Abstract
Status epilepticus is a neurological emergency that is commonly encountered by the neurohospitalist. Successful treatment depends upon the recognition of prolonged seizure activity and the acute mobilization of available resources. Pharmacologic treatment regimens have been shown to decrease the time needed for successful control of seizures and have provided for the rapid administration of anticonvulsant medications. Treatment strategies have evolved so that clinicians can administer effective doses of medication by whatever routes of administration are immediately available. Traditional algorithms for the treatment of status epilepticus have used a stepwise approach to the administration of first-, second-, and third-order medications. More recent options have included aggressive anesthetic doses of medications while second-line medications are being titrated.
Keywords: seizures, status epilepticus, neurological emergencies
Treatment of refractory status epilepticus (RSE) requires anesthetic doses of anticonvulsant medication. Early administration of these medications may allow for more successful treatment of very recalcitrant forms of status epilepticus. Significant questions remain as to the depth and duration of treatment for RSE.
Definition
Status epilepticus (SE) can be defined as a seizure of sufficient duration to overcome the inherent cellular mechanisms designed to terminate the seizure.1 Thus, SE becomes a self-sustaining process. The International League against Epilepsy originally defined SE as 30 minutes of sustained seizure activity or 2 or more seizures during this time period without the full recovery of the patient.2 Pathologically, however, hippocampal neurons begin to die after 30 minutes of sustained seizure activity.3 The limitation of this definition thus suggests that treatment should not be initiated until pathological damage has already been demonstrated.
Newer operational definitions have suggested that seizures lasting longer than 5 minutes are unlikely to discontinue spontaneously and should be treated.4 This is a more useful definition since treatment for SE is not delayed. In addition, individual unprovoked seizures can on occasion be observed and may not warrant aggressive treatment.
Classification and Recognition
SE can be categorized into convulsive and nonconvulsive status epilepticus (NCSE). Generalized convulsive status epilepticus (GCSE) is the most commonly encountered form of SE. Nonconvulsive SE can represent an evolved stage of previous GCSE or can occur in complex partial status epilepticus (CPSE).
GCSE is easily recognizable, presenting with generalized tonic or clonic activity. As seizures continue, the overt signs and symptoms of GCSE may become more repressed and difficult to recognize. Treiman5 described this as subtle NCSE. In this form of SE, clinical signs may be difficult to ascertain, thus requiring close neurological assessment. Subtle signs of NCSE may include nystagmus, eye twitching, and/or subtle rhythmic finger or toe movements. Husain et al6 reported a sensitivity of 100% of NCSE in patients with remote risk factors for seizures and ocular movement disorders and recommended urgent electroencephalographic (EEG) monitoring in these patients. Lothman7 described electrographic and physiological changes that may parallel the development of these subtle neurological findings.
Identification of patients with NCSE has also grown in importance since the report that a significant percentage of patients with head trauma will develop nonconvulsive seizures during their ICU stay. Vespa et al8 recommended continuous EEG monitoring in unconscious patients with head trauma during their first week. Claassen9 described electrographic seizures in one third of patients with intracerebral hemorrhage undergoing continuous EEG monitoring. Seizures were associated with cortical and expanding hemorrhages and had worse outcomes.
CPSE, the most common form of NCSE, starts focally but spreads rapidly to involve other portions of the brain. Patients may present confused or combative or in a “twilight state” characterized by bizarre behavior and automatisms.10 Initially CPSE was thought to be benign, but more recent evidence has suggested that aggressive treatment is necessary to avoid cellular loss and long-term neuropsychological consequences.3
Myoclonic SE presents as multifocal myoclonus. It occurs after severe neurological insults including anoxia and in toxic and metabolic states. Prognosis is generally considered poor and there is a debate as to whether this represents a true form of SE or is a marker for dying and degenerating neurons.1 A recent study, however, reported neurological improvement beyond a vegetative state in 6 patients with postanoxic status after therapeutic hypothermia.11
Absence and focal forms of SE are generally benign and will not be addressed. However, the neurohospitalist on occasion may need to differentiate nonepileptic or pseudostatus from SE. Clinical features that may distinguish pseudostatus from SE include nonrhythmic movements, pelvic thrusting, and biting of the tip of the tongue compared with the side of the tongue, which is most commonly encountered with SE.
