Abstract
Fulminant demyelinating disease is a heading that covers acute disseminated encephalomyelitis and its variant acute hemorrhagic leukoencephalitis (Hurst disease), severe relapses of multiple sclerosis (MS), variants of MS (tumefactive MS, Marburg variant, Balo concentric sclerosis, myelinoclastic diffuse sclerosis), and neuromyelitis optica-spectrum disorders associated with aquaporin autoimmunity. These categories of inflammatory demyelinating disease often prompt hospital admission and many necessitate intensive care monitoring due to the aggressive nature of the illness and associated neurologic morbidity. In this review, we highlight the discriminating clinical, radiographic, and pathologic features of these disorders. Acute management is often accomplished with use of high-dose intravenous steroids and plasma exchange. Aggressive disease may respond to immunosuppression. Prognosis for recovery varies among the disorders but most patients improve. Factors influencing outcome are also discussed.
Keywords: ADEM, fulminant MS, neuromyelitis optica, Marburg, tumefactive MS
Introduction
Fulminant demyelinating disease may be best described as a range of inflammatory demyelinating disorders (IDDs) associated with rapid progression to disability within several days to weeks, culminating in the need for hospital admission and aggressive therapy for an acute attack. Hospital admission is often indicated due to substantial neurologic impairment, diagnostic uncertainty, or need for supportive care. Patients presenting with an alteration in mental status, signs of increased intracranial pressure, respiratory or cardiac compromise, and seizures may warrant admission to an intensive care unit (ICU).
The fulminant demyelinating diseases include acute disseminated encephalomyelitis (ADEM) and its variant acute hemorrhagic leukoencephalitis (AHLE or Hurst disease), severe relapses of multiple sclerosis (MS), variants of MS (tumefactive MS, Marburg variant, Balo concentric sclerosis, myelinoclastic diffuse sclerosis), and neuromyelitis optica (NMO)-spectrum disorders associated with aquaporin autoimmunity. This review will focus on the clinical and diagnostic features of these disorders and standard treatment for an acute IDD attack.
ADEM
Acute disseminated encephalomyelitis is a monophasic, multifocal IDD of the brain and spinal cord with distinct pathology and clinical course from MS. ADEM is often preceded by an immunologic trigger in the form of a systemic infection or vaccination 1 to 2 weeks prior to the onset of neurological symptoms.1,2 The most common association is with a viral upper respiratory tract infection and clustering of cases may occur in winter months.3,4
ADEM occurs most often in the pediatric population, where MS is less common. The incidence ranges from 0.4 to 0.8/100 000 per year and is highest in children younger than 10 years of age.1,5,6 The incidence in the adult population is unknown, and distinguishing these cases from an atypical MS presentation may be difficult. The reason for the higher incidence in children has been attributed to immature myelin, relative immaturity of the immunological response, and increased frequency of viral infections in this age group when compared to adults.7
Often one of the primary differential diagnostic considerations in these cases is that of a first demyelinating event of MS. This distinction has important implications regarding prognosis and the need for long-term treatment. In some cases, this may be difficult or impossible to distinguish at the onset of the first clinical syndrome. Close clinical and radiographic follow-up may be required. In those patients deemed high risk of developing MS, early initiation of immunomodulatory treatment is desirable.
Clinical Features
Neurological symptoms typically evolve over several days, but encephalopathy and/or alteration in consciousness is usually present early in the course.1,4,5,8 This may range from mild irritability or somnolence to prominent behavioral change or coma. Additional presenting features are variable and may include motor, sensory, visual, and cerebellar symptoms. Atypical MS symptoms occurring with greater frequency in ADEM include bilateral optic neuritis, seizures, aphasia, vomiting, fever and headache, and confusion.2,4,5,8–10
In 2007, the International Pediatric MS Study Group (IPMSSG) proposed diagnostic criteria to discriminate ADEM from a first attack of MS.11 These are listed in Table 1. The monophasic illness should not progress beyond 3 months. It is critical that there be no prior history to suggest an attack and no radiographic evidence of prior demyelination (ie, associated T1 hypointensity).11 These criteria have been validated in a study of biopsy-proven pediatric and adult ADEM and MS and were found to be 80% sensitive and 91% specific for a pathologic diagnosis of ADEM.12 A depressed level of consciousness may be even more specific for ADEM than encephalopathy.12 Despite the fact that an immunologic trigger is often reported, it is not required as part of the diagnostic criteria and may also precede a typical MS attack.9Other useful clinical features that may help to discriminate ADEM from an initial attack of MS have been the presence of fever and meningeal signs in the absence of central nervous system (CNS) infection.4
Table 1.
