Abstract
Advances in critical care have resulted in improved intensive care unit (ICU) mortality. However, improved ICU survival has resulted in a growing number of ICU survivors living with long-term sequelae of critical illness, such as impaired physical function and quality of life (QOL). In addition to critical illness, prolonged bed rest and immobility may lead to severe physical deconditioning and loss of muscle mass and muscle weakness. ICU-acquired weakness is associated with increased duration of mechanical ventilation and weaning, longer ICU and hospital stay, and increased mortality. These physical impairments may last for years after ICU discharge. Early Physical Medicine and Rehabilitation (PM&R) interventions in the ICU may attenuate or prevent the weakness and physical impairments occurring during critical illness. This article reviews the evidence regarding safety, feasibility, barriers, and benefits of early PM&R interventions in ICU patients and discusses the limited existing data on early PM&R in the neurological ICU and future directions for early PM&R in the ICU.
Keywords: bed rest, deconditioning, early ICU rehabilitation, early Neuro-ICU rehabilitation, feasibility, immobility, muscle weakness, outcomes, safety
Introduction
Advances in critical care have resulted in improved intensive care unit (ICU) mortality. As a consequence, there are a growing number of ICU survivors living with long-term sequelae of critical illness, such as impaired physical function and quality of life (QOL).
In addition to critical illness, prolonged bed rest and immobility in the ICU lead to a loss of muscle mass and muscle weakness. Intensive care unit–acquired weakness (ICUAW) may occur in 25% of patients requiring mechanical ventilation for >1 week1 and is associated with increased duration of mechanical ventilation and weaning,2–5 longer ICU and hospital stay,2,3 and increased mortality.6–8 Impairments in physical function and exercise capacity may last for years after ICU discharge.9 Thus, there is a need for interventions that may attenuate or prevent weakness and physical impairments occurring during critical illness.
This article first reviews the etiology, risk factors, and long-term sequelae of muscle weakness in the ICU as well as the clinical features of ICUAW and a diagnostic approach. We then discuss the role of early Physical Medicine and Rehabilitation (PM&R) interventions in the ICU to address these physical sequelae, including evidence regarding its safety, feasibility, barriers, and benefits. This article concludes by discussing the limited existing data on early PM&R in the neurological ICU and future directions for early PM&R in the ICU.
ICU-Acquired Weakness
Muscle weakness and physical impairments after critical illness may be due to (1) a preexisting neuromuscular disorder, such as myasthenia gravis, (2) a new neurological disease causing the need for ICU admission, such as Guillain-Barré syndrome, (3) a complication of critical illness (ICUAW), or (4) post-ICU mood changes or persistent pain that may affect survivors’ capacity or willingness to perform physical activities.10 ICU-acquired weakness refers to the acute onset of diffuse, symmetric, generalized muscle weakness that develops after the onset of critical illness without other identifiable cause.11
Etiology and Risk Factors Associated With Muscle Weakness After Critical Illness
The etiology of ICUAW is multifactorial and may include (1) prolonged bed rest and immobility leading to deconditioning and disuse atrophy, (2) critical illness polyneuropathy (CIP), critical illness myopathy (CIM), or a combination of these conditions, known as critical illness neuromyopathy (CINM), or (3) prolonged neuromuscular blockade.11 Commonly cited risk factors for ICUAW and CINM include the systemic inflammatory response syndrome, sepsis, and multiple organ dysfunction syndrome (MODS); hyperglycemia; and medications, such as corticosteroids and neuromuscular blocking agents (NMBAs).8
Bed rest and immobilization
Prolonged bed rest leads to decreased protein synthesis, enhanced proteolysis, and a net loss in the muscle mass and muscle strength.12 In healthy, well-nourished volunteers, there is a 4% to 5% loss of muscle strength for each week of bed rest.13 In critically ill patients, the onset of muscle atrophy is rapid and severe, beginning within days of hospitalization.14 Both oxidative stress and proinflammatory cytokines have been investigated as potential causes for myopathy during critical illness. Limited data suggest that prolonged inactivity contributes to imbalances in the inflammatory response with increased proinflammatory cytokine and oxidative stress activity.15,16 In 3 studies, the duration of mechanical ventilation, as a potential surrogate measure of bed rest and immobility, was associated with ICUAW.1,17,18
Systemic inflammatory response, sepsis, and MODS
The systemic inflammatory response and MODS have been associated with the loss of muscle mass19 and neuromuscular abnormalities.2,20 Clinically detectable muscle weakness has been associated with the number of days with organ dysfunction in 2 or more systems and the number of days receiving vasoactive drugs.17 These neuromuscular abnormalities are regarded as part of sepsis-associated MODS.21
Hyperglycemia
Hyperglycemia is an identified risk factor for neuromuscular abnormalities. Two large randomized controlled trials of intensive insulin therapy for tight glycemic control (blood glucose target 80-110 mg/dL) demonstrated a strong association between hyperglycemia and the presence of abundant spontaneous electrical activity with needle electromyography (EMG); however, no clinical evaluation of muscle strength was performed to supplement this limited EMG evaluation.22,23
NMBAs and corticosteroids
Controversy exists regarding the associations of NMBAs and/or use of corticosteroids with ICUAW. For instance 2 prospective studies, which adjusted for potential confounders, found a significant independent association of NMBAs with ICUAW2,24 whereas in other studies NMBAs were not associated with ICUAW.25 Similarly, a prospective cohort study demonstrated a significant independent association of corticosteroids with ICUAW but could not detect an association with dose or duration of corticosteroid therapy. However, other prospective studies could not identify corticosteroids as an independent factor for CINM.2,17,20,26 Conversely, 1 trial of intensive insulin therapy reported that corticosteroids had a protective effect against the presence of EMG abnormalities in critically ill patients receiving intensive insulin therapy.24
Clinical Presentation and Evaluation
Critically ill patients who demonstrate clinically evident weakness and/or prolonged ventilator dependence should be evaluated for ICUAW. Physical findings show diffuse symmetric weakness and decreased tone. Deep tendon reflexes may be normal, reduced, or absent.11 Table 1 provides a comparison of the clinical, electrophysiological, and pathological features of common ICU neuromuscular abnormalities.