Epidemiology
The incidence of SE has been estimated to be about 60 cases per 100 000 population with a mortality of 9 patients per 100 000 population.7–10 This extrapolates to approximately 150 000 cases per year in the United States, accounting for 40 000 deaths per year,12 making SE one of the most commonly encountered neurological emergencies.13,14
There is a bimodal distribution of SE, with most cases occurring in patients less than 1 year of age or older than 60 years of age. Mortality varies between 7% in the pediatric group up to 28% in the elderly.15 Mortality increases with age, the duration of SE, and the underlying cause. Worse outcomes have been documented after global anoxia, acute stroke, trauma, infections, and metabolic disturbances. Patients with alcohol or anticonvulsant withdrawal, tumors, and previous epilepsy appear to have better outcomes.15
It is difficult to ascertain how much of the above-listed morbidity and mortality can be directly attributed to SE. The underlying disease process is oftentimes the primary source of morbidity and mortality. The consequences of SE alone, however, can be significant. The primary concern is that epilepsy will develop in about 20% to 40% of patients after a single episode of SE. In addition, prolonged seizure activity has profound neuropsychological consequences. A chronic encephalopathy has been described with marked global and hippocampal atrophy.1
Pathophysiology
Brain slice preparations suggest that the development of SE includes both an initiation and a maintenance phase.16 SE can be initiated through excessive excitatory stimulation but is maintained through the lack of appropriate γ-aminobutyric acid (GABA)–mediated neuronal suppression. This failure to suppress the initial excitatory focus may be due to the development of changing GABA isoforms. With sustained seizure activity, hippocampal GABAA isoforms with different pharmacologic properties may develop. This has clinical importance since it has been postulated to explain the resistance to benzodiazepines that develops during SE over time.17
Alternatively, SE may be sustained through excitatory N-methyl-D-aspartate (NMDA)–mediated neuronal stimulation. NMDA antagonists have been suggested as a possible pharmacologic strategy in the treatment of SE.18
Treatment of Status Epilepticus: Practical Aspects
General Principles
The primary focus of treatment is the immediate termination of the seizure. Accumulating clinical evidence and overwhelming experimental evidence have shown that early seizure control improves long-term outcome.1,19,20 In addition, seizures become more recalcitrant to treatment as SE progresses.21 Seizures can be terminated with relatively low doses of medications if treated early. Alldredge et al22 reported that almost 60% of SE was controlled with 2 to 4 mg of lorazepam when given by emergency medical services prior to arrival to the emergency department.
Adequate resources need to be mobilized quickly with a sense of urgency from the involved physicians and support staff. Since the most effective method to terminate seizures is early treatment, rapid response teams, emergency medicine, critical care, or hospitalist personnel should be contacted immediately. For personnel not familiar with SE, a useful comparison is to suggest that the reverberating circuit encountered in SE is similar to the cardiac circuit involved in ventricular tachycardia.1
Treatment needs to be initiated as soon as medication is available by whatever route is available. Good intravenous access is imperative but in some circumstances may not be immediately available. Several medications can be given intramuscularly, rectally, or sublingually. Table 1 provides the dosages of medications that can be given acutely and by the most common routes used for those medications when intravenous access is not immediately available.
Table 1.
Medication Dosages and Routes of Administration for the Treatment of Status Epilepticus
| Medication | Route | Dose |
|---|---|---|
| Lorazepam | Intravenous | 4-8 mg initial 0.1-0.2 mg/kg loading |
| Diazepam | Intravenous | 5-20 mg initial 0.15 mg/kg loading |
| Diazepam | Rectal gel | 0.2-0.5 mg/kg initial |
| Midazolam | Intramuscular | 0.07-0.3 mg/kg initial |
| Phenytoin | Intravenous | 20-30 mg/kg loading |
| Fosphenytoin | Intravenous | 20-30 PE/kg loading |
| Fosphenytoin | Intramuscular | 500-1500 PE initial |
| Phenobarbital | Intravenous | 20-30 mg/kg loading |
| Valproate | Intravenous | 20 mg/kg loading |
| Levetiracetam | Intravenous | 1000-2500 mg initial |
PE, phenytoin equivalents.
An evaluation to simultaneously identify the underlying cause of the seizure and the treatment of secondary complications should be initiated. Seizure history and details surrounding the onset and initiation of the seizure can provide helpful diagnostic clues. Changes in medication or alcohol or illicit drug use should be ascertained. An exam should focus on possible signs of trauma. Routine laboratory studies should be sent including toxicology and antiepileptic drug levels. SE secondary to electrolyte abnormalities (ie, hyponatremia, hypocalcemia) can only be successfully treated by correcting the underlying electrolyte abnormality. A bedside glucose can be obtained quickly. Thiamine and glucose can be administered quickly if hypoglycemia is present. Neuroimaging should be obtained as soon as the patient is able to travel and seizures have been controlled. There should be a low threshold to obtain cerebrospinal fluid.1
Management Principles
The management of SE follows the principles of life support. Airway management may be as simple as positioning the patient to allow for adequate ventilation.23 Oral or nasopharyngeal airways can be placed to ensure airway patency. Bag mask ventilation with supplementary oxygen is usually adequate to avoid significant hypoxemia. Most single seizures will resolve by the time resources are mobilized, leaving the decision at that point for observation or elective endotracheal intubation.24 Prolonged seizures, however, may lead to acute airway obstruction and carry a high risk of aspiration. The timing of intubation is a clinical decision but usually occurs after large doses of sedative medications are given. Rhabdomyolysis can occur with SE. Succinyl choline should be avoided in this circumstance, given the risk of developing life-threatening hyperkalemia. Neuromuscular paralysis may mask subclinical seizure activity, and thus the shortest acting neuromuscular agent available should be used.
Adequate intravenous access is crucial for medication administration but can be difficult to obtain in the seizing patient. Several medications can be given intramuscularly, rectally, or sublingually until intravenous access can be initiated. Central access may be needed if vasopressor support is required.