Discriminating Features of Demyelinating Diseases.
| ADEM | RRMS | NMO | |
|---|---|---|---|
| Demographics | Most commonly in children <10 yrs | Most common in 2nd and 3rd decades | Most common in 3rd and 4th decades |
| Distinguishing presenting symptoms | Encephalopathy, depressed level of consciousness, meningeal signs, polyfocal presentation, ataxia, seizures | Unilateral ON, partial myelitis, INO, brainstem event | Recurrent ON, unilateral or bilateral and generally severe; recurrent myelitis, generally severe; brainstem syndrome; nausea and vomiting; hiccups; narcolepsy; endocrine disorders |
| Clinical diagnostic criteria | IPMSSG 2007:a polysymptomatic, must include encephalopathy, multifocal radiographic lesions, no history of previous demyelination | 2010 revised McDonald criteria for RRMS:b dissemination in time (DIT) and space (DIS) required: DIT: asymptomic and symptomatic enhancing lesions or new T2 lesion on follow-up MRI or 2nd clinical attack DIS: 2 or more T2 lesions in at least 2 of 4 locations: periventricular, juxtacortical, infratentorial, and spinal | Revised criteria 2006:c optic neuritis, acute myelitis, and 2 of 3 of the following supporting criteria: contiguous cord lesions 3 or more vertebral segment in length, MRI fails to meet MS diagnostic criteria,d NMO IgG seropositivity |
| Radiographic features | Multifocal diffuse WM lesions with poor margins, bilateral thalamic/basal ganglia involvement; sparing of periventricular white matter, absence of T1 hypointensity | Ovoid, periventricular lesions oriented perpendicular to the long axis of the ventricle, spinal cord lesions <3 vertebral segments in length, often involving lateral or posterior columns, posterior fossa, juxtacortical | Contiguous spinal cord lesions over 3+ vertebral segments; extensive ON and chiasmal lesions, subcortical or subcallosal “nonspecific” WM disease, NMO SD brain lesions: hypothalamus/thalamus, corticospinal tract, periependymal, brainstem, and cervicomedullary junction |
Abbreviations: RRMS, relapsing remitting multiple sclerosis; ON, optic neuritis; INO, internuclear ophthalmoplegia; NMO SD, neuromyelitis optica-spectrum disorder; IPMSSG, International Pediatric MS Study Group; WM, white matter; ADEM, acute disseminated encephalomyelitis; IgG, immunoglobulin G; MRI, magnetic resonance imaging; MS, multiple sclerosis.
a Krupp et al.11
b Pollman et al, 2011.
c Wingerchuk et al, 2006.
d Brain lesions that meet MS criteria can be seen in NMO SD.
The 2007 IPMSSG criteria classified a recurrence of similar symptoms after 3 months without new radiographic findings as “Recurrent ADEM” and a second episode with new symptoms, new magnetic resonance imaging (MRI) lesion(s), and meeting the initial criteria for ADEM as “Multiphasic ADEM.”11 These classifications are somewhat controversial, and some experts would argue that recurrence implies MS pathology.12
Radiographic Features
MRI is the imaging modality of choice for visualizing demyelinating lesions. In early ADEM, MRI findings may lag behind the clinical presentation for up to several weeks.13 Characteristic features of ADEM include multifocal and diffuse T2/FLAIR hyperintensities involving the gray and white matter of the brain and spinal cord, with poorly defined margins. More than 50% of cases have infratentorial involvement, and 30% to 85% may have spinal cord involvement.14,15 Brain lesions may be large, and at least one lesion is often greater than 1 cm in diameter.3 Diffusion-weighted imaging (DWI) can help to stage demyelinating lesions, but does not distinguish between MS and ADEM. In the acute period (0-7 days), apparent diffusion coefficient may show restricted diffusion due to the presence of dense inflammatory infiltrates, reduced vascular supply, and edema of the myelin sheaths. In the subacute stage (7-14 days), lesions show increased diffusivity due to an expanded extracellular space, edema, and myelin loss.16
It may not be possible to distinguish ADEM from a first attack of MS on the basis of radiographic findings alone, but certain features may favor a diagnosis of ADEM. These are highlighted in Table 1. Historically, these have included a multifocal and diffuse lesion pattern with indistinct margins, the majority of lesions demonstrating contrast enhancement, symmetric involvement of the basal ganglia and thalamus (Figure 1), sparing of the corpus callosum, and the absence of periventricular lesions.9,17 Gray matter involvement is more frequent in ADEM and may exclusively involve the cortex.1,6,9
Figure 1.

Acute disseminated encephalomyelitis (ADEM). Brain magnetic resonance imaging (MRI) demonstrates classic symmetric T2/FLAIR signal abnormality involving the basal ganglia and thalamus in a pediatric patient.