Table 1.
Features of Neuromuscular Abnormalities of Critical Illnessa
| Critical Illness Polyneuropathy | Critical Illness Myopathy | Disuse Atrophy | |
|---|---|---|---|
| Setting | Sepsis, SIRS, MODS | Most common with NMBAs, steroids, asthma, organ transplant | Prolonged bed rest/immobility Prolonged sedation |
| Clinical features | Generalized or distal muscle weakness Cranial nerves usually spared Distal sensory deficit Preserved or depressed DTRs | Generalized or predominantly proximal muscle weakness No sensory impairment Preserved or depressed DTRs Elevated CK levels: 10- to 100-fold | Muscle wasting with flaccid weakness Normal or reduced strength with marked diminished endurance No sensory deficits Preserved DTRs |
| Nerve conduction studies | CMAP decreased to <80% of LLN in >2 nerves SNAP decreased to <80% of LLN in > 2 nerves Normal conduction velocity | CMAP decreased to <80% of LLN in > 2 nerves SNAPs are >80% of LLN in >2 nerves NCVs normal or near-normal without conduction block | Normal NCS, or Modest decreased CMAP with preserved SNAP |
| Needle EMG | Abnormal spontaneous activity Large polyphasic MUAPs Reduced recruitment/interference pattern | Variable spontaneous activity Small polyphasic MUAPs in >2 muscle groups, Low-amplitude, full interference pattern | Abnormal/absence of spontaneous activity Polyphasic MUAP |
| Direct muscle stimulation | Reduced ne-CMAP Normal dm-CMAP ne-CMAP to dm-CMAP ratio <0.532 | Reduced ne-CMAP and dm-CMAP ne-CMAP: dm-CMAP ratio > 0.5 in >2 muscle groups | |
| Biopsy | Axonal degeneration of motor and sensory nerve fibers | Thick filament (myosin) loss Necrotizing myopathy Inflammatory infiltrate may be present | Shift from slow twitch (type I) to fast twitch (type II) muscle fibers Decreased muscle fiber size Little or no inflammatory infiltrates |
Abbreviations: CK, serum creatine kinases; CMAP, compound muscle action potential; dm-CMAP, direct muscle stimulated muscle action potential; DTRs, deep tendon reflexes; LLN, lower limit of normal; MODS, multiple organ dysfunction; MUAP, motor-unit action potential; ne-CMAP, nerve stimulated compound muscle action potential; NMBAs, neuromuscular blocking agents; NCVs, nerve conduction velocities; SIRS, systemic inflammatory response syndrome; SNAP, sensory nerve action potential.
Electrophysiological studies
Electromyography (EMG) and nerve conduction studies (NCSs) can help identify abnormalities in sedated or uncooperative patients who cannot be clinically examined for muscle strength. Electrophysiological abnormalities are present early and precede clinical manifestations of muscle weakness.28 Typically, CIP is characterized by a sensorimotor axonopathy with markedly reduced compound muscle action potentials (CMAPs) and sensory nerve action potentials (SNAPs), whereas nerve conduction velocities and distal latencies are minimally affected.29 Needle EMG demonstrates abnormal spontaneous activity with fibrillation potentials and positive sharp waves.29,30 Typical features of CIM include low-amplitude, sometimes broadened or absent, CMAPs and relatively preserved SNAPs,31 and needle EMG demonstrates low amplitude, short duration, and polyphasic motor unit action potentials, with good recruitment pattern despite the pronounced weakness.32 Fibrillation potentials and positive sharp potentials, which are usually indicative of axonal injury, may also occur in CIM (PM-T).32 Routine EMG/NCS may be unable to discriminate between CIP and CIM in unconscious, uncooperative, or extremely weak patients without additional testing, such as direct muscle stimulation.33,34 Participants with CIM demonstrate reduced or absent direct muscle excitability.35 Electrophysiological studies require resources and expertise and therefore are not universally available in all ICUs. Moreover, it is currently unclear whether all abnormal EMG/NCS findings have clinical importance to patients.11
Muscle and nerve biopsy
Histological studies have contributed substantially to our understanding of neuromuscular impairments in critical illness. In patients with CIP, nerve biopsy demonstrates an axonal degeneration of motor and sensory nerve fibers,36 resulting in extensive denervation atrophy of limb and respiratory muscles.37 Muscle biopsies have demonstrated 3 morphologic subtypes of ICU-acquired myopathies. Thick filament myopathy is characterized by selective proteolysis and loss of myosin filaments. Acute necrotizing myopathy is characterized by muscle fiber vacuolization and phagocytosis of myocites. In cachectic myopathy, the muscle fibers show caliber variations, angulations, internalized nuclei, rimmed vacuoles, fatty degeneration, and fibrosis.37 A biopsy may be helpful to help determine an etiology or pathological process when specific diagnostic information is required for clinical care.
