Abstract
The neurologic presentation of limbic encephalitis is variable and when it occurs due to a rare cause the diagnosis may be problematic. We present a case of autoimmune limbic encephalitis due to glutamic acid decarboxylase antibody and consider the magnetic resonance imaging and antineural antibody screening aspects in the diagnosis of this entity.
Keywords: epilepsy, neuroimmunology, neuroradiology, seizures
Introduction
Limbic encephalitis (LE) is characterized by acute or subacute development of seizure, short-term memory loss, irritability, hallucinations, and psychiatric symptoms with a pathogenesis related to inflammation of the medial temporal lobes. Magnetic resonance imaging (MRI) and electroencephalogram (EEG) are abnormal in the majority of patients, yet seizures are not an absolute requirement for LE. In the majority of patients the cerebrospinal fluid (CSF) shows a mild-to-moderate pleocytosis, increased protein concentration, normal glucose concentration, and frequently elevated immunoglobulin G (IgG) index and oligoclonal bands. Changes in MRI most commonly indicate that the medial temporal lobes are affected bilaterally with varying intensity, rarely show enhancement, are rarely limited to the temporal lobe, and best seen as increased signal on coronal MR-fluid attenuated inversion recovery (FLAIR). Up to 25% of cases have normal MRI with a syndrome typical of LE, and EEG is almost always abnormal showing epileptic activity or slowing.1
Limbic encephalitis may be of viral or autoimmune etiology with the latter being either paraneoplastic or idiopathic. Herpes simplex virus (HSV) encephalitis is the classic form of viral encephalitis and rarely human herpes virus 6 can mimic this condition in the immunosuppressed patient. Autoimmune LE can be classified according to the presence of autoantibodies into 2 broad categories, one associated with antibodies to intracellular neuronal antigens and the other with antibodies to cell-surface antigens. Antigens with the former include Hu, Ma2, collapsin response-mediator protein 5 (CRMP5), and amphiphysin and with the latter the voltage-gated potassium channel (VGKC), N-methyl-d-aspartate receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPAR), and others. Patients with neuronal intracytoplasmic targets with LE are less frequent, more highly associated with cancer, and generally have a poor outcome with limited response to treatment compared to neuronal cell-surface antigen patients.2 Paraneoplastic LE has been associated with a number of cancers including small cell lung cancer (SCLC), testicular tumors, thymoma, breast cancer, and Hodgkin lymphoma, while the second group is predominately nonparaneoplastic.1,3
When the clinical features suggest LE, the evaluation should include MRI, EEG, and CSF analysis to exclude viral causes, primarily HSV encephalitis. Once a viral cause has been ruled out by polymerase chain reaction (PCR) and serologies, the clinician must determine the autoimmune etiology and whether the condition is paraneoplastic or not. The autoimmune form of LE may respond to immunotherapy if diagnosed and treated early.4 New onset temporal lobe epilepsy (TLE), particularly when intractable and associated with memory loss, behavioral, or psychiatric features should raise suspicion for autoimmune LE.5 Features of increased signal on MR-FLAIR in the medial temporal lobes, hyponatremia, dysthyroidism, and/or neurologic findings associated with cerebellar dysfunction mandate antineuronal antibody screening for autoimmune LE that includes glutamic acid decarboxylase antibodies (GAD-Abs).
Case History
A 57-year-old woman was in her usual state of health when her husband noted “increased seizure activity” characterized by confusion, agitation, and behavior change. He noted several-minute episodes of jaw clenching, muscle stiffening, and labored breathing in bed followed the next morning by difficulty arousing and flat affect. She was admitted to an outside hospital where MRI showed increased signal on MR-FLAIR of the medial temporal lobe on the left-hand side. She was treated for HSV encephalitis with acyclovir that was discontinued after several days. Serum sodium was 107 mmol/L (N = 136-145) and EEG showed diffuse slowing of 5 to 6 Hz. The CSF-PCR for HSV was negative and she was transferred to our hospital to rule out a brain tumor. She is a retired nurse with formal training in music and voice. Her husband stated that during the past 3 to 4 years she has shown less drive, required increased assistance, and has not been as sharp mentally but that there had been no progression during this time. She is a vegetarian and her only medication was thyroid replacement.
