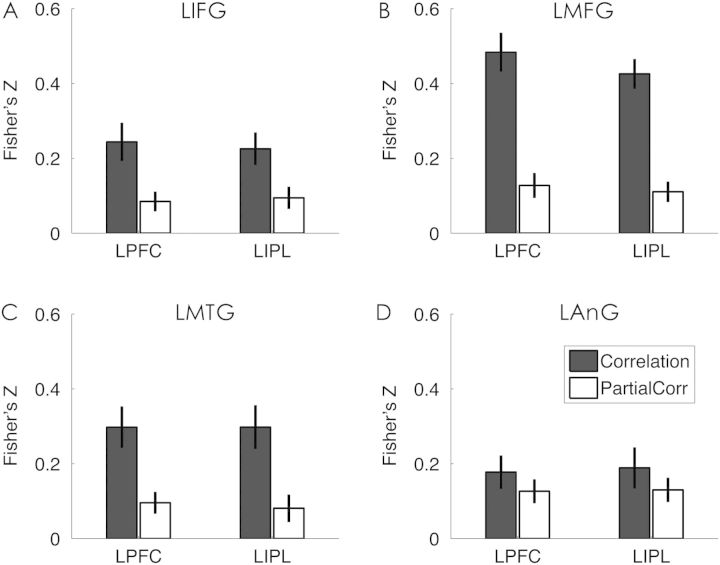
The existence of the 2 independent effects was clearly illustrated by the ROI analysis focussing on the regions showing the SME in PS (Fig. 4). We revealed significant partial correlations between LPFC and LIPL activity and PS in these regions after controlling the activation level, with the mean Fisher's z-score ranging from 0.08 (r-score of 0.08) to 0.13 (r-score of 0.13). These correlations, while significantly greater than 0, were also significantly lower than those obtained when the activation was not controlled [between 0.19 (r-score of 0.19) and 0.47 (r-score of 0.44), P< 0.001 for LMFG, LIFG, and LMTG, except for LAnG: P = 0.18]. No differences between the 2 seed regions were found in any of the 4 ROIs (P> 0.22).
Figure 4.
ROI results of the effect of frontoparietal activity on PS. The bar graphs represent the mean Pearson correlation (Fisher's z-score) between LPFC or LIPL activation and PS in the 4 regions showing SME in PS (as shown in Fig. 2A), including (A) LIFG, (B) LMFG, (C) LMTG, and (D) LAnG. The dark bars represent the correlation coefficients (Fisher's z-score) when the activation level was not controlled. The light bars represent the partial correlation coefficients when the activation level was controlled. Error bars represent the standardized errors of the mean.
PS Predicted Memory Encoding After Controlling for Frontoparietal Activity
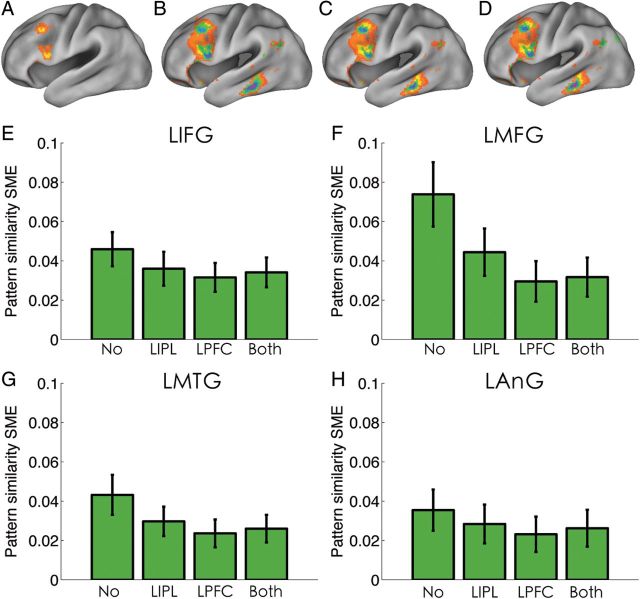
The above analysis suggests that the frontoparietal activity implemented in the LPFC and LIPL could modulate PS in other brain regions, including the regions showing SME in PS. Thus, it is important to examine whether the SME in PS was simply driven by the frontoparietal activity. In other words, could PS still predict subsequent memory performance after controlling for effects of frontoparietal activity? To address this issue, we reexamined the SME in PS by controlling the activity level of the LPFC or the LIPL or both. We found that the SME in PS in the LMFG and LIFG survived the whole-brain correction after controlling the LIPL activity (Fig. 5A). Using an uncorrected threshold (z> 2.3), the 4 regions showing PS in SME without controlling the frontoparietal activity still showed SME in PS, although at a reduced significance level (Fig. 5B–D). This was confirmed by the ROI analysis (Fig. 5E–H), which revealed significant SME in PS in these ROIs after controlling activation levels of the LPFC, LIPL, and both (P < 0.019), although the effect size was smaller when no covariates were added [P < 0.03, except in the LAnG, where no significant reduction in effect size was found after controlling the activity of LIPL (P = 0.28), LPFC (P = 0.12), or both (P = 0.22)].