Diagnostic Strategy
The diagnosis of ICUAW is based on clinical presentation, predisposing factors, and clinically detectable muscle weakness, on physical examination, in a critically ill patient in whom there is no plausible etiology other than critical illness. Given the challenges of EMG/NCS in the ICU, EMG/NCS are generally conducted in patients for whom an alternative cause of muscle weakness is being considered, particularly in patients with unexpected severe muscle weakness or slow recovery. These tests may be sometimes performed in patients who cannot have a complete physical examination or are having difficulties with weaning from mechanical ventilation.
Outcomes of Patients With ICUAW
ICU-acquired weakness is independently predictive of poor patient outcomes. Liberation from mechanical ventilation may be 2 to 7 times longer2–4 with prolonged ICU and hospital stays.2,3 Furthermore, clinically detectable ICUAW at the time of awakening has been independently associated with increased hospital mortality in 2 studies.6,7 Clinical evidence of recovery of muscle strength may occur within weeks in mild cases and within months in severe cases of ICUAW. In 1 study, the median duration of ICUAW after awakening was 21 days, with 94% of the ICU survivors having a meaningful clinical recovery by 9 months.1 In more severe cases, however, recovery is incomplete or does not occur at all. Persistent muscle weakness and electrophysiological abnormalities have been reported up to 5 years after ICU discharge.38 Survivors of severe critical illness frequently report persistent functional limitations and impaired QOL for months and years after ICU, which they attribute to neuromuscular issues, including muscle wasting and weakness and residual motor and sensory deficits.9,39
Strategies for Prevention and Treatment of ICUAW
While there is growing knowledge about the epidemiology and mechanisms of ICUAW, to date there are few options for its prevention and/or treatment.40 Timely and intensive treatment of sepsis and MODS are key to critical care medicine and may help reduce neuromuscular abnormalities. Although tight glycemic control with intensive insulin therapy may decrease the incidence of electrophysiological neuromuscular abnormalities, in large multicenter studies, this intervention has been associated with an increased risk of hypoglycemia and in-hospital death.41 Avoidance of corticosteroids and/or NMBAs, where possible, may be prudent until future studies can clarify their role in ICUAW. The next section of this article will discuss the role of early PM&R interventions for improving ICUAW and physical function.
PM&R in the ICU
Early PM&R in the ICU refers to all physical therapy (PT), occupational therapy (OT), and other rehabilitation activities initiated immediately upon achieving physiologic stability and continued throughout the ICU stay. Such activities may start within 1 or 2 days of initiation of mechanical ventilation. During PM&R activities, patients may still require mechanical ventilation, vasopressors, and dialysis. The goal of early PM&R is to prevent or attenuate neuromuscular complications arising from immobility and prolonged bed rest.
Feasibility
ICU patients are frequently perceived as “too ill” to participate in early PM&R therapies.42 However, trials have shown that early PM&R activities are feasible within 24 to 48 hours of initiation of mechanical ventilation.43,44 In a large case series of 103 patients, 41% of all mobilization events occurred in intubated patients, with ambulation occurring in 42% of these events.45 In addition, a prospective trial of 280 medical ICU patients requiring mechanical ventilation on admission was successful in using a dedicated mobility team to initiate physical therapy within 48 hours of admission. This trial demonstrated that many more patients assigned to the mobility team (vs usual care) received physical therapy in the ICU (73% vs 6%, P < .001).44 Figure 1 illustrates the feasibility of early mobilization of mechanically ventilated patients.
Figure 1.
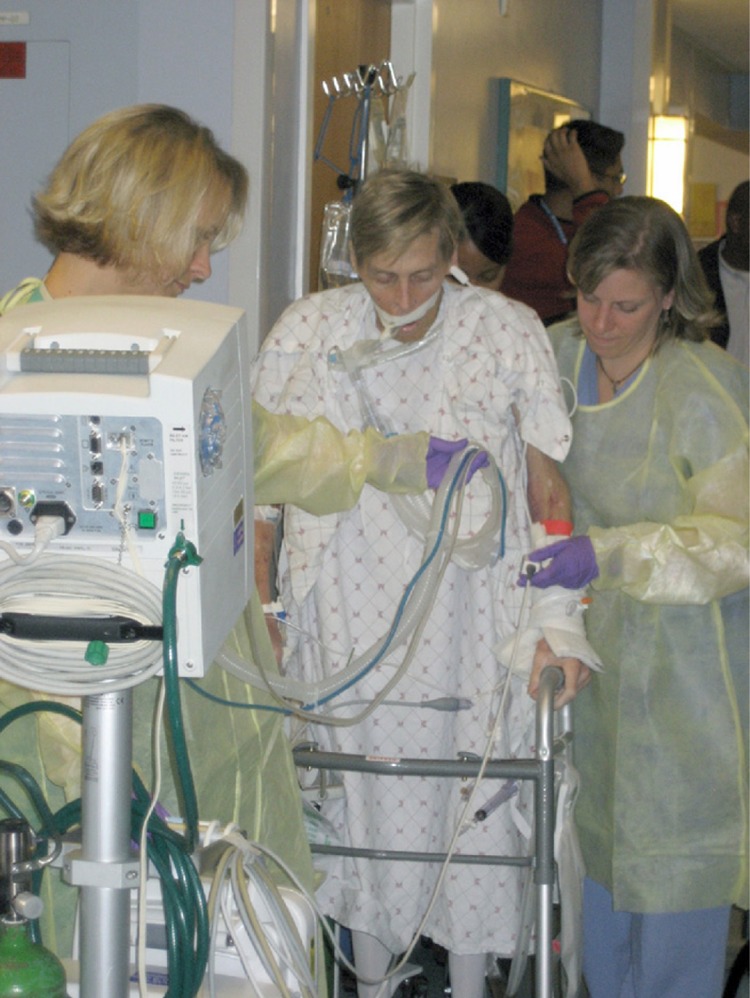
The feasibility of early ambulation is illustrated in this 56-year-old man admitted to The Johns Hopkins Medical Intensive Care Unit with respiratory and renal failure. The patient is being ambulated while on mechanical ventilation, with the assistance of a physical therapist, respiratory therapist, nurse, and a physical therapy technician. Additional equipment included a portable ventilator, a portable cardiac monitor, a wheeled pole with intravenous infusion pumps, and a wheeled walker. A wheelchair is being pushed behind the patient by a physical therapy technician (not seen). Reprinted with permission from Needham et al.46
