Abstract
Neurologists and emergency department physicians are frequently involved in the comprehensive evaluation of a first generalized seizure. An important aspect of this evaluation is a detailed history which can identify a provoked seizure secondary to drug toxicity and hence avoid unnecessary treatment with antiepileptic drugs. “Spice” is an umbrella term for a variety of synthetic cannabinoid products whose inhalation has been associated with an increasing number of toxic side effects resulting in emergency department visits. These side effects (including psychosis, tachyarrhythmia, and seizures) are not typically seen with marijuana (Cannabis sativa) use. We report 2 patients with no prior history of neurological disease that experienced their first generalized tonic–clonic seizure after smoking Spice. The mechanism behind the possible proconvulsant effect of synthetic cannabinoids is not known, but it may be due to their effects at the cannabinoid receptor CB1. Although the US Drug Enforcement Administration placed 5 synthetic cannabinoids into schedule 1 for a 12-month period beginning March 2011, new Spice products containing different synthetic cannabinoids continue to emerge. Because synthetic cannabinoids are not detectable on commercial drug screens it is important that neurologists and emergency department physicians consider Spice inhalation in their differential diagnosis of a first generalized seizure.
Keywords: Spice, synthetic cannabinoid, cannabis toxicity, marijuana toxicity, first generalized seizure, provoked seizure, toxic seizure
Introduction
Initial evaluations of a first generalized seizure are commonly performed in the emergency department (ED). The most important aspect of the evaluation is a detailed history, usually obtained from paramedics and witnesses as the patient can be in a postictal state. Neurologists are consulted to aid in the comprehensive evaluation of the seizure, namely identification of the underlying pathogenesis and assessment of the need for treatment. First generalized seizures can be broadly classified as unprovoked or provoked (also known as acute symptomatic seizures). Approximately half of patients with an unprovoked first generalized seizure meet criteria for the diagnosis of epilepsy at time of presentation.1 Causes for a provoked seizure include stroke, traumatic brain injury, malignancy, central nervous system (CNS) infection, anoxia, alcohol withdrawal, drug toxicity, and metabolic derangement.2 Provoked seizures may or may not require treatment. Therefore, identifying a first generalized seizure as provoked can prevent unnecessary treatment with antiepileptic drugs (AEDs) and their potential side effects. With the exception of stroke, traumatic brain injury, malignancy, and CNS infection, the underlying medical condition can generally be reversed, preventing further seizures unless the condition recurs.3
Drug toxicity is a common cause for provoked seizures. A wide variety of illicit drugs induce seizure, such as phencyclidine (PCP), inhalants, cocaine, and psychostimulants.4 Recently EDs in the United States and Europe have reported an increase in the number of patients presenting with toxic side effects from the inhalation of synthetic cannabinoid products called Spice.5,6 Despite being a cannabinoid, Spice can cause psychosis, tachyarrhythmia, and seizure, which are not typically seen after marijuana (Cannabis sativa) use.5,6 Spice products are a variety of generally inert dried plant matter, such as damiana, rosehip, or dwarf skullcap, which is sprayed or infused with a synthetic cannabinoid and other adulterants. Sold as incense products in smoke shops, convenience stores, or on the Internet, one of the initially popular brands was named Spice and the name has become an umbrella term for other synthetic cannabinoid products such as black mamba, funky monkey, k2, popeye, demon smoke, purple haze, dank, Hawaiian harvest, and vanilla sky (Figure 1 ). Inhalers of Spice products report psychoactive experiences similar to marijuana use, which is expected because synthetic cannabinoids are also cannabinoid-receptor agonists.
Figure 1.
Examples of currently legal Spice products. Note the physical similarity to marijuana and the “not for human consumption” and “incense” labelling.
Several countries in Europe have banned certain synthetic cannabinoids7 and on March 1, 2011, the US Drug Enforcement Administration (DEA) announced it had formally placed 5 synthetic cannabinoids into schedule 1 for a 12-month period.8 These compounds include JWH-018 (1-pentyl-3-(1-naphthoyl)indole) and its homologue JWH-073, JWH-200 (1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole), CP-47,497 (5-(1,1-dimethylheptyl)-2-[(1R,3S)-3 hydroxycyclohexyl]-phenol), and CP-47,497 C8 homologue (5-(1,1-dimethyloctyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol).