Medications given in doses to treat SE may cause hemodynamic compromise. The neurohospitalist needs to be aware of the hemodynamic effects of the medications given and be prepared for cardiac arrhythmias, hypotension, or bradycardia. Frequent monitoring of blood pressure is important. Electrocardiographic monitoring should also be available. Most patients will be treated in the emergency department or in a critical care unit.
It is important to give medications as soon as possible in doses adequate to terminate seizures. Since early termination of seizures is crucial to prevent the development of SE, it is more important to start a medication that is immediately available than to delay treatment while waiting for a medication to arrive from pharmacy. The neurohospitalist should maximize the dosing of a single drug to high therapeutic or supratherapeutic drug levels before adding a second or third agent. Standardized protocols for anticonvulsant medications have been shown to decrease the time needed to control seizure activity.25
One of the most difficult decisions facing the neurohospitalist is when and how to obtain EEG monitoring. GCSE over time may lose the physical features that identify continual seizure activity. In several instances, overt physical signs of seizure activity will become attenuated and eventually lost despite ongoing electrical seizure activity. In addition, CPSE can be clinically difficult to identify and may require EEG monitoring. The decision to obtain an emergent EEG is thus a clinical one and is oftentimes based on the resources available at the hospital. In general if all clinical signs of SE can be aborted quickly with first- or second-line drugs, it is unlikely that continued seizure activity will be present. However, prolonged treatment and any subtle signs of possible activity should prompt urgent EEG evaluation. Patients who remain pharmacologically paralyzed should similarly be monitored.
The availability of EEG monitoring is often a problem. When EEG monitoring is not available and there are concerns that a patient may be in NCSE, it may be prudent to place the patient under a short-acting anesthetic (ie, propofol, thiopental) until monitoring can be arranged and the sedative medications discontinued. The decision to do this must be weighed against the potential side effects of this treatment.
Pharmacological Treatment
First-Line Medications
Benzodiazepines are the first medications to be used and are the mainstay of treatment. They function by stimulating GABAA receptor subunits. This leads to inhibition of neural transmission through chloride channel–induced hyperpolarization of the resting cell membrane.17 At high levels, benzodiazepines function in a manner similar to phenytoin.26
The 3 benzodiazepines used in the treatment of SE are diazepam, lorazepam, and midazolam. Each drug has slightly different properties and routes of administration. Diazepam achieves higher brain concentrations with an onset of action of about 30 seconds. It is, however, highly lipid soluble, leading to rapid redistribution and decreases in brain concentrations. Clinical effectiveness is only about 20 minutes. Relapse rate is high and thus a second drug is required if diazepam is used as a first-line drug.27 Diazepam can be given rectally in gel form and intramuscularly.
Midazolam is rarely used as a first-line drug for SE but is commonly used as a continuous intravenous infusion for RSE. It can be used acutely and is frequently used because of physician familiarity and its many routes of administration. These include the intramuscular, rectal, sublingual, and nasal routes. Midazolam is commonly used out of hospital or with children since parents can be trained in administration of this medication. It has, however, a very short half-life with a high recurrence rate of seizures.27
Lorazepam is considered the benzodiazepine of choice for the treatment of individual seizures and SE. It has a slightly longer onset of action, approximating 2 minutes; however, it is less lipid soluble than diazepam and has a duration of action greater than 12 hours. The results of the Veterans Affairs Status Epilepticus Cooperative Study Group suggested improved seizure control with lorazepam,28 although direct comparisons of benzodiazepines have shown little difference.29 Lorazepam has fewer hypotensive effects and is generally better tolerated than diazepam. Initial doses are usually 4 to 10 mg with a maximum dose of 0.2 mg/kg. The loading dose for seizure control in the VA Cooperative Study was 0.1 mg/kg. Dosages for all medications are provided in Table 1.
Second-Line Medications
Phenytoin or its phosphate ester prodrug fosphenytoin are considered the second-line medications with the most use in the treatment of SE. Phenytoin is a barbiturate-like compound that controls seizure activity by decreasing the recovery rate of voltage-activated sodium channels. It is highly protein bound, and only the free portion is active. Phenytoin is metabolized by the liver and has saturable pharmacokinetics.30 Other medications that can affect protein binding can have significant effects on free phenytoin activity. Thus, levels need to be followed carefully.
The initial loading dose for phenytoin is 20 mg/kg given in non–glucose-containing solutions. If seizures have not been terminated by the completion of the loading dose, another dose of 10 mg/kg can be given. The goal is to achieve supratherapeutic levels (25-30 μg/mL) before considering an additional medication.31 Concerns of overdosing phenytoin are probably overstated, and most side effects will abate as levels decrease. Patients previously on phenytoin should be given half of the loading doses until phenytoin levels can be obtained.