Gadolinium enhancement is variable and often absent; however, when present, multiple lesions may enhance simultaneously reflecting the similar stage of evolution.14 In MS, there is often a combination of nonenhancing and enhancing lesions. This may also occur in ADEM.18 The 2010 McDonald MS criteria allow “dissemination in time” to be met on a single MRI if it demonstrates both an asymptomatic enhancing lesion and a simultaneous nonenhancing lesion.19 Caution should be exercised in cases where there is a high suspicion of ADEM, as patients could meet these radiographic MS criteria after a single MRI.
The Callen MRI criteria (listed in Table 1) help to distinguish a first attack of MS from ADEM in children.17 These criteria have a sensitivity of 75% to 81% and specificity of 95% for development of pediatric MS.17,20 New radiographic lesions and evolution of symptoms should not occur beyond 3 months in order to be considered part of the monophasic ADEM illness.1,6,9–11 When suspicion of MS is high, follow-up imaging may be performed at 3 and 6 months. If new lesions are detected at 6 months or any time thereafter, a diagnosis of MS is more likely. The strongest predictors for development of MS are the presence of one or more T1 hypointense or periventricular lesions on baseline MRI.17,18
Cerebrospinal Fluid (CSF)
CSF testing is indicated to rule out infection. Common CSF abnormalities include lymphocytic pleocytosis and elevated protein. The presence of oligoclonal bands does not discriminate ADEM from MS but is less common in ADEM, occurring in 12.5% to 20% of the cases.1,4–6,9 When present, oligoclonal banding is more likely to resolve over time, in contrast to MS where band persistence is generally the rule.9
Pathologic Findings
Pathologic findings are distinct from MS. ADEM lesions demonstrate prominent perivenous demyelination in “sleeves” with infiltrates of lymphocytes and macrophages.21 These infiltrates can involve both the white and gray matter, are often widespread, and have indistinct margins. Lesions appear of similar age. In contrast, MS lesions demonstrate confluent demyelination of varying age.21 Cases of pathologic overlap have been reported with a relapsing course typical of MS.12 These findings contribute to the speculation that ADEM and MS may be part of a variable spectrum of IDD.
Prognosis
The majority of patients recover with minimal to no neurological disability, even after coma or prolonged intracranial hypertension.22–24 Mortality rates have ranged from 10% to 30%, with the majority of deaths attributed to respiratory failure, pneumonia, sepsis, and refractory intracranial hypertension.15 Persistent cognitive impairment has been reported in up to one-third of the pediatric and adult cases.20
The prognosis in adult-onset ADEM is less favorable and associated with a longer hospital stay, higher incidence of ICU admission, higher mortality rate, and greater morbidity.20 There have been no significant differences in terms of clinical presentation and CSF findings between adult and pediatric groups. Radiographic findings are similar, but the presence of 3 or more periventricular lesions is more common in adult patients with an ADEM-like event.20
In a study of 20 adult patients with ADEM in the ICU, reasons for ICU admission included severely impaired consciousness, recurrent seizures, and symptoms prompting initial concern for CNS infection.15 The average length of ICU stay was 19 days. Mechanical ventilation was necessary in 70% of the cases, and the mortality rate was 25%. Predictors of a good outcome at the time of admission included a Glasgow Coma Scale score of 7 or greater and fewer seizures. Despite the fulminant presentation, 70% of the patients were able to ambulate independently after 3- to 9-month follow-up.15 This underscores the importance of aggressive therapy and supportive care in these patients who have good potential for substantial neurological recovery. These aspects are discussed later in this review.
Approximately one-fourth of the patients initially diagnosed with ADEM will develop MS over a 2- to 5-year period.4,9,10,18,20With this in mind, it is important to counsel patients about the risk of a subsequent relapse. Radiographic criteria may be helpful in this regard, but the importance of longitudinal follow-up and serial imaging is also emphasized. Close follow-up and consultation with a MS specialist may be worthwhile when formulating a discharge plan.
Acute Hemorrhagic Leukoencephalitis
AHLE, or Hurst disease, is a rare variant of ADEM presenting with rapid neurologic deterioration associated with perivascular hemorrhage and edema, often within hours of onset. In one pediatric ADEM series, it occurred in 2% of the cases.8 It is also frequently preceded by a respiratory tract infection. Symptoms are often indistinguishable from classic ADEM and include depressed level of consciousness, confusion, headache, and fever. The CSF findings may show a polymorphonuclear pleocytosis in contrast to the lymphocytic pleocytosis seen in ADEM and can be mistaken for CNS infection.25 Case reports describe presentations with a fulminant and fatal course and others improving with corticosteroids and plasma exchange (PLEX).25Larger series of ADEM do indicate that there is a radiographic spectrum, with some ADEM cases demonstrating hemorrhagic involvement.8
MS Variants
Multiple sclerosis is the most common IDD of the CNS, occurring with an incidence of 7.5 of 100 000 per year in one population-based study.26 Approximately 7% of the patients presenting with MS have radiographic features of fulminant disease.27 Historically, Marburg variant, Balo concentric sclerosis, and tumefactive MS have been considered variants of MS, with a clinical course distinct from that of relapsing-remitting MS. This may include monophasic presentations that by definition would not meet criteria for dissemination in time and space. Most commonly, fulminant attacks present as the first demyelinating event in the course of MS but may also occur less frequently in an individual with previously established MS.28Significant overlap exists among clinical and pathologic characteristics of these disorders.