Safety
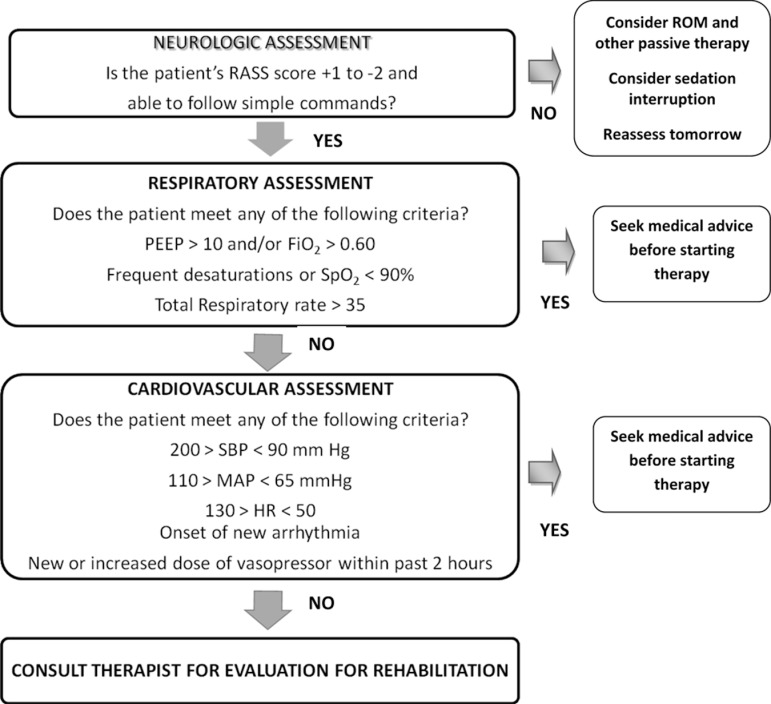
In experienced ICUs, early PM&R interventions are safe, even for mechanically ventilated patients. One study with prospective monitoring of safety events reported an event rate of <1% in 1449 activity events. None of these safety events resulted in extubation, additional therapy, higher cost, or longer hospital stay.45 Another trial reported no adverse events among 114 patients who received early PT in the ICU.44 Safety of early PM&R is aided by careful patient screening and trained staff who carefully evaluate patients’ physiological changes during graduated interventions.47 Figure 2 provides an example screening algorithm for active rehabilitation therapy for ICU patients.
Figure 2.
Screening algorithm to evaluate for appropriateness for PM&R activity. Fio 2 indicates fractional of inspired oxygen; HR, heart rate; MAP, mean arterial pressure; PEEP, positive end-expiratory pressure; RASS, Richmond Agitation Sedation Scale; ROM, range of motion; SBP, systolic blood pressure; SpO2, saturation of peripheral oxygen. Modified from Korupolu et al.47
Benefits
Early PM&R has been consistently associated with improvement in patients’ mobility and physical function. In a large case series45 of 89 ICU survivors, 69% were able to ambulate >100 feet at the time of ICU discharge. Moreover, a nonrandomized trial demonstrated that mechanically ventilated patients that received early PT (vs usual care) were out of bed earlier (5 vs 11 days, P = <.001).44
The strongest evidence for benefit of early PM&R comes from a 2-site, randomized controlled trial of 104 mechanically ventilated patients receiving very early PT and OT started at the beginning of respiratory failure.48 Both the intervention and usual care groups received daily sedation interruption and daily spontaneous breathing trials. Participants in the intervention group received progressive mobilization and activities of daily living interventions started at 1.5 days after intubation (vs 7.3 days in the usual care group, P = <.01). This trial demonstrated that early intervention led to better outcomes at hospital discharge, including return to independent function (59% vs 35%, P = .02), physical function (median Barthel Index 75 vs 55, P = .05), and ventilator-free days (median 23.5 vs 21.1, P = .05).48
Early PM&R is also associated with shorter ICU and hospital stays and potentially improved outcomes after hospital discharge. In a nonrandomized trial of an early mobility team, there was a shorter adjusted mean ICU and hospital length of stay ([LOS]; 5.5 vs 6.9 days, P = .025 and 11.2 vs 14.5 days, P = .006, respectively)44 and a significantly greater odds of surviving without hospital readmissions during 1-year follow-up (odds ratio 1.77, P = .0362).49 In addition, a quality improvement project with before–after comparison demonstrated that intensive PT and OT in mechanically ventilated MICU patients was associated with a 30% and 18% decrease in ICU and hospital LOS, respectively, and a 20% increase in ICU admissions.46
Perceived Barriers
There are potential perceived barriers to implementing early PM&R in ICU patients. Commonly perceived patient-related barriers include delirium, cardiopulmonary instability, and obesity. Both ICU- and institution-related barriers include sedation practices, safety concerns, local beliefs and attitudes, and lack of staffing or expertise.50 In particular, sedation substantially reduces patients’ ability to engage in active PM&R activities and prolongs the duration of mechanical ventilation and ICU and hospital LOS.45,46,51 In many ICUs, it is a customary for mechanically ventilated patients to be deeply sedated and immobile.52,53 However, existing studies evaluating early PM&R in the ICU consistently demonstrate that deep sedation is not necessary for the comfort and safety of mechanically ventilated patients, even when ventilated via an oral endotracheal tube.49,54 Trained staff and a supportive ICU culture are important for successfully introducing early PM&R. One study of mechanically ventilated patients noted that the odds of ambulation were 2.5 greater after transfer to an ICU in which early mobilization was a priority.51 This increase in ambulation could not be accounted for by differences in patients’ severity of illness, suggesting that many ICU patients are subjected to unnecessary immobilization due to the routine care practices and culture of the ICU.