We report 2 cases of patients with no prior history of neurological disease that experienced their first generalized tonic–clonic (GTC) seizure after smoking Spice.
Case Description
Case 1 is a 24-year-old male with a history of paranoid schizophrenia and hypertension who presented to the ED after having a witnessed GTC seizure lasting approximately for 3 to 5 minutes followed by a prolonged postictal state. Clinical and laboratory evaluations showed no signs of metabolic abnormality or infection. A comprehensive serum and urine drug screen9 that tests for more than 30 compounds and their metabolites was only positive for escitalopram and hydrocodone, both prescribed to the patient. A contrasted magnetic resonance imaging (MRI) of the brain at the time of admission showed diffuse, supratentorial sulcal cerebrospinal fluid (CSF), fluid-attenuated inversion recovery (FLAIR) hyperintensity and postcontrast enhancement of the pia and arachnoid (Figure 2 ). The electroencephalogram (EEG) was mildly encephalopathic with no focal features or epileptiform discharges. Cerebrospinal fluid analysis was normal, including a comprehensive meningoencephalitis panel.10
Figure 2.
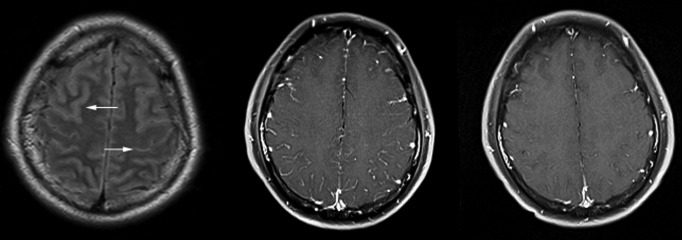
On the far left, the initial magnetic resonance imaging (MRI) of brain shows bilateral sulcal cerebrospinal fluid (CSF), fluid-attenuated inversion recovery (FLAIR) hyperintensity highlighted by the white arrows. In the middle, the initial T1 postcontrast shows diffuse pial and arachnoid enhancement, often seen in the setting of infection, postictal states, or drug reactions. On the right, the follow-up MRI brain T1 postcontrast obtained 2 weeks later, which shows resolution of the enhancement.
After admission, the patient had a second GTC seizure lasting 1 minute with urinary incontinence and postictal confusion. Upon further questioning, he reported smoking funky monkey Spice that day and denied other recreational drug use. A contrasted MRI of the brain performed 13 days after admission demonstrated resolution of the aforementioned abnormalities. The patient was not started on a maintenance AED and 3 months later when seen in clinical follow-up he reported no further seizure activity or Spice use.
Case 2 is a 36-year-old female with a history of polysubstance abuse in remission, depression, and migraine headaches, who had a GTC seizure that progressed to status epilepticus. Rescue services inadvertently placed the endotracheal tube in the esophagus and the patient continued to convulse until she was reintubated at an outside hospital ED and given lorazepam, etomidate, vecuronium, propofol, levetiracetam, and phenytoin in rapid sequence. Laboratory analysis obtained outside the ED included a negative drug screen for ethanol, tetrahydrocannabinol (THC), opiates, amphetamines, and cocaine. Upon transfer, the only metabolic abnormality was a white blood cell count of 12.4 k/μL, which resolved within 48 hours. The patient remained afebrile with a normal chest X-ray, urinalysis, blood cultures, and CSF analysis. A noncontrasted MRI of the brain was unremarkable. The EEG was mildly encephalopathic, with no focal features or epileptiform discharges.
The patient was extubated 12 hours after admission and reported smoking black mamba Spice for the first time immediately before the seizure. She was not started on a maintenance AED and had no additional seizure activity during her hospital stay. In a follow-up phone call 6 months later, the patient reported no further seizure activity or Spice use.