The side effect profile of phenytoin is primarily cardiovascular, with hypotension, bradycardia, and QT prolongation being common. A drop in systolic blood pressure of greater than 10 mm Hg occurred in a majority of patients loaded with fosphenytoin in one retrospective study.32 Most effects are correlated with the infusion rate (maximum rate 50 mg/min) and are decreased as the infusion rate is decreased. Physiological monitoring including a continuous electrocardiogram (ECG) is required during infusion. These effects are largely attributable to the diluent propylene glycol used to increase the solubility of phenytoin. There is also a direct medication effect on the cardiovascular system.31 The most worrisome concern with the use of phenytoin is the severe tissue necrosis that can occur with extravasation. The “purple glove syndrome” has been reported in almost 6% of patients receiving phenytoin infusions.33
Fosphenytoin is a phosphate ester prodrug of phenytoin that was developed to attenuate the significant side effect profile of phenytoin. It is water soluble and therefore can be given intramuscularly. It can be administered at a rate of 150 mg/min. Loading doses are the same as phenytoin and may have slightly fewer cardiovascular effects.34 There has been some debate about the bioavailability of fosphenytoin to phenytoin since the conversion time is about 15 minutes. More recent data, however, suggest increased bioavailability of fosphenytoin in direct comparison to phenytoin.35 Given that fosphenytoin can be given intramuscularly and at a faster rate with fewer side effects, it is now preferred over phenytoin despite the increased cost. Intramuscular use of fosphenytoin has not been directly tested in SE.
Other medications that could possibly be used in the treatment of SE include valproic acid and levetiracetam. Valproic acid is a short-chain fatty acid that decreases seizure activity by prolonging the recovery of voltage-gated sodium channels and through effects on GABA metabolism.30 Valproic acid can be administered intravenously or rectally. Intravenous loading doses of 20 mg/kg have been given at a maximum rate of 6 mg/kg/min without hemodynamic compromise.36 Experience in SE is limited to small series.36–38 In one series valproic acid was as effective in terminating SE as phenytoin.39 Since valproate appears to have few cardiovascular effects, it may serve as a second-line drug that could be administered in recalcitrant SE prior to giving phenobarbital or initiating treatment for RSE.40
Levetiracetam inhibits burst firing of neurons without affecting normal neural excitability. The exact mechanism of this is unknown, but levetiracetam appears to prevent hypersyncronization of burst firing and thus propagation of seizures. It is metabolized by enzymatic hydrolysis and is excreted renally. Dosing needs to be adjusted for renal failure.41 Levetiracetam has recently been introduced in intravenous form and, similar to valproate, appears to have few cardiovascular side effects. Side effects are primarily neuropsychiatric, with both agitation and sedation being noted.41 Use in SE has been limited to a few case reports.42,43 Doses of 2500 mg have been shown to be safe and effective when used as an additional drug to treat SE.44 Levetiracetam may be particularly effective in absence seizures and CPSE.45 Randomized trials comparing levetiracetam to other first- and second-line drugs will need to be performed.
Third-Line Medications
Phenobarbital is now generally considered a third-line drug in the treatment of SE. Phenobarbital is a barbiturate with similar properties as the benzodiazepines but is believed to activate a varying isoform of the GABAA receptor.21 Phenobarbital is used less often due to its long half-life and significant cardiorespiratory depressant effects. Dosing is similar to phenytoin.
Refractory Status Epilepticus
The definition of RSE varies according to the number of medications used and the time needed to control seizures. For practical purposes, RSE occurs when standard loading doses of anticonvulsants have proven to be ineffective. RSE has been reported to occur in almost one third of patients who are being treated for SE.46,47 This percentage may be high because of delays in treatment. Some authors have suggested that RSE may be avoided with earlier and more aggressive treatment.46 RSE requires anesthetic dosages of short-acting barbiturates, benzodiazepines, and/or propofol. The dosages for these medications are provided in Table 2 .
Table 2.
Intravenous Medications and Dosages for the Treatment of Refractory Status Epilepticus
| Medication | Dosage |
|---|---|
| Midazolam | 0.2 mg/kg load 0.05-2.0 mg/kg/h infusion rate |
| Pentobarbital | 5-15 mg/kg load 0.5-10 mg/kg/h infusion rate |
| Thiopental | 75-125 mg load 1-5 mg/kg/h infusion rate |
| Propofol | 3-5 mg/kg load 1-15 mg/kg/h |
Midazolam is a short-acting benzodiazepine that is considered the first drug of choice of treatment for RSE. It is rapidly inducible and has cardiorespiratory side effects that are significant but considerably less than encountered with propofol or the short-acting barbiturates.48,49 A loading dose of 0.2 mg/kg is given with maintenance doses ranging between 0.05 and 2.0 mg/kg/h. An escalation of dosing is common, as patients may develop a rapid tolerance. Similarly, withdrawal of this medication will depend upon the amount and duration of treatment since benzodiazepine withdrawal can complicate treatment if treatment is maintained for an extended period of time. One study comparing midazolam to propofol for treatment of RSE found improved outcome with midazolam.50 Cost can be limiting. Some pharmacies will recommend lorazepam as an alternative benzodiazepine. However, clinical experience is limited for the use of lorazepam in RSE.1
Propofol is a short-acting nonbarbiturate hypnotic used for induction. It has the advantages of rapid induction and elimination. Since propofol is commonly used for sedation, it is usually immediately available in most emergency rooms or intensive care units. Loading dose is 3 to 5 mg/kg with maintenance of 1 to 15 mg/kg/h.48,49
Propofol has fallen into relative disfavor because of increasing anecdotal reports of deaths associated with prolonged or excessive use.51–55 Use of propofol in children is a black box contraindication. This has been attributed to the development of a metabolic acidosis associated with hypotension, rhabdomyolysis, and hyperlipidemia. This has been labeled the propofol infusion syndrome and may be related to a mitochondrial enzymatic deficiency.55 Feeding regimens need to account for the large amount of fat administered with propofol.