Her past medical history was significant for hypothyroidism and a history of seizures beginning 4 years ago and the MRI was normal and the 24-hour video EEG monitoring recorded 2 seizures. There was also a history of hyponatremia 1 year prior. During the past 4 years, she had tried various antiepileptic medications with limited success and was not taking any for a period of 6 months prior to her recent hospitalization because of adverse reactions.
Neurologic Examination
She was alert, oriented 3 times, cooperative, and fluent but tangential and hazy on detail of why she was in the hospital and the circumstances leading to hospitalization at the facility she was transferred from. She remembered 1 of 3 items after 5 minutes and gave the name of the president correctly but could not give the name of vice president or prior president. Mini-Mental Status Examination was 28. Cranial nerve testing showed visual fields, funduscopic examination, extraocular movements, pupils, hearing, and facial sensation to be intact. Vertical nystagmus was noted on upgaze and downgaze and horizontal nystagmus was appreciated on lateral gaze. Motor, sensory, cerebellar, and gait testing was normal. Deep tendon reflexes were +3/4 symmetric and pendular at the knees. Plantar response was flexor bilaterally.
Laboratory Studies
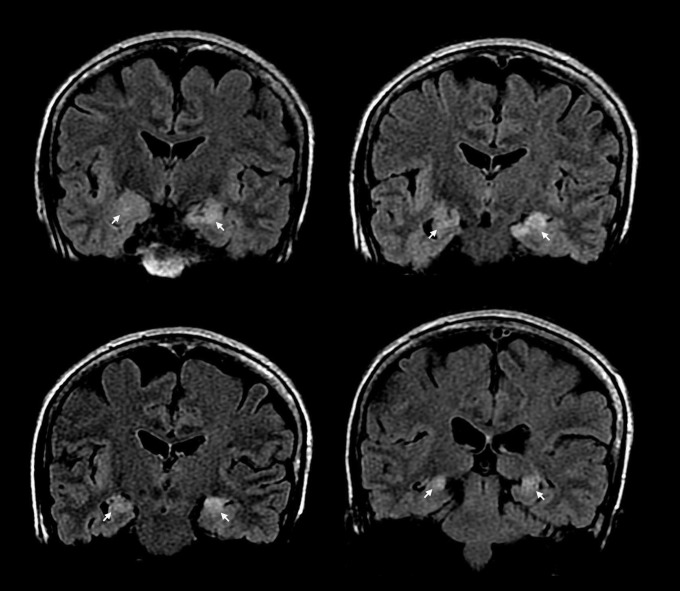
Complete blood count, thyroid-stimulating hormone, thyroxine, serum and urine osmolality, electrolytes, and routine blood chemistries were normal. Serum Venereal Disease Research Laboratory (VDRL) test, HIV, and Lyme serology were nonreactive and the B12 level = 2000 ng/L. Electroencephalogram was normal. Cerebrospinal fluid protein = 26 mg/dL, glucose = 65 mg/dL, cell count = 1 lymphocyte/mm3, and the viral antibody panel was negative as was PCR for HSV. The CSF oligoclonal bands/IgG index was not tested. The VDRL and Lyme serology in the CSF was nonreactive. Computed tomography of chest, abdomen, and pelvis was normal. Whole body CT and positron emission tomography (PET) scan were negative for malignancy. Magnetic resonance-FLAIR showed bilateral medial temporal lobe lesions (Figure 1). Serum and CSF were negative for voltage-gated potassium channel, NMDAR, CRMP5, Anti-Yo, Hu, and Ri, Ma, and Purkinje cell cytoplasmic antibodies (PCA), antineuronal nuclear and amphiphysin antibodies. Glutamic acid decarboxylase-Abs showed a serum level of 630 nmol/L and CSF of 54.3 nmol/L (N ≤ 0.02 for serum/CSF). Radioimmunoassay was performed at Mayo Medical Laboratories which suggest a cutoff of 20 nmol/L for major neurologic disease. Levetiracetam was started and increased to 2500 mg daily with improved seizure control. Repeat MR at 4 months showed persistent increased signal in the temporal lobes however, with diminished intensity. After 6 months, the patient’s husband reported increased episodes of “seizure” activity and the patient underwent inpatient video EEG monitoring that showed no electrographic correlate of the 10 to 12 episodes of shaking. She did have approximately 20 subclinical seizures during sleep over the right hemisphere.