Figure 5.
PS predicts subsequent memory after controlling for frontoparietal activity. (A) Whole-brain-corrected effect (z > 2.3) after controlling for LIPL activity. The uncorrected (z > 2.3) results (blue-pink color) after regressing out the LIPL(B), the LPFC (C), or both (D) are displayed on top of the result that did not regress out the effect of frontoparietal activity (red-yellow color). (E–H) Bar graphs of the differences in PS (Pearson's correlation) between recalled and forgotten items (termed as PS SME) in the 4 regions showing SME in PS (as shown in Fig. 2A), separated by how the frontoparietal activity effect was controlled.
Control Analysis: PS Estimation was not Affected by Repetition Lag
Due to the nature of temporal autocorrelation of the BOLD response, the PS analysis might be affected by the temporal lag of each pair. Still, psychological factors, such as temporal context drift, might also affect the PS estimation (Jenkins and Ranganath 2010). In the present study, we used a slow event-related design (12 s per trial) and a challenging visual judgment task in between trials to interrupt the further processing of the material, which would have significantly reduced the autocorrelation of BOLD signal due to psychological factors such as extended processing of the stimulus. In our analysis, we have also ensured that the repetition lag for recalled and forgotten items did not differ (mean intertrial interval, 6.4 vs. 6.3). In addition, the lag for within-item pairs was strictly matched with that for between-item pairs.
We did 2 additional analyses to examine the relationship between repetition lag and PS. First, we did a correlational analysis to examine the relationship between PS and repetition lag. The result revealed no significant correlation (P> 0.52), including the regions whose PS correlated with subsequent memory (Supplementary Fig. S7). Secondly, we did a categorical analysis to compare the PS for trials with different repetition lags (ranging from 4 to 8). Again, we found no differences between lags (all P> 0.26) (Supplementary Fig. S8). No differences were found even when we compared the 2 lags showing the biggest differences, for example, lags 5 and 7 in the LIFG (P = 0.18) or lags 6 and 8 in the LMTG (P = 0.14).
We think that these results are in general agreement with the work of Jenkins and Ranganath (2010), which revealed no significant increase in PS from lag 3 to 4 (the minimal repetition lag is 4 trials in our study). In addition, they found that bigger pattern distance was associated with better memory, which is consistent with our finding that items with more unique and consistent representation during learning would be better remembered.
Discussion
The present study aimed to address 2 questions. First, does frontoparietal activity correlate with the reproducibility of item-specific cortical representations during repeated encoding of the same item? Secondly, if so, would PS still make a unique contribution to memory encoding after controlling the effects of frontoparietal activity? Our results provided positive answers to both questions, which not only provide a mechanistic account of the role of frontoparietal activity in memory encoding, but also reveal an important contributor to the variances in cortical representations of category- and/or item-specific information. In addition, our results suggest that PS can capture additional mechanisms beyond frontoparietal activity that contribute to episodic memory encoding.
Activation, Representation, and Episodic Memory Encoding
Functional imaging studies have consistently revealed that stronger encoding-related brain activities in the PFC (Brewer et al. 1998; Wagner et al. 1998, 2000; Henson et al. 1999; Davachi et al. 2001; Otten et al. 2001; Kensinger and Schacter 2005; Blumenfeld and Ranganath 2006; Xue, Mei et al. 2010) and dorsal PPC (Kirchhoff et al. 2000; Davachi et al. 2001; Otten et al. 2001; Garoff et al. 2005; Kao et al. 2005; Sommer et al. 2005) were associated with better later memory performance. Using activation- and information-based analyses, we found that both stronger frontoparietal activity and higher PS in a more distributed network are associated with better subsequent memory. These results are in agreement with the observation that MVPA is more sensitive to distributed coding of information, whereas activation-based analysis is more sensitive to global engagement in ongoing tasks (Jimura and Poldrack 2012).