Pentobarbital, a short-acting barbiturate, is often used to treat RSE.49 The initial loading dose is 5 to 15 mg/kg over 1 hour. Infusion rates can be maintained at 0.5 to 15 mg/kg/h. Pentobarbital is considered relatively short acting; however, with prolonged usage its duration of action approaches the longer lasting phenobarbital. Pentobarbital has significant cardiovascular effects and will most likely require additional vasopressor support. All barbiturates are also immunosuppressive, which may lead to an increase in nosocomial infections.1 Thiopental is metabolized to pentobarbital. It has the advantage of oftentimes being readily available and can be given in 75- to 125-mg boluses. Infusion rates are 1 to 5 mg/kg/h.56
Seizures refractory to the above medication regimens may respond to ketamine. Ketamine is a noncompetitive NMDA antagonist that can serve both neuroprotective and anticonvulsant roles.57 Animal models have suggested a benefit in RSE58; however, the effect of ketamine may be time dependent, with this agent becoming more effective after 60 minutes of seizure activity.59 Clinical experience is limited to a few case reports.60,61 Ketamine may be used as an adjunctive treatment; however, its use remains controversial.
Isoflurane is a volatile anesthetic that can be used for seizures resistant to all treatments. It has significant cardiovascular effects and requires gas scavenging equipment. It can increase intracranial pressure. Nonpharmacological treatments that have been used at the case report level have included vagal nerve stimulation, cortical resection, plasmapheresis, hypothermia, and electroconvulsive therapy.62–66
Treatment of Status Epilepticus
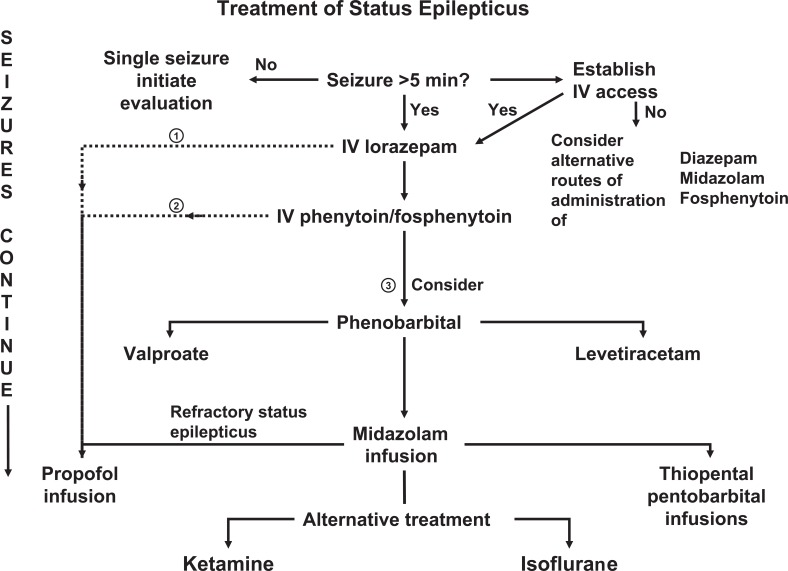
The goal of treatment is the immediate cessation of all seizure activity. Simultaneously, treatment should focus on preventing the recurrence of seizures, identifying the underlying cause, and treating any secondary complications.67,68 Resources need to be mobilized including nursing, pharmacy, rapid response teams, and/or other available medical staff. The approach to treatment and diagnosis needs to be deliberate and methodical. One possible pharmacological algorithm is provided in Figure 1 .
Figure 1.
Algorithm for the treatment of status epilepticus. Treatment can proceed along several different pathways to obtain the most immediate seizure control. See Table 1 and 2 for medication routes and dosages.
The first task is to determine whether the patient is in SE. As mentioned, it is unlikely that continuous seizure activity will spontaneously abort after 5 minutes, and any prolonged seizure activity should prompt treatment. A prolonged postictal period after a single or recurrent seizure can make this a difficult decision. If there is any suspicion of subclinical seizure activity, treatment for SE should continue until EEG monitoring can be arranged.
Lorazepam is considered the benzodiazepine of choice for the initial treatment of seizures given its pharmacokinetic and safety profile. Initial doses should be 4 to 10 mg intravenously. A loading dose of 0.1 to 0.2 mg/kg should be given if seizures are not aborted within 2 to 3 minutes. Diazepam or midazolam can also be given but will require the addition of a second-line medication. If intravenous access is not immediately available, rectal, sublingual, or intramuscular benzodiazepines can be administered. Similarly, fosphenytoin can be given intramuscularly while intravenous access is being obtained.