Figure 1.
Coronal magnetic resonance-fluid attenuated inversion recovery (MR-FLAIR) showing bilateral increased signal of the medial temporal lobe (arrows).
It was postulated her shaking events were a behavioral response to a simple partial seizure that was not large enough to be seen on EEG. Her levetiracetam was increased to 2000 mg twice daily with no change in her clinical status after 2 months and intravenous Ig (IVIg) therapy was planned.
Discussion
Glutamic acid decarboxylase-Abs are described in association with a number of neurologic conditions including stiff-person syndrome, refractory seizures, nystagmus, and cerebellar syndromes,3 and more recently with autoimmune LE both paraneoplastic and nonparaneoplastic. Paraneoplastic LE with GAD-Ab has been described in several patients in association with SCLC and with thymoma.3,6 Nonparaneoplastic LE related to GAD-Ab has been noted in 21 cases, some of which are case reports of 1 or 2 patients, 17 of whom manifest MR-FLAIR signal abnormality of the medial temporal lobe.5,7–11 In addition to whole body CT or PET scan guidelines with caveats for workup of patients with suspected paraneoplastic syndrome for tumors have recently been published.12 Antineural antibody studies include VGKC (leucine-rich, glioma-inactivated 1),13 NMDAR, CRMP5, Anti-Yo, Hu, Ri and Ma, and PCA, antineuronal nuclear, amphiphysin, GAD, AMPAR, and novel cell membrane antigen. Recently, paraneoplastic LE has been described with γ-aminobutyric acid-B receptor antibody (GABABR-Ab) in association with SCLC and screening for this antibody was suggested in all patients with LE suspected to be related to GAD-Ab.6 The screening panel is not always inclusive and can vary based on physician, hospital, or reference laboratory protocol. If malignancy is still suspected, a bone marrow biopsy should be considered, particularly for lymphoma. Additionally, blood tests that include electrolytes, thyroid function tests, and a screen for diabetes are to be included.1 Patients with GAD-Ab LE may have drug-resistant TLE for a number of years prior to diagnosis.
Considering the 4-year history of seizures, an inadequate antiepileptic drug (AED) regimen trial, a normal brain PET scan, absence of clear clinical progression, and lack of electrographic correlation on video EEG monitoring, it was decided to optimize the AED regimen in our patient prior to initiating immunotherapy. These factors along with cost, associated risk with long-term immunotherapy4 and variable outcome tempered our treatment plan. Despite an initial reduction in seizure frequency after increased dose of levetiracetam, her spells gradually increased after 4 months. Because of this and her overall deterioration of well-being treatment with IVIg was considered.