Although the LIFG result is consistent with the role of ventrolateral PFC in semantic encoding of single items (Blumenfeld and Ranganath 2007; Kim 2011), the present study also revealed strong SME in the dorsolateral PFC (i.e. LMFG). The LMFG plays a specific role in organizing the material in working memory during encoding, which facilitates later retrieval (Blumenfeld and Ranganath 2007). Consistently, the LMFG is involved in relational memory (Dolan and Fletcher 1997; Murray and Ranganath 2007), memory encoding involving organization (Fletcher et al. 1998; Blumenfeld and Ranganath 2006), and encoding process that supports semantic clustering in later free recall (Long et al. 2010). Other studies suggest that the dorsolateral PFC is involved in reflective attention (e.g. refreshing just-activated information) that benefits subsequent memory (Raye et al. 2002; Miller et al. 2008; Chun and Johnson 2011). In the present study, the LIFG and LMFG showed qualitatively similar SME, although the effects appear to be greater in the LMFG. As we did not require subjects to organize or refresh learning materials, it is unclear whether these 2 regions play differential roles under the current learning condition (i.e. semantic judgment of single item). Presumably, as subjects needed to perform the semantic judgment task and then a distracting perceptual judgment task, voluntary refreshing implemented by the LMFG might help to keep the just learnt information active in working memory, which facilitates later free recall. Future studies are certainly required to examine this issue.
Although our previous study using ROI-based PS calculation found more regions showing SME in PS and most of these regions showed no differences in the activation level, the searchlight method in the present study tends to pick up regions that also showed subtle differences in the activation level. Nevertheless, by repeating the same item multiple times and separating items according to their subsequent memory status, our PS analysis could dissociate item-specific representation and engagement of common processes, and the significant interaction between item specificity (within- vs. cross-item) and subsequent memory provides direct evidence to suggest that the consistency of item-specific representation could contribute to better subsequent memory. Furthermore, the PS in the PCC regions correlated with individuals’ memory performance, even after controlling overall activation level. They together suggest that frontoparietal activity and item-specific representation play complementary roles in episodic memory encoding.
The Effect of Frontoparietal Activity on PS
We further found that frontoparietal activity, which likely reflects goal-directed processing such as selection and attention, can enhance the fidelity of cortical representations of a single item. Consistently, neurophysiological data show that attention increases the reliability of neuronal responses, especially for interneurons, which allow reliable information encoding by neuronal signals (Mitchell et al. 2007). This is also consistent with human imaging studies, showing that more conscious cognitive processing is associated with more reproducible neural patterns (Schurger et al. 2010).
Our results further suggest that the top-down effect on reproducibility might be achieved both by increasing the engagement of common processes and by enhancing goal-directed feature representations. On the one hand, the positive correlation between LPFC and LIPL activity and activity in other brain regions is consistent with many observations showing increased neural responses as a result of attention (Brefczynski and DeYoe 1999; Somers et al. 1999; Jehee et al. 2011). Neurophysiological data also suggest that goal-directed processing can improve behavioral performance by increasing the neuronal firing rate (Motter 1993) and gamma-band synchronization (Fries et al. 2001). The causal role of top-down modulation on cortical activity has been observed using transcranial magnetic stimulation (Ruff et al. 2006). Meanwhile, we found strong correlations between PS and activation level (note that the PS calculation, Pearson's correlation, is not affected by the activation level). Thus, this positive correlation might reflect increased engagement of common task processes.
On the other hand, after controlling the activation level, LPFC and LIPL activations were still correlated with PS in the cortical areas that showed SME. This is consistent with the observation that attention can enhance goal-directed feature representations in a way that is independent of activation levels (Jehee et al. 2011). At least 2 mechanisms can contribute to this enhanced item representation. First, frontoparietal activity can reduce the baseline correlation between neurons (Cohen and Maunsell 2009; Mitchell et al. 2009), thus increasing the amount and uniqueness of the information encoded by a given population of neurons. Secondly, frontoparietal activity can help reduce noise and interference, thus enhancing gains in signal (Lu et al. 2011), and also the neuronal representations of targets in the face of distracters (Zhang et al. 2011).