If a loading dose of a benzodiazepine is given, it is most likely that airway management will require endotracheal intubation. At this point if seizures continue, one option is to move immediately to treatment of RSE (pathway 1 in Figure 1). Patients could be given an inducing dose of either propofol or thiopental and be maintained with an infusion until a second-line drug can be added and EEG monitoring can evaluate the effectiveness of therapy.
A second option is to add a second-line medication prior to moving to treatment for RSE (pathway 2 in Figure 1). This option has been advocated by some authors in an attempt to avoid endotracheal intubation.23 However, I warn against excessive delays in effective treatment while second-line drugs are administered to avoid intubation. Support for this argument is provided by the observation that second- and third-line medications have decreasing effectiveness for the treatment of SE.67
A third option is to move immediately to loading doses of phenobarbital if second-line medications are ineffective (pathway 3 in Figure 1). Mayer et al46 have argued that the ineffectiveness of second- and third-line medications probably reflects a delay in treatment that has made control of seizure activity more difficult. This pathway represents the historical pathway used in the treatment of SE. A loading dose of phenobarbital, however, mandates a prolonged hospital course.
If second- and third-line medications are ineffective, the treatment regimen proceeds to treatment for RSE. This mandates EEG monitoring and the titrating of anesthetic levels of medications. Opinion has moved away from prolonged treatment with propofol in favor of midazolam; however, large comparison trials have not been undertaken. In my experience, propofol in low doses may serve as a relatively safe adjunctive mediation.
Many questions remain as to the optimal treatment of RSE. The depth of induction of anesthetic doses of medications is debatable. In practice, medication is usually titrated to a burst suppression pattern on EEG monitoring. Smith and Bleck,69 however, have argued that all seizure activity should be suppressed and that treatment is not adequate until the EEG has been rendered flat line.
The duration of treatment is similarly unknown. Traditional teaching has suggested 24 to 48 hours of treatment. However, my colleagues and I have had successful treatment of patients in SE for 2 to 3 months.69–71
Conclusions
Status epilepticus is a neurological emergency that will be encountered commonly by the neurohospitalist. Successful treatment will depend upon the mobilization of available resources and the rapid administration of anticonvulsant medications. Pharmacologic treatment regimens have been shown to decrease the time needed for successful control of seizures. Treatment strategies have evolved to administer effective doses of medication by whatever routes of administration are immediately available. Treatment of refractory SE requires anesthetic doses of anticonvulsant medication. Early administration of these medications may allow for more successful treatment of very recalcitrant forms of SE.
Footnotes
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
The author received no financial support for the research and/or authorship of this article.
References
- 1. Manno EM. New management strategies in the treatment of status epilepticus. Mayo Clin Proc. 2003;78:508–518 [DOI] [PubMed] [Google Scholar]
- 2. Treatment of convulsive status epilepticus: recommendations of the Epilepsy Foundation of America’s Working Group on Status Epilepticus JAMA. 1993;270:854–859 [PubMed] [Google Scholar]
- 3. Nevander G, Ingvar M, Auer R, Siesjo BK. Status epilepticus in well-oxygenated rats causes neuronal necrosis. Ann Neurol. 1985;18:281–290 [DOI] [PubMed] [Google Scholar]
- 4. Theodore WH, Porter RJ, Albert P, et al. The secondarily generalized tonic-clonic seizure: a videotape analysis. Neurology. 1994;44:1403–1407 [DOI] [PubMed] [Google Scholar]
- 5. Treiman DM. Status epilepticus. Baillieres Clin Neurol. 1996;5:821–839 [PubMed] [Google Scholar]
- 6. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003;74:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(suppl 2):13–23 [PubMed] [Google Scholar]
- 8. Vespa PM, Newer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365 [DOI] [PubMed] [Google Scholar]
- 10. Drislane FW. Types of status epilepticus. Definitions and classification. In: Drislane FW. ed. Status Epilepticus: A Clinical Perspective. Totowa, NJ: Humana Press; 2005: 11–31 [Google Scholar]
- 11. Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72:744–749 [DOI] [PubMed] [Google Scholar]