Despite the absence of randomized controlled studies, treatment with immunotherapy regimens that include steroid, IVIg, plasma exchange (PE), and immunosuppressive agents in varying combinations for nonparaneoplastic autoimmune LE4,5,8,9,11 is based on case reports and prospective case series and response to treatment is variable. Results varied depending on the antineuronal antibody involved, with a more favorable response with VGKC-Ab5,6 than with GAD-Ab.5,8,9,11 Mata et al8 reported dramatic improvement of the seizure frequency and memory in association with decreased antibody titers in a patient with GAD-Ab LE treated with PE. The benefit however was of short duration. Mazzi et al11 reported a young women with multiple-daily partial drug-resistant seizures for 7 years unresponsive to high-dose steroids and IVIg infusion. The use of PE resulted in a long disease-free period however, when PE was reduced seizures reappeared and with termination of treatment clinical status worsened. Malter et al5 screened 53 patients who met the criteria for LE, 9 of whom had high titers of GAD-Ab and 10 had VGKC-Abs. In addition, suggesting GAD-Ab should be looked for in new onset TLE with memory impairment they treated these patients with monthly IV methylprednisolone and individualized AED. None of the 9 GAD-Ab-treated patients became seizure free while all patients treated with VGKC-Ab became seizure free. Blanc et al9 reported a 30-year-old man with GAD-Ab LE treated with corticosteroids, IVIg, immunosuppressive agents, and AED who showed improved memory status and disappearance of seizures associated with decreased GAD-Ab titers. One recent open-labeled prospective series using combined immunotherapy of PE, IVIg, and IV methylprednisolone proved effective for the clinical, cognitive, and immunological features in 9 patients with VGKC-Ab LE with radiologic improvement seen in the majority.4
The natural history of autoimmune LE is not fully understood, nevertheless, a substantial number of patients may experience severe and nonremitting neurologic impairment. As such studies as reported by Wong et al4 despite the high associated treatment complications, provide an immunotherapy benchmark for a condition with variable results depending on the antineuronal antibody involved.
The goal of treatment with autoimmune LE is immunomodulating therapy to suppress the autoimmune response. Steroids, IVIg, and PE have shown variable effectiveness and the use of other immunotherapies that include monoclonal antibody treatment may be a future consideration.5 Early diagnosis is important and in the appropriate clinical setting particularly when abnormal MR-FLAIR signal of the temporal lobe is apparent, LE should be a primary diagnostic consideration with GAD included in the antineural antibody screen.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tuzun E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13(5):261–271 [DOI] [PubMed] [Google Scholar]
- 2. Honnorat J. Autoimmune limbic encephalitis: an expanding concept. Lancet Neurol. 2010;9(1):24–25 [DOI] [PubMed] [Google Scholar]
- 3. Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(pt 10):2553–2563 [DOI] [PubMed] [Google Scholar]
- 4. Wong SH, Saunders MD, Lamer AJ, Das K, Hart IK. An effective immunotherapy regimen for VGKC antibody-positive limbic encephalitis. J Neurol Neurosurg Psychiatry. 2010;81(10):1167–1168 [DOI] [PubMed] [Google Scholar]
- 5. Malter MP, Helmstaedter C, Urbach H, et al. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470–478 [DOI] [PubMed] [Google Scholar]
- 6. Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABAB receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76(9):795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akman CI, Petterson MC, Rubinstein A, et al. Limbic encephalitis associated with anti-GAD antibody and common variable immune deficiency. Dev Med Child Neurol. 2009;51(7):563–567 [DOI] [PubMed] [Google Scholar]
- 8. Mata S, Muscas GC, Naldi I, et al. Non-paraneoplastic limbic encephalitis associated with anti-glutamic acid decarboxylase antibodies. J Neuroimmunol. 2008;199(1-2):155–159 [DOI] [PubMed] [Google Scholar]
- 9. Blanc F, Ruppert E, Kleitz C, et al. Acute limbic encephalitis and glutamic acid decarboxylase antibodies: a reality? J Neurol Sci. 2009;287(1-2):69–71 [DOI] [PubMed] [Google Scholar]
- 10. Cianci V, Labate A, Lanza P, et al. Non-paraneoplastic limbic encephalitis characterized by mesio-temporal seizure and extratemporal lesions: a case report. Seizure. 2010;19(7):446–449 [DOI] [PubMed] [Google Scholar]
- 11. Mazzi G, DeRoia D, Cruciatti B, Mata S, Catapano R. Plasma exchange for anti GAD associated nonparaneoplastic limbic encephalitis. Transfus Apher Sci. 2008;39(3):229–233 [DOI] [PubMed] [Google Scholar]
- 12. Titulaer MJ. Soffieti R, Dalmau J, et al. Screening for tumors in paraneoplastic syndromes: report of EFNS task force. Eur J Neurol. 2011;18(1):19–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai M, Huybers MGM, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]