Our results thus provide a mechanistic explanation of the effect of frontoparietal activity on memory. Specifically, frontoparietal activity can enhance subsequent memory not only by engaging stronger task processing, but also by sharpening neural representations and thus increasing the distinctiveness and reproducibility of cortical patterns, which can help optimize the inputs to the MTL regions and enhance memory. On the one hand, highly consistent representation would suggest high fidelity of cortical representation of memory materials. This could increase the distinctiveness of cortical representation of learning materials, and the unique inputs to the hippocampus could facilitate pattern separation and avoid interference in later retrieval. On the other hand, this sharpening neural representation can also increase the reproducibility of cortical patterns, as noisy representations are less reproducible at both category and item levels. The consistent pattern of activation might result in more effective pattern completion at the neural level that helps strengthen the memory trace.
PS Captures Additional Mechanisms Underlying Memory Encoding
Another important finding of this study is that frontoparietal activity could only account for a portion of the variance in PS, and PS could still predict SME after the effect of frontoparietal activity is controlled. In particular, the PS in the left angular gyrus (AG) was only slightly correlated with frontoparietal activity, and controlling frontoparietal activity did not significantly reduce its effect size in predicting subsequent memory, suggesting that the left AG might play a different role in memory encoding during repeated studies.
The left AG has been implicated in episodic memory retrieval (Wagner et al. 2005; Cabeza et al. 2008; Ciaramelli et al. 2008; Hutchinson et al. 2009). Stronger AG activation has been found for strong, recollected, and high-confidence memory when compared with weak, familiar, and low-confidence memory (Wagner et al. 2005; Cabeza et al. 2008). It should be noted that although the attention-to-memory (AtoM) model suggests that the AG plays a role in attending to retrieved memory, meta-analysis and resting-state functional connectivity MRI-based parcellations of the PPC convergently suggest finer functional dissociation between attention and memory retrieval within the PPC: the ventral attention region corresponds to the supramarginal gyrus, whereas the retrieval region corresponds to the AG/pIPL regions (Hutchinson et al. 2009; Nelson et al. 2010; Uncapher et al. 2010).
The left AG identified in this study falls into the memory retrieval region and does not overlap with the LIPL. More importantly, the pattern of left AG activation shows several characteristics that fit well with the retrieval account. First, whereas other regions showed repetition suppression, the AG showed repetition enhancement, but only for subsequently recalled items. Secondly, the AG activation only weakly correlated with its PS, suggesting that it might represent more item-specific features than common processes. Finally, the PS in AG was slightly affected by the frontoparietal activity, and the effect size of AG PS in predicting subsequent memory was barely reduced after controlling the frontoparietal activity. Taken together, they suggest that PS in the left AG likely reflects item-specific PS as a result of pattern reinstatement during study-phase retrieval (Kuhl et al. 2011).
Behavioral studies have consistently suggested that study-phase retrieval can benefit memory encoding (Bjork 1975; Thios and D'Agostino 1976; Appleton-Knapp et al. 2005; Kuhl et al. 2010, 2011). In particular, retrieval practice has been shown to be more effective than simply repetition in forming long-lasting memories (Karpicke and Roediger 2008; Karpicke and Blunt 2011). Imaging studies have shown that successful memory retrieval is accompanied by reinstatement of the neural activation patterns during encoding (Wheeler et al. 2000; Nyberg et al. 2001; Wheeler and Buckner 2003; Kahn et al. 2004; Smith et al. 2004; Kuhl et al. 2011). This category-specific or sequence-specific activation reinstatement precedes memory (Polyn et al. 2005) and is associated with performance in free recall (Gelbard-Sagiv et al. 2008) and cued-retrieval (Kuhl et al. 2011). Our results suggest that the benefit of retrieval practice may partly arise from the pattern reinstatement during retrieval practice. The greater involvement of goal-directed processing during retrieval might further enhance the PS.