Discussion
First identified in the early 1990s, the cannabinoid receptors CB1 and CB2 are targets of endogenous cannabinoid-like substances (endocannabinoids). The CB1 receptor is found predominantly in the CNS, while the CB2 receptor is almost exclusively in circulating immune cells.11 Cannabinoid receptors are targets of drug development for applications ranging from pain control to antiemesis and hundreds of synthetic cannabinoids have been synthesized.12 They share basic structural similarity with THC and cannabidiol (CBD), the most abundant of the numerous cannabinoids present in marijuana.11,13 Like THC and CBD, synthetic cannabinoids have nonpolar, lipid-soluble structures that are relatively volatile. Several categories exist, including THC analogues (eg, HU-210), cyclohexylphenol compounds (eg, CP-47,497), and aminoalkylindole compounds developed by J. W. Huffman (eg, JWH-018).13 Despite the basic structural similarities with THC, the heterogeneous synthetic cannabinoids will not cross-react with traditional THC detection methods.14
Marijuana and its active ingredients possess anticonvulsant effects in animal models and humans.15 For centuries, prior to the development of AEDs, marijuana and hashish were used to treat epilepsy and are still utilized in countries with limited access to AEDs.16 Through mechanisms that have not been fully elucidated cannabinoids decrease both glutamate and γ-aminobutyric acid (GABA) synaptic transmission in the brain.17 Decreasing the excitatory neurotransmitter glutamate decreases seizure susceptibility, while decreasing the inhibitory neurotransmitter GABA increases seizure susceptibility. Tetrahydrocannabinol is a partial agonist of the CB1 receptor and, as such, its anticonvulsant effect is not entirely understood. Cannabidiol possesses anticonvulsant effects both in vitro and in vivo independently of the CB1 receptor.18,19 When wild-type mice are administered a synthetic cannabinoid, there is an expected decrease in GABA synaptic transmission, while CB1 receptor knockout mice have no decrease in GABA synaptic transmission. Interestingly, there is a decrease in the level of glutamate synaptic transmission in both wild-type and CB1-receptor knockout mice. This suggests a novel cannabinoid-sensitive receptor is responsible for the inhibition of glutaminergic neurotransmisson.20 The synthetic cannabinoids recently banned by the DEA are all full agonists of the CB1 receptor.6 Their full agonism of the GABA CB1 receptor may lead to a more potent epileptogenic decrease in GABA synaptic transmission without the concurrent effects of CBD and the novel cannabinoid-sensitive glutamate receptor.
Chemical analysis of Spice products continues to yield synthetic cannabinoids that are distinct from the 5 compounds recently banned.11 In 2009, 1 month after Germany passed legislation banning JWH-018 and several other compounds, Spice products emerged that contained JWH-073, a synthetic cannabinoid that was not included in the legislation.21 A large inventory of previously synthesized cannabinoids are candidates for future Spice production. The toxicity of the synthetic cannabinoids, plant matter, and unknown adulterants used in the production of Spice causes side effects which are not typically encountered following marijuana use, such as psychosis, elevated blood pressure, tachyarrhythmia, hyperactive agitation, and seizure.5,6 As a result, Spice products are receiving an increasing amount of attention in EDs and poison control centers, but numerous cases are missed because commercially available drug tests do not detect synthetic cannabinoids, which may be a large part of the products' appeal.
Conclusion
When Spice is smoked, it releases a variety of different chemicals in a combination distinct to each brand or even batch. This heterogeneity makes it impossible to test specifically for Spice as the etiological agent in our 2 cases, but the case history and workup suggest an association. The other notable abnormality in case 1 was the transient pial and arachnoid enhancement on MRI, which may have been the result of a GTC seizure or a chemically induced temporary breakdown of the blood–brain barrier (ie, Spice). The history of polysubstance abuse in case 2 should also be taken into account. Even though her limited drug screen was negative, it did not test for a wider range of commonly abused epileptogenic substances, such as PCP or inhalants. Although there was no evidence of anoxic brain injury from the esophageal intubation, it is possible the hypoxia was a contributing factor in her presentation of status epilepticus. In addition to the temporal relationship of the Spice consumption and seizure, both patients had extensive neurological workups, including MRI imaging, CSF analysis, and EEG testing that failed to reveal a compelling alternate etiology for their generalized seizure.