Implementing an Early ICU Physical Medicine and Rehabilitation Program
Initiating an early ICU PM&R program requires a multifaceted approach which includes (1) assembling a multidisciplinary team of physicians, nursing, and respiratory, physical, and occupational therapists, (2) identifying local barriers to PM&R implementation, and (3) engaging key hospital administrators and ICU leaders and frontline clinicians who will champion changes in PM&R practices and provide support for the program.46,50
Equipment and Resources
Equipment and resources are required to introduce early PM&R in ICU patients. Standard equipment includes rolling intravenous poles with infusion pumps, walkers, and wheelchairs.46,55 A portable ventilator, cardiac monitor, and pulse oximeter can also facilitate ambulation of mechanically ventilated patients. When a portable ventilator is not available, other approaches include using a traditional (nonportable) ventilator on battery power or using a bag valve mask (BVM) with positive end-expiratory pressure valve and supplemental oxygen.
Personnel and Teamwork
Effective communication and coordination among ICU staff is important for early PM&R. In addition to rehabilitation clinicians, such as PT and OT, early PM&R requires teamwork from ICU physicians, nurses, and respiratory therapists. Additional PM&R members may include a speech language pathologist, physiatrist, and a technician or assistant for the PT and OT.56,57
Adjuvant Interventions and Strategies
Several PM&R interventions are available, which may be useful for patients who are unable to participate in exercise programs due to physical limitations such as profound muscle weakness or are deeply sedated or in comatose, as described in this section.
Cycle ergometer
A cycle ergometer is a stationary bicycle that allows patients to receive passive, active-assisted, and active-resistive exercise. Bedside cycle ergometry has been used safely in patients with severe COPD58 and end-stage renal disease during dialysis.59 In the ICU, cycle ergometry may be used with sedated, immobile, or awake patients to provide a range of motion (ROM) and strength muscle training.55 In a randomized controlled trial of 90 mechanically ventilated patients with expected prolonged ICU stays, a 20-minute daily leg cycling session 5 days/week resulted in improved isometric quadriceps force, higher self-perceived functional status, and higher exercise capacity as measured by the 6-minute walk test at hospital discharge.60
Fixed and dynamic tilt tables
A tilt table can allow partial weight bearing in a gravity-reduced environment for patients who require a graded transition from bed rest to supporting their full body weight. A dynamic tilt table allows movement of the table using a sled mechanism to permit flexion and extension of hips and knees against a partial body weight workload created by partial elimination of gravity when the device is tilted to <90° upright (eg, the table could be tilted as little as 10° above the horizontal plane). Although rigorous clinical research evaluations of such interventions in ICU patients have not yet been performed, they may help facilitate weight bearing, improve lower limb strength, and prevent ankle contractures.61–64
Neuromuscular electrical stimulation
Neuromuscular electrical stimulation (NMES) has been used to reduce muscle atrophy and enhance muscle strength and endurance. This technique has been used as an alternative form of exercise in patients with severe chronic heart failure and chronic obstructive pulmonary failure.65 In critically ill patients, NMES may elicit a systemic effect on peripheral microcirculation,66decrease muscle protein breakdown,67 preserve muscle mass,68,69 and reduce ICUAW and weaning time.70 Additional studies are needed to confirm these early findings.
Inspiratory muscle training
Inspiratory muscle training (IMT) may be beneficial in selected patients who fail ventilator weaning. There is some evidence that IMT improves maximum inspiratory pressure and reduces weaning time in selected ICU patients with prolonged mechanical ventilation.71 However, one study showed no benefit72 and IMT may not be generalizable to other ICU populations, such as those with chronic pulmonary obstructive pulmonary disease or congestive heart failure.73 Further research is required to explore the patients who may most benefit from this intervention.