In addition to study-phase retrieval, other factors such as the features of the material and the interaction between stimulus presentation and spontaneous brain oscillation might also affect the reproducibility of cortical representations (Busch et al. 2009). Future studies are definitely required to understand other sources of variances in PS and their contribution to memory encoding. The present study provides a useful theoretical and methodological framework to examine how behavioral manipulations, such as attention (Turk-Browne et al. 2006; Uncapher et al. 2011), spacing (Xue et al. 2011), and encoding strategy (Otten et al. 2001), retrieval practice (Karpicke and Roediger 2008), neural manipulations, such as brain stimulations (Ruff et al. 2006; Floel et al. 2012), and pharmacological manipulation (Knecht et al. 2004) could affect PS and memory encoding, which can help establish a causal relationship between PS and episodic memory formation.
Conclusion
Taken together, the present study reveals interactive and complementary role of frontoparietal activity (as revealed by univariate analysis) and cortical item representations (as revealed by representation similarity analysis) in forming episodic memory. The strong positive correlation between frontoparietal activity and PS suggests that frontoparietal activity can enhance memory encoding by increasing PS, which can help memory encoding by providing consistent and unique inputs to the MTL. Still, other factors such as study-phase-retrieval-induced pattern reinstatement can also enhance PS and memory. These results provide new insights into the cognitive and neural mechanisms underlying successful memory encoding.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfourdjournals.org/.
Funding
This work is supported by the National Natural Science Foundation of China (31130025), NSF (BCS 0823624 and BCS 0823495) and NIH (HD057884-01A2).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Alexander M, Stuss D, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non frontal lesions. Brain. 2003;126:1493. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Technical Report TR07JA1. 2007a. www.fmrib.ox.ac.uk/analysis/techrep .
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB Technical Report TR07JA2. 2007b. www.fmrib.ox.ac.uk/analysis/techrep .
- Appleton-Knapp S, Bjork R, Wickens T. Examining the spacing effect in advertising: encoding variability, retrieval processes, and their interaction. J Cons Res. 2005;32:266–276. [Google Scholar]
- Badre D, Poldrack RA, ParÈ-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bjork R. Retrieval as a memory modifier. In: Grunberg MM, Morris PE, Sykes RN, editors. Information processing and cognition: the Loyola Symposium. London: Wiley; 1975. pp. 396–401. [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Brewer J, Zhao Z, Desmond J, Glover G, Gabrieli J. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:61–62. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Johnson MK. Memory: enduring traces of perceptual and reflective attention. Neuron. 2011;72:520–535. doi: 10.1016/j.neuron.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R, Fletcher P. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 1998;121:1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Floel A, Suttorp W, Kohl O, Kurten J, Lohmann H, Breitenstein C, Knecht S. Non-invasive brain stimulation improves object–location learning in the elderly. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.05.007. Forthcoming 2012 http://dx.doi.org/10.1016/j.neurobiolaging.2011.05.007 . [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Armbruster DJN, Panagiotidi M. Similarity between brain activity at encoding and retrieval predicts successful realization of delayed intentions. J Cogn Neurosci. 2012;24:93–105. doi: 10.1162/jocn_a_00094. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P, Selemon L, Schwartz M. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46:1756–1766. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg M, Shallice T, Josephs O, Dolan R. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerl A, Kennard R. Ridge regression: biased estimation for nonorthogonal problems. Technometrics. 1970;12:69–82. [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehee JFM, Brady DK, Tong F. Attention improves encoding of task-relevant features in the human visual cortex. J Neurosci. 2011;31:8210–8219. doi: 10.1523/JNEUROSCI.6153-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jimura K, Poldrack RA. Analyses of regional-average activation and multivoxel pattern information tell complementary stories. Neuropsychologia. 2012;50:544–552. doi: 10.1016/j.neuropsychologia.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Davis ES, Gabrieli J. Neural correlates of actual and predicted memory formation. Nat Neurosci. 2005;8:1776–1783. doi: 10.1038/nn1595. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Blunt JR. Retrieval practice produces more learning than elaborative studying with concept mapping. Science. 2011;331:772–775. doi: 10.1126/science.1199327. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Roediger HL. The critical importance of retrieval for learning. Science. 2008;319:966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Emotional content and reality-monitoring ability: fMRI evidence for the influences of encoding processes. Neuropsychologia. 2005;43:1429–1443. doi: 10.1016/j.neuropsychologia.