The C. Sativa plant appears to have anticonvulsive properties.15,16 More research into synthetic cannabinoids is needed to determine whether, compared to THC, their more potent full agonism of the CB1 receptor induces an epileptogenic inhibition of GABA synaptic transmission without the concurrent anticonvulsant effects of CBD and decrease in glutamate synaptic transmission mediated by novel cannabinoid receptors. Continued production of new Spice products suggests they will persist despite the DEA ban of 5 synthetic cannabinoids in March 2011. Because synthetic cannabinoids are not detectable on commercial drug screens, it is important that neurologists and ED physicians consider Spice products in their differential diagnosis of a first generalized seizure and avoid unnecessary use of AEDs.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 2001;42(4):464–475 [DOI] [PubMed] [Google Scholar]
- 2. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49(suppl 1):8–12 [DOI] [PubMed] [Google Scholar]
- 3. Hesdorffer DC, Benn EKT, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50(5):1102–1108 [DOI] [PubMed] [Google Scholar]
- 4. Wyllie E, Gupta A, Lachwan D. The Treatment of Epilepsy: Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2006:578–580 [Google Scholar]
- 5. Wehrman J. Fake marijuana spurs more than 2,000 calls to U.S. Poison centers this year alone. American Association of Poison Control Centers. November 22, 2010. http://aapcc.org/dnn/Portals/0/Nov22revisedk2release.pdf Accessed April 23, 2011
- 6. Nutt D. Consideration of the Major Cannabinoid Agonists. Advisory council on the misuse of drugs. July 16, 2009. http://www.namsdl.org/documents/ACMDMajorCannabinoidReport.pdf Accessed April 23, 2011
- 7. Sedefov R, Gallegos A, King L, et al. Understanding the ‘Spice’ phenomenon. European monitoring centre for drugs and drug addiction. March 6, 2009. http://www.emcdda.europa.eu/attachements.cfm/att_80086_EN_Spice%20Thematic%20paper%20—%20final%20version.pdf Accessed April 23, 2011
- 8. Docket No. DEA-345F Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I. Drug Enforcement Administration (DEA), U.S. department of justice. http://edocket.access.gpo.gov/2011/2011-4428.htm Accessed April 23, 2011
- 9.http://www.aruplab.com/guides/ug/tests/0090560.jsp Drug Screen (Nonforensic), Comprehensive, serum & urine: 0090560. Accessed April 23, 2011.
- 10.http://www.aruplab.com/guides/ug/tests/2001765.jsp Meningoencephalitis Panel, CSF with Reflex to HSV Type 1 & Type 2 Glycoprotein G-Specific Ab, IgG: 2001765. Accessed April 23, 2011.
- 11. De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23(1):1–15 [DOI] [PubMed] [Google Scholar]
- 12. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids [published online ahead of print Feb 21 2011]. Drug Test Anal. 2011. http://www.ncbi.nlm.nih.gov/pubmed/21337724 Accessed April 23, 2011 [DOI] [PubMed]
- 13. Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157–162 [DOI] [PubMed] [Google Scholar]
- 14. Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwärter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46(2):163–171 [DOI] [PubMed] [Google Scholar]
- 15. Mortati K, Dworetzky B, Devinsky O. Marijuana: an effective antiepileptic treatment in partial epilepsy? A case report and review of the literature. Rev Neurol Dis. 2007;4(2):103–106 [PubMed] [Google Scholar]
- 16. Grinspoon L, Bakalar J. Marihuana: The Forbidden Medicine. New Haven, CT: Yale University Press;1997:66–81 [Google Scholar]
- 17. Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68(9):1691–1698 [DOI] [PubMed] [Google Scholar]
- 18. Jones NA, Hill AJ, Smith I, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332(2):569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428(1):51–57 [DOI] [PubMed] [Google Scholar]
- 20. Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106(1):1–4 [DOI] [PubMed] [Google Scholar]
- 21. Lindigkeit R, Boehme A, Eiserloh I, et al. Spice: a never ending story? Forensic Sci Int. 2009;191(1-3):58–63 [DOI] [PubMed] [Google Scholar]