- 12. DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–325 [PubMed] [Google Scholar]
- 13. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035 [DOI] [PubMed] [Google Scholar]
- 14. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50:735–741 [DOI] [PubMed] [Google Scholar]
- 15. Waterhouse EJ. Epidemiology of status epilepticus. In: Drislane FW. ed. Status Epilepticus: A Clinical Perspective. Totowa, NJ: Humana Press; 2005:55–75 [Google Scholar]
- 16. Rafiq A, Zhang YF, DeLorenzo RJ, Coulter DA. Long-duration self-sustained epileptiform activity in the hippocampal-parahippocampal slice: a model of status epilepticus. J Neurophysiol. 1995;74:2028–2042 [DOI] [PubMed] [Google Scholar]
- 17. Noe K, Manno EM. Mechanisms underlying status epilepticus. Drugs Today. 2005;41:257–266 [DOI] [PubMed] [Google Scholar]
- 18. Bleck TP. Refractory status epilepticus in 2001 [editorial]. Arch Neurol. 2002;59:188–189 [DOI] [PubMed] [Google Scholar]
- 19. Sloviter RS. Status epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999;40(suppl 1):S34–S39 [DOI] [PubMed] [Google Scholar]
- 20. Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34 [DOI] [PubMed] [Google Scholar]
- 21. Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185 [DOI] [PubMed] [Google Scholar]
- 22. Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus [published correction appears in N Engl J Med. 2001;345:1860]. N Engl J Med. 2001;345:631–637 [DOI] [PubMed] [Google Scholar]
- 23. Chapman MG, Smith M, Hirsch NP. Status epilepticus. Anaesthesia. 2001;56:648–659 [DOI] [PubMed] [Google Scholar]
- 24. Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976 [DOI] [PubMed] [Google Scholar]
- 25. Gilbert KL. Evaluation of an algorithm for treatment of status epilepticus in adult patients undergoing video/EEG monitoring. J Neurosci Nurs. 2000;32:101–107 [DOI] [PubMed] [Google Scholar]
- 26. Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures: focus on delivery routes. Clin Pharmacokinet. 1999;36:409–424 [DOI] [PubMed] [Google Scholar]
- 27. Greenblatt DJ, Divoll M. Diazepam versus lorazepam: relationship of drug distribution to duration of clinical action. Adv Neurol. 1983;34:487–491 [PubMed] [Google Scholar]
- 28. Treiman DM, Meyers PD, Walton NY, et al; Veterans Affairs Status Epilepticus Cooperative Study Group A comparison of four treatments for generalized status epilepticus. N Engl J Med. 1998;339:792–798 [DOI] [PubMed] [Google Scholar]
- 29. Levy RJ, Krall R. Treatment of status epilepticus with lorazepam. Arch Neurol. 1984;41:605–611 [DOI] [PubMed] [Google Scholar]
- 30. McNamara JO. Drugs effective in the therapy of the epilepsies. In: Hardman JG, Limbird LE, Gilman AG. eds. Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001;521–547 [Google Scholar]
- 31. Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976 [DOI] [PubMed] [Google Scholar]
- 32. Kassala MY, Lobeck IN, Majid A, Xie Y, Farooq MV. Blood pressure changes after intravenous fosphenytoin and levitiracetam in patients after acute cerebral symptoms. Epilepsy Res. 2009;87:268–271 [DOI] [PubMed] [Google Scholar]
- 33. O’Brien TJ, Cascino GD, So EL, Hanna DR. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998;51:1034–1039 [DOI] [PubMed] [Google Scholar]
- 34. Browne TR, Kugler AR, Eldon MA. Pharmacology and pharmacokinetics of fosphenytoin. Neurology. 1996;46(6, suppl 1):S3–S7 [DOI] [PubMed] [Google Scholar]
- 35. Fischer JH, Patel TV, Fischer PA. Fosphenytoin: clinical pharmacokinetics and comparative advantages in the acute treatment of seizures. Clin Pharmacokinet. 2003;42:33–58 [DOI] [PubMed] [Google Scholar]
- 36. Venkataraman V, Wheless JW. Safety of rapid intravenous infusion of valproate loading doses in epilepsy patients. Epilepsy Res. 1999;35:147–153 [DOI] [PubMed] [Google Scholar]
- 37. Venkataraman V, Wheless JW. Safety of rapid intravenous infusion of valproate loading doses in epilepsy patients. Epilepsy Res. 1999;35:147–153 [DOI] [PubMed] [Google Scholar]
- 38. Gilad R, Izkovitz N, Dabby R, et al. Treatment of status epilepticus and acute repetitive seizures with i.v. valproic acid vs phenytoin. Acta Neurol Scand. 2008;118:296–300 [DOI] [PubMed] [Google Scholar]
- 39. Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16:527–532 [DOI] [PubMed] [Google Scholar]
- 40. Sinha S, Naritoku DK. Intravenous valproate is well tolerated in unstable patients with status epilepticus. Neurology. 2000;55:722–724 [DOI] [PubMed] [Google Scholar]
- 41. Product information Keppra ® oral tablet solution. Smyrna, Ga: UCB Inc;2008 [Google Scholar]
- 42. Farooq MU, Naravetia B, Majid A, Gupta R, Pysh JJ, Kassab MY. IV levetiracetam in the management of non-convulsive status epilepticus. Neurocrit Care. 2007;7:36–39 [DOI] [PubMed] [Google Scholar]