Early PM&R in the Neuro-ICU
The efficacy of inpatient rehabilitation is well established for neurological patients with stroke, spinal cord injury, multiple sclerosis, brain tumors, acquired brain injuries, and Guillain-Barré syndrome.74–77 However, early PM&R in the Neuro-ICU may not be routinely implemented due, in part, to the lack of research investigating critical issues such as safety, feasibility, and overall benefits. The existing evidence supporting early PM&R has generally occurred within medical or medical–surgical ICUs. Given positive results in these ICUs, new investigation in the neuro-ICU is anticipated. In this section, we discuss some selected considerations in evaluating early PM&R in the Neuro-ICU setting.
Perceived Barriers
Neuro-ICUs may experience many of the same perceived barriers previously discussed. Important information is required regarding the safety of early PM&R in neurological patients as demonstrated in an anonymous survey of stroke clinicians evaluating their opinion on early mobilization after stroke. The survey demonstrated greater discomfort with mobilization of patients with hemorrhagic versus ischemic strokes, with 60% of respondents believing that there would be ill effects if mobilization occurred within 24 hours of stroke. Thus, establishing safety data in neuro-ICU patients is important for informing changes in practice.
Safety, Feasibility, and Benefit in Neuro-ICU
The effects of mobility on key neuro-ICU issues, such as intracranial pressure (ICP), cerebral blood flow (CBF), and cerebral perfusion pressure, have not been rigorously evaluated in prior studies. However, preliminary information supports early PM&R in certain populations. For instance, in a study of 20 participants with mild or moderate vasospasm after aneurysmal subarachnoid hemorrhage, gradual head of bed elevation of 20° and 45° did not result in significant changes in CBF when measured by transcranial Doppler.78 Similarly, in a study of comatose and noncomatose neurosurgical participants with normal (<15 mm Hg) and elevated (> 15 mm Hg) ICP and stable neurologic status, passive and active ROM in a 30° head-up position had no effect or caused a modest decrease on ICP.79 This preliminary information suggests that ROM and limb exercises can be performed safely in patients with normal or increased ICP provided there is no Valsalva-like maneuvers.79
Researchers from Taiwan demonstrated that early and intensive rehabilitation of 154 participants with acute ischemic (60%) or hemorrhagic (40%) strokes in a neuro-ICU was feasible.80 In this study, 44% and 45% of patients had moderate and severe strokes, respectively, and half of the patients started rehabilitation therapy within 4 days, receiving a mean of 0.6 (±0.4) sessions/d.80 This early and intensive rehabilitation resulted in improved functional outcomes with a mean improvement in the Barthel Index score of 33 points and 57% of patients walking independently at hospital discharge.80 Data on early PM&R in other neuro-ICU populations are keenly awaited.
Finally, the A Very Early Rehabilitation Trial for Stroke (AVERT) demonstrated the feasibility and safety of very early mobilization (VEM) for patients with acute stroke. In this trial, 71 patients with acute stroke admitted to specialized non-ICU acute stroke units were randomized to standard care (standard PT) versus standard care plus VEM (upright and out of bed twice daily within 24 hours of stroke for up to 14 days or until discharge).81 The median time to first mobilization after symptoms onset was significantly lower in the VEM group (18.1 vs 30.8 hours, P < .001), without any significant differences in the number of serious adverse events (15 vs 14, P = .846). Moreover, the VEM group returned to walking 50 m more quickly (median 3.5 vs 7.0 days, P = .032); and by 2 weeks, 67% of the VEM group (vs 50% of control group, P = .032) was able to walk unassisted.82
Conclusions and Future Directions
Muscle weakness and impaired physical function are common and long lasting complications of critical illness. Currently, there are limited interventions to prevent these complications. However, in recent years, there is growing evidence that early PM&Rs are feasible, safe, and beneficial in critically ill patients. Such early interventions result in better functional outcomes at hospital discharge with further studies needed to evaluate the potential long-term benefits.
There is great potential for exploring the benefit of adjunctive interventions, such as use of cycle ergometry, dynamic tilt table, NMES, and IMT. Early and intensive mobilization of patients with acute stroke appears safe and leads to improved functional outcomes. However, greater investigation in neuro-ICU patients is required in future studies to understand whether the results of studies in other critically ill populations are applicable in the neuro-ICU patient populations.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
References
References
- 1. De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867 [DOI] [PubMed] [Google Scholar]
- 2. Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia J, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296 [DOI] [PubMed] [Google Scholar]
- 3. Garnacho-Montero J, Amaya-Villar R, Garcia-Garmendia JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med. 2005;33(2):349–354 [DOI] [PubMed] [Google Scholar]
- 4. De Jonghe B, Sharshar T, Hopkinson N, Outin H. Paresis following mechanical ventilation. Curr Opin Crit Care. 2004;10(1):47–52 [DOI] [PubMed] [Google Scholar]
- 5. De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–1121 [DOI] [PubMed] [Google Scholar]
- 6. Ali NA, O’Brien JM, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–268 [DOI] [PubMed] [Google Scholar]
