Abstract
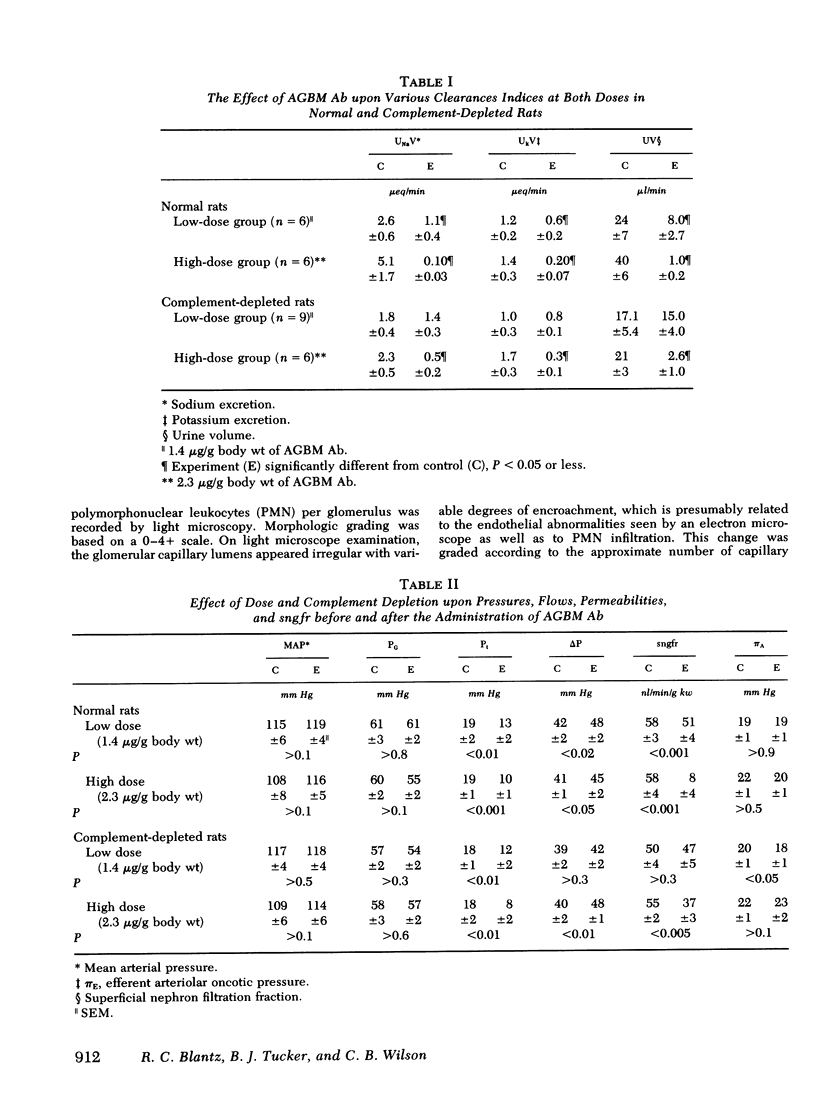
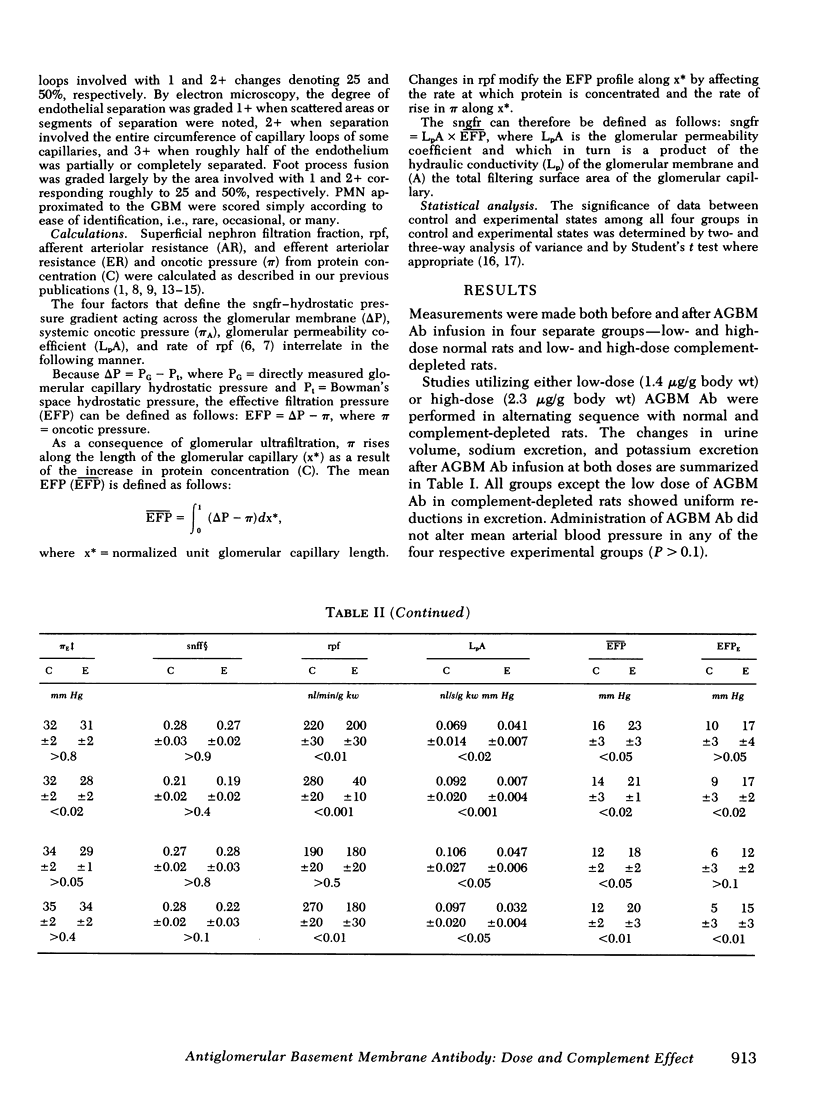
Recent studies from this laboratory have revealed that single nephron filtration rate (sngfr) decreases significantly within 1 h of the administration of large doses of complement-fixing antiglomerular basement membrane antibody (AGBM Ab) in plasma-expanded Munich-Wistar rats. This reduction in sngfr was due to decreases in nephron plasma flow (rf) and the glomerular permeability coefficient (LpA) utilizing direct evaluation of all pertinent pressures, flows, and permeabilities. With identical micropuncture techniques, we have determined (a) the respective influences of rpf and LpA upon sngfr by examining the effects of differing doses of AGBM Ab, and (b) the specific effect of complement fixation upon the reduction in sngfr. In normal rats, low dose (1.4 microgram/g body wt) AGBM Ab decreased sngfr from 57.9 +/- 3.4 to 50.8+/- 3.9 nl/min per g kidney wt (kw) (P less than 0.001), and this was due to a 10% reduction in rpf and a decrease in LpA FROM 0.069 +/- 0.014 in control to 0.041 +/- 0.007 nl/s per g kw per mm Hg (P less than 0.02). At the high dose (2.3 microgram/g body wt), sngfr fell dramatically from 58.4 +/- 4.0 to 7.6 +/- 3.8 nl/min per g kw (P less than 0.001), and this effect upon filtration was the result of an 86% reduction in rpf and a decrease in LpA from 0.092 +/- 0.020 to 0.007 +/- 0.004 nl/s per g kw mm Hg (P less than 0.001). Therefore, at lower doses sngfr fell primarily as a result of a 40% reduction in LpA and a 10% decrease in rpf; however, at the high dose massive reductions in both rps and LpA led to the large decrease in sngfr. In complement-depleted rats, receiving identical doses, low-dose AGBM Ab no longer reduced the sngfr, but a reduction in LpA persisted (other factors compensating to maintain sngfr). At the high dose, complement depletion ameliorated the reduction in sngfr (55.1 +/- 2.4 to 37.2 +/- 3.4 nl/min per g kw mm Hg) by nearly eliminating the vasoconstriction but only partially diminished the reduction in LpA (0.097 +/- 0.020 to 0.032 +/- 0.004 nl/s per g kw mm Hg, P less than 0.05). Complement depletion prevented the migration of polymorphonuclear leukocytes (present in larger numbers after the high dose of AGBM Ab) into the capillary and eliminated vasoconstriction. Complement depletion resulted in a lesser effect of high-dose AGBM Ab upon LpA than in normal rats, and this is likely due to lesser polymorphonuclear leukocyte effects upon capillary surface area. The persistent reduction in LpA observed in complement-depleted rats correlated with separation of the endothelial cell from the glomerular basement membrane after AGBM Ab, AGBM Ab diminished glomerular ultrafiltration by decreasing LpA and altering the endothelial surface of the glomerular membrane, and this effect is not totally dependent upon the fixation of complement.