- 43. Knake S, Gruener J, Hattemer K, et al. Intravenous levetiracetam in the treatment of benzodiazepine refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2008;79:588–589 [DOI] [PubMed] [Google Scholar]
- 44. Uges JW, van Huizem MD, Wilms EB, Touw DJ, Peeters E, Vecht CJ. Safety and pharmacokinetics of iv levetiracetam as an add on in status epilepticus. Epilepsia. 2009;50:415–421 [DOI] [PubMed] [Google Scholar]
- 45. Wheless JW, Treiman DM. The role of the newer antiepileptic drugs in the treatment of generalized convulsive status epilepticus. Epilepsia. 2008;49(suppl 10):74–78 [DOI] [PubMed] [Google Scholar]
- 46. Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–210 [DOI] [PubMed] [Google Scholar]
- 47. Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036–1042 [DOI] [PubMed] [Google Scholar]
- 48. Hanley DF, Kross JF. Use of midazolam in the treatment of refractory status epilepticus. Clin Ther. 1998;20:1093–1105 [DOI] [PubMed] [Google Scholar]
- 49. Bleck TP. Management approaches to prolonged seizures and status epilepticus. Epilepsia. 1999;40(suppl 1):S59–S63 [DOI] [PubMed] [Google Scholar]
- 50. Prasad A, Worrall BB, Bertram EH, Bleck TP. Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia. 2001;42:380–386 [DOI] [PubMed] [Google Scholar]
- 51. Stecker MM, Kramer TH, Raps EC, O’Meeghan R, Dulaney E, Skaar DJ. Treatment of refractory status epilepticus with propofol: clinical and pharmacokinetic findings. Epilepsia. 1998;39:18–26 [DOI] [PubMed] [Google Scholar]
- 52. Cannon ML, Glazier SS, Bauman LA. Metabolic acidosis, rhabdomyolysis, and cardiovascular collapse after prolonged propofol infusion. J Neurosurg. 2001;95:1053–1056 [DOI] [PubMed] [Google Scholar]
- 53. Kelly DF. Propofol-infusion syndrome [editorial]. J Neurosurg. 2001;95:925–926 [DOI] [PubMed] [Google Scholar]
- 54. Cremer OL, Moons KG, Bouman EA, Kruijswijk JE, de Smet AM, Kalkman CJ. Long-term propofol infusion and cardiac failure in adult head-injured patients [letter]. Lancet. 2001;357:117–118.0 [DOI] [PubMed] [Google Scholar]
- 55. Friedman JA, Manno EM, Fulgham JR. Propofol use in the neuro ICU. J Neurosurg. 2002;96:1161–1162 [PubMed] [Google Scholar]
- 56. O’Brien MD. Management of major status epilepticus in adults. BMJ. 1990;301:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fujikawa DG. Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia. 1995;36:186–195 [DOI] [PubMed] [Google Scholar]
- 58. Santi SA, Cook LL, Persinger MA. Normalization of spatial memory following post seizure treatment with ketamine: selective damage attenuates memory deficits in brain damaged rodents. Int J Neurosci. 2001;107:63–75 [DOI] [PubMed] [Google Scholar]
- 59. Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res. 2000;42:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nathan BR, Smith TL, Bleck TP. The use of ketamine in the treatment of refractory status epilepticus [abstract]. Neurology. 2002;58(suppl 3):9711781412 [Google Scholar]
- 61. Sheth RD, Gidal BE. Refractory status epilepticus: response to ketamine. Neurology. 1998;51:1765–1766 [DOI] [PubMed] [Google Scholar]
- 62. Winston KR, Levisohn P, Miller BR, Freeman J. Vagal nerve stimulation for status epilepticus. Pediatr Neurosurg. 2001;34:190–192 [DOI] [PubMed] [Google Scholar]
- 63. Lousa M, Sanchez-Alonso S, Rodriguez-Diaz R, Dalmau J. Status epilepticus with neuron-reactive serum antibodies: response to plasma exchange. Neurology. 2000;54:2163–2165 [DOI] [PubMed] [Google Scholar]
- 64. Carrasco Gonzales MD, Palomar M, Rovira R. Electroconvulsive therapy for status epilepticus [letter]. Ann Intern Med. 1997;127:247–248 [DOI] [PubMed] [Google Scholar]
- 65. Correy JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9:189–197 [DOI] [PubMed] [Google Scholar]
- 66. Gorman DG, Shields WD, Shewmon DA, et al. Neurosurgical treatment of refractory status epilepticus. Epilepsia. 1992;33:546–549 [DOI] [PubMed] [Google Scholar]
- 67. Shih T, Bazil CW. Treatment of generalized convulsive status epilepticus. In: Drislane FW. ed. Totowa, NJ: Humana Press; 2005:265–288 [Google Scholar]
- 68. Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43(3, pt 1):483–488 [DOI] [PubMed] [Google Scholar]
- 69. Smith TL, Bleck TP. Treatment of refractory status epilepticus. In: Drislane FW. ed. Totowa, NJ: Humana Press; 2005:289–298 [Google Scholar]
- 70. Dara SI, Tungpalan LA, Manno EM, et al. Prolonged coma after refractory status epilepticus. Neurocrit Care. 2006;4:40–42 [DOI] [PubMed] [Google Scholar]
- 71. Zubkov AY, Rabinstein AA, Manno EM, Wijdicks EF. Prolonged refractory status epilepticus related to thrombotic thrombocytopenic purpura. Neurocrit Care. 2008;9:361–365 [DOI] [PubMed] [Google Scholar]