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kirchhoff B, Wagner A, Maril A, Stern C. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Flöel A, Zwitserlood P, Ringelstein EB. Levodopa: faster and better word learning in normal humans. Ann Neurol. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA. 2006;103:3863. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl B, Shah A, DuBrow S, Wagner A. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci USA. 2011;108:5903–5908. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Wagner AD. Multi-voxel patterns of visual category representation during episodic encoding are predictive of subsequent memory. Neuropsychologia. 2012;50:458–469. doi: 10.1016/j.neuropsychologia.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, öztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Li X, Tjan BS, Dosher BA, Chu W. Attention extracts signal in external noise: a BOLD fMRI study. J Cogn Neurosci. 2011;23:1148–1159. doi: 10.1162/jocn.2010.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, Verstynen T, Johnson MK, D'Esposito M. Prefrontal and parietal contributions to refreshing: an rTMS study. Neuroimage. 2008;39:436–440. doi: 10.1016/j.neuroimage.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, et al. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Petersson K, Nilsson L, Sandblom J, Aberg C, Ingvar M. Reactivation of motor brain areas during explicit memory for actions. NeuroImage. 2001;14:521–528. doi: 10.1006/nimg.2001.0801. [DOI] [PubMed] [Google Scholar]
- Otten L, Henson R, Rugg M. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Knight RT. Prefrontal cortex and episodic memory: integrating findings from neuropsychology and event-related functional neuroimaging. In: Parker A, Wildng E, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. Philadelphia: Psychology Press. 2003:83–99. [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. Neuroimaging a single thought: dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage. 2002;15:447–453. doi: 10.1006/nimg.2001.0983. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, Babiloni C, Rossini PM. Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2006;23:793–800. doi: 10.1111/j.1460-9568.2006.04600.x. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes J-D, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niklas C, Kaufmann J, Bodammer NC, Machts J, Schütze H, Düzel E. Fiber density between rhinal cortex and activated ventrolateral prefrontal regions predicts episodic memory performance in humans. Proc Natl Acad Sci USA. 2011;108:5408. doi: 10.1073/pnas.1013287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurger A, Pereira F, Treisman A, Cohen J. Reproducibility distinguishes conscious from nonconscious neural representations. Science. 2010;327:97–99. doi: 10.1126/science.1180029. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RNA, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RBH. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:1663. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Rose M, Weiller C, Büchel C. Contributions of occipital, parietal and parahippocampal cortex to encoding of object–location associations. Neuropsychologia. 2005;43:732–743. doi: 10.1016/j.neuropsychologia.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Thios S, D'Agostino P. Effects of repetition as a function of study-phase retrieval. J Verb Learn Verb Behav. 1976;15:529–536. [Google Scholar]
- Thompson-Schill SL, DíEsposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi D-J, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. Dissociable effects of top-down and bottom-up attention during episodic encoding. The Journal of Neuroscience. 2011;31:12613–12628. doi: 10.1523/JNEUROSCI.0152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. A roadmap to brain mapping: toward a functional map of human parietal cortex. Neuron. 2010;67:5–8. doi: 10.1016/j.neuron.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: when priming hinders new episodic learning. J Cogn Neurosci. 2000;12:52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter D, Rotte M, Koutstaal W, Maril A, Dale A, Rosen B, Buckner R. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Petersen S, Buckner R. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen C, Lu Z, Mumford JA, Poldrack RA. Greater neural pattern similarity across repetitions is associated with better memory. Science. 2010;330:97. doi: 10.1126/science.1193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Ghahremani DG, Poldrack RA. Neural substrates for reversing stimulus-outcome and stimulus-response associations. J Neurosci. 2008;28:11196–11204. doi: 10.1523/JNEUROSCI.4001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu Z, Poldrack R. Facilitating memory for novel characters by reducing neural repetition suppression in the left fusiform cortex. PLoS ONE. 2010;5:e13204. doi: 10.1371/journal.pone.0013204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu Z, Poldrack R, Dong Q. Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. J Cogn Neurosci. 2011;23:1624–1633. doi: 10.1162/jocn.2010.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyers EM, Bichot NP, Serre T, Poggio TA, Desimone R. Object decoding with attention in inferior temporal cortex. Proc Natl Acad Sci USA. 2011;108:8850–8855. doi: 10.1073/pnas.1100999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.