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. E., Wilson C. B., Gottschalk C. W. Pathophysiology of experimental glomerulonephritis in rats. J Clin Invest. 1974 May;53(5):1402–1423. doi: 10.1172/JCI107689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Balavitch D. Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science. 1976 Mar 26;191(4233):1275–1276. doi: 10.1126/science.56780. [DOI] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Blantz R. C. Effect of mannitol on glomerular ultrafiltration in the hydropenic rat. J Clin Invest. 1974 Nov;54(5):1135–1143. doi: 10.1172/JCI107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Glomerular filtration response to elevated ureteral pressure in both the hydropenic and the plasma-expanded rat. Circ Res. 1975 Dec;37(6):819–829. doi: 10.1161/01.res.37.6.819. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Rector F. C., Jr, Seldin D. W. Effect of hyperoncotic albumin expansion upon glomerular ultrafiltration in the rat. Kidney Int. 1974 Oct;6(4):209–221. doi: 10.1038/ki.1974.102. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. The mechanism of acute renal failure after uranyl nitrate. J Clin Invest. 1975 Mar;55(3):621–635. doi: 10.1172/JCI107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Wilson C. B. Acute effects of antiglomerular basement membrane antibody on the process of glomerular filtration in the rat. J Clin Invest. 1976 Oct;58(4):899–911. doi: 10.1172/JCI108543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The alternative pathway of complement activation. Adv Immunol. 1976;24:1–35. doi: 10.1016/s0065-2776(08)60328-4. [DOI] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McPhaul J. J., Jr, Mullins J. D. Glomerulonephritis mediated by antibody to glomerular basement membrane. Immunological, clinical, and histopathological characteristics. J Clin Invest. 1976 Feb;57(2):351–361. doi: 10.1172/JCI108286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The complement system and nephritis. Adv Nephrol Necker Hosp. 1974;4:3–14. [PubMed] [Google Scholar]
- PRESSMAN D., DAY E. D., BLAU M. The use of paired labeling in the determination of tumor-localizing antibodies. Cancer Res. 1957 Oct;17(9):845–850. [PubMed] [Google Scholar]
- UNANUE E. R., DIXON F. J. EXPERIMENTAL GLOMERULONEPHRITIS. V. STUDIES ON THE INTERACTION OF NEPHROTOXIC ANTIBODIES WITH TISSUE OF THE RAT. J Exp Med. 1965 May 1;121:697–714. doi: 10.1084/jem.121.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Dixon F. J. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973 Feb;3(2):74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]