- 7. Sharshar T, Bastuji-Garin S, Stevens RD, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37(12):3047–3053 [DOI] [PubMed] [Google Scholar]
- 8. De Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10 suppl):S309–S315 [DOI] [PubMed] [Google Scholar]
- 9. Herridge M, Tansey C, Matté A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304 [DOI] [PubMed] [Google Scholar]
- 10. Hough CL, Needham DM. The role of future longitudinal studies in ICU survivors: understanding determinants and pathophysiology of weakness and neuromuscular dysfunction. Curr Opin Crit Care. 2007;13(5):489–496 [DOI] [PubMed] [Google Scholar]
- 11. Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 suppl):S299–S308 [DOI] [PubMed] [Google Scholar]
- 12. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270(4 pt 1):E627–E633 [DOI] [PubMed] [Google Scholar]
- 13. Berg H, Larsson L, Tesch P. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997;82(1):182. [DOI] [PubMed] [Google Scholar]
- 14. Helliwell TR, Wilkinson A, Griffiths RD, et al. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol Appl Neurobiol. 1998;24(6):507–517 [DOI] [PubMed] [Google Scholar]
- 15. Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit--from pathophysiology to clinical trials. Crit Care. 2009;13(4):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winkelman C. Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23(1):21–34 [DOI] [PubMed] [Google Scholar]
- 17. Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353 [DOI] [PubMed] [Google Scholar]
- 18. Witt N, Zochodne D, Bolton C, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99(1):176–184 [DOI] [PubMed] [Google Scholar]
- 19. Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 suppl):S354–S367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Letter MCJ, Schimtz PIM, Visser LHH, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–2286 [DOI] [PubMed] [Google Scholar]
- 21. Friedrich O. Critical illness myopathy: sepsis-mediated failure of the peripheral nervous system. Eur J Anaesthesiol. 2008;25(suppl 42):73–82 [DOI] [PubMed] [Google Scholar]
- 22. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367 [DOI] [PubMed] [Google Scholar]
- 23. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461 [DOI] [PubMed] [Google Scholar]
- 24. Hermans G, Wilmer A, Meersseman W, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–489 [DOI] [PubMed] [Google Scholar]
- 25. Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–1891 [DOI] [PubMed] [Google Scholar]
- 26. Bednarík J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol. 2005;252(3):343–351 [DOI] [PubMed] [Google Scholar]
- 27. Fan E, Zanni JM, Dennison CR, Lepre SJ, Needham DM. Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit. AACN Adv Crit Care. 2009;20(3):243–253 [DOI] [PubMed] [Google Scholar]
- 28. Latronico N, Shehu I, Guarneri B. Use of electrophysiologic testing. Crit Care Med. 2009;37(10 suppl):S316–S320 [DOI] [PubMed] [Google Scholar]
- 29. Bolton CF, Laverty DA, Brown JD, et al. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1986;49(5):563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bolton CF. Electrophysiologic studies of critically ill patients. Muscle Nerve. 1987;10(2):129–135 [DOI] [PubMed] [Google Scholar]
- 31. Lacomis D, Zochodne DW, Bird SJ. Critical illness myopathy. Muscle Nerve. 2000;23(12):1785–1788 [DOI] [PubMed] [Google Scholar]
- 32. Dhand UK. Clinical approach to the weak patient in the intensive care unit. Respir Care. 2006;51(9):1024–1040 [PubMed] [Google Scholar]
- 33. Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve. 1997;20(6):665–673 [DOI] [PubMed] [Google Scholar]
- 34. Weber-Carstens S, Koch S, Spuler S, et al. Nonexcitable muscle membrane predicts intensive care unit-acquired paresis in mechanically ventilated, sedated patients. Crit Care Med. 2009;37(9):2632–2637 [DOI] [PubMed] [Google Scholar]
- 35. Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46(3):731–736 [DOI] [PubMed] [Google Scholar]
- 36. Bolton C, Gilbert J, Hahn A, Sibbald W. Polyneuropathy in critically ill patients. Jf Neurol Neurosurg Psychiatry. 1984;47(11):1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zink W, Kollmar R, Schwab S. Critical illness polyneuropathy and myopathy in the intensive care unit. Nat Rev Neurol. 2009;5(7):372–379 [DOI] [PubMed] [Google Scholar]
- 38. Fletcher SN, Kennedy DD, Ghosh IR, et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31(4):1012–1016 [DOI] [PubMed] [Google Scholar]
- 39. Herridge M, Cheung A, Tansey C, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693 [DOI] [PubMed] [Google Scholar]
- 40. Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev. 2009;(1):CD006832. [DOI] [PubMed] [Google Scholar]
- 41. Finfer S, Chittock D, Su S, Blair D, Foster D. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297 [DOI] [PubMed] [Google Scholar]
- 42. Gosselink R, Bott J, Johnson M, et al. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008;34(7):1188–1199 [DOI] [PubMed] [Google Scholar]
- 43. Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38(11):2089–2094 [DOI] [PubMed] [Google Scholar]
- 44. Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243 [DOI] [PubMed] [Google Scholar]
- 45. Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145 [DOI] [PubMed] [Google Scholar]
- 46. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542 [DOI] [PubMed] [Google Scholar]
- 47. Korupolu R, Gifford J, Needham D. Early mobilization of critically ill patients: reducing neuromuscular complications after intensive care. Contemp Critl Care. 2009;6(9):1–11 [Google Scholar]
- 48. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehab. 2010;17(4):271–281 [DOI] [PubMed] [Google Scholar]
- 51. Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36(4):1119–1124 [DOI] [PubMed] [Google Scholar]
- 52. Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit*. Crit Care Med. 2007;35(2):393–401 [DOI] [PubMed] [Google Scholar]
- 53. Needham DM, Wang W, Desai SV, et al. Intensive care unit exposures for long-term outcomes research: development and description of exposures for 150 patients with acute lung injury. J Crit Care. 2007;22(4):275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Korupolu R, Needham DM. Series on early mobilisation of critically ill patients Part One: screening and safety issues. ICU Manage. 2009;9(3):27 [Google Scholar]
- 55. Needham DM, Truong AD, Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 suppl):S436–S441 [DOI] [PubMed] [Google Scholar]
- 56. Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690 [DOI] [PubMed] [Google Scholar]
- 57. Reina ML, Reina DS, Rushton CH. Trust: the foundation for team collaboration and healthy work environments. AACN Adv Crit Care. 2007;18(2):103–108 [DOI] [PubMed] [Google Scholar]
- 58. Galetke W, Randerath W, Pfeiffer M. Spiroergometry in patients with severe chronic obstructive pulmonary disease confined to bed. Pneumologie. 2002;56(2):98–102 [DOI] [PubMed] [Google Scholar]
- 59. Torkington M, Macrae M, Isles C. Uptake of and adherence to exercise during hospital haemodialysis. Physiotherapy. 2006;92(2):83–87 [Google Scholar]
- 60. Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505 [DOI] [PubMed] [Google Scholar]
- 61. Pryor J, Webber B. Physiotherapy techniques. In: Pryor J, Prasad S, eds. Physiotherapy for Respiratory and Cardiac Disorders. 3rd ed London: Churchill Livingstone; 2002:161–242 [Google Scholar]
- 62. Bourdin G, Barbier J, Burle JF, et al. The feasibility of early physical activity in intensive care unit patients: a prospective observational one-center study. Respir Care. 2010;55(4):400–407 [PubMed] [Google Scholar]
- 63. Trees DW, Ketelsen CA, Hobbs JA. Use of a modified tilt table for preambulation strength training as an adjunct to burn rehabilitation: a case series. J Burn Care Rehabil. 2003;24(2):97–103 [DOI] [PubMed] [Google Scholar]
- 64. Chang AT, Boots R, Hodges PW, Paratz J. Standing with assistance of a tilt table in intensive care: a survey of Australian physiotherapy practice. Aust J Physiother. 2004;50(1):51–54 [DOI] [PubMed] [Google Scholar]
- 65. Sillen MJH, Speksnijder CM, Eterman RMA, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest. 2009;136(1):44–61 [DOI] [PubMed] [Google Scholar]
- 66. Gerovasili V, Tripodaki E, Karatzanos E, et al. Short-term systemic effect of electrical muscle stimulation in critically ill patients. Chest. 2009;136(5):1249–1256 [DOI] [PubMed] [Google Scholar]
- 67. Bouletreau P, Patricot M, Saudin F, Guiraud M, Mathian B. Effects of intermittent electrical stimulations on muscle catabolism in intensive care patients. JPEN J Parenter Enteral Nutr. 1987;11(6):552. [DOI] [PubMed] [Google Scholar]
- 68. Gruther W, Benesch T, Zorn C, et al. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40(3):185–189 [DOI] [PubMed] [Google Scholar]
- 69. Gerovasili V, Stefanidis K, Vitzilaios K, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13(5):R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Routsi C, Gerovasili V, Vasileiadis I, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14(2):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin AD, Smith BK, Davenport PD, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15(2):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Caruso P, Denari SD, Ruiz SA, et al. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics (Sao Paulo). 2005;60(6):479–484 [DOI] [PubMed] [Google Scholar]
- 73. Nava S, Fasano L. Inspiratory muscle training in difficult to wean patients: work it harder, make it better, do it faster, makes us stronger. Crit Care. 2011;15(2):1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Freeman JA, Langdon DW, Hobart JC, Thompson AJ. The impact of inpatient rehabilitation on progressive multiple sclerosis. Ann Neurol. 1997;42(2):236–244 [DOI] [PubMed] [Google Scholar]
- 75. Cullen N, Chundamala J, Bayley M, Jutai J. The efficacy of acquired brain injury rehabilitation. Brain Inj. 2007;21(2):113–132 [DOI] [PubMed] [Google Scholar]
- 76. Bode R, Heinemann A. Course of functional improvement after stroke, spinal cord injury, and traumatic brain injury. Arch Phys Med Rehabil. 2002;83(1):100–106 [DOI] [PubMed] [Google Scholar]
- 77. Carroll A, McDonnell G, Barnes M. A review of the management of Guillain-Barré syndrome in a regional neurological rehabilitation unit. Int J Rehabil Res. 2003;26(4):297–302 [DOI] [PubMed] [Google Scholar]
- 78. Blissitt PA, Mitchell PH, Newell DW, Woods SL, Belza B. Cerebrovascular dynamics with head-of-bed elevation in patients with mild or moderate vasospasm after aneurysmal subarachnoid hemorrhage. Am J Crit Care. 2006;15(2):206–216 [PubMed] [Google Scholar]
- 79. Brimioulle S, Moraine JJ, Norrenberg D, Kahn RJ. Effects of positioning and exercise on intracranial pressure in a neurosurgical intensive care unit. Phys Ther. 1997;77(12):1682–1689 [DOI] [PubMed] [Google Scholar]
- 80. Hu MH, Hsu SS, Yip PK, Jeng JS, Wang YH. Early and intensive rehabilitation predicts good functional outcomes in patients admitted to the stroke intensive care unit. Disabil Rehabil. 2010;32(15):1251–1259 [DOI] [PubMed] [Google Scholar]
- 81. Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39(2):390–396 [DOI] [PubMed] [Google Scholar]
- 82. Cumming TB, Thrift AG, Collier JM, et al. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. 2011;42(1):153–158 [DOI] [PubMed] [Google Scholar]