Tumefactive MS Variant
Tumefactive MS refers to the presentation of a large demyelinating lesion, often associated with mass effect and edema, mimicking a brain tumor. The incidence is estimated as 0.3 of 100 000 per year.29 Tumefactive demyelination may occur at any age, but is more frequent in the 20s and 30s.28,30 Lesions may occur as a solitary mass, multiple large lesions, or in conjunction with classic MS lesions. In a study of biopsy-proven tumefactive MS, 5% of the patients met diagnostic criteria for MS prior to biopsy, and 70% had multiple lesions on MRI at the time of biopsy.28
Clinical Features
Clinical presentation is often polysymptomatic and suggestive of multifocal or diffuse involvement of the CNS. Symptoms develop over days to weeks. Motor, cognitive, sensory, and cerebellar symptoms predominate.27,28 Other symptoms include aphasia, seizures, impaired consciousness, and visual field deficits.27 Rarely, a peripheral demyelinating polyradiculoneuropathy mimicking Guillain-Barre syndrome may accompany central demyelination.2,31 Mass effect and edema may result in symptoms of increased intracranial pressure and/or cerebral herniation, which require aggressive management. The clinical history should be scrutinized for signs or symptoms to indicate previous demyelinating attacks, which can narrow the differential diagnosis. Common differential diagnostic considerations include primary CNS malignancy, metastasis, abscess, vasculitis, and granulomatous disease.28
Radiographic Features
Tumefactive lesions are usually greater than 2 cm in diameter. Mass effect is present in 45% and edema in 77% of the cases.28 Figure 2 illustrates classic MRI features of tumefactive lesions. Other MRI findings may include peripheral restriction on DWI, and venous enhancement or dilatation surrounding the lesion on brain angiography.32
Figure 2.
Tumefactive multiple sclerosis (MS). A, Large tumefactive lesion is seen on T2/FLAIR imaging. B, T1 postcontrast study of the lesion in Figure A demonstrating a characteristic open-ring enhancement pattern. C, Tumefactive demyelinating lesion in the midbrain (arrow). D, Patchy, heterogeneous enhancement of the lesion in Figure C (arrow).
Contrast enhancement of tumefactive lesions occurs in 95% to 100% of the cases but patterns are variable.28–30 Patterns of enhancement include open or closed rings, diffuse, homogeneous, punctate, or concentric.28 When present, an open ring with the broken portion abutting the gray matter of the cortex or deep nuclei may be a useful clue to a demyelinating etiology.29,33A T2 hypointense rim often colocalizes with the area of ring enhancement.28,33 The majority of lesions do demonstrate some degree of associated T1 hypointensity.27 At follow-up, most lesions demonstrate moderate to marked improvement or complete resolution.27
If there are other lesions that appear typical for MS, the diagnosis may be clear. Suspicious locations for demyelinating plaques are located in the periventricular or juxtacortical white matter, posterior fossa, or spinal cord. Spinal imaging can be a useful screening tool to identify asymptomatic spinal cord lesions that are common in MS and may point toward a demyelinating etiology.34
Ancillary Testing
The CSF findings may be normal or demonstrate elevation in the immunoglobulin G (IgG) index and oligoclonal bands. These findings have been demonstrated in 11% to 33% of the cases.28,35 The reason for the paucity of CSF abnormalities may relate to the fulminant nature of the demyelinating process and presumed short disease duration.
Evoked potentials may provide useful paraclinical evidence to a demyelinating etiology. Abnormal visual evoked potentials occur in up to one-third of the cases, and abnormal somatosensory-evoked potentials are present in 60% of the tumefactive cases.28
When a lesion presents in isolation, distinguishing it from a neoplasm, abscess, or other inflammatory process is critical. In cases with atypical radiographic features for demyelination, high index of suspicion for alternative pathology, failure to respond to aggressive therapy, or urgent need to confirm the diagnosis, a brain biopsy should be considered. It is also worth noting that biopsy results can be misleading if not interpreted by an experienced neuropathologist. Initial biopsy results may be incorrect in as many as 31% of the cases, most frequently misdiagnosing the lesion as a low-grade astrocytoma.28 The presence of astrocytes with nuclear inclusions, known as Creuztfelt-Peters cells, can be misinterpreted as evidence of mitotic glial cells and falsely lead to diagnosis of a glial tumor.21 This has important implications, as radiation therapy can exacerbate demyelinating disease.36
Marburg Variant
Marburg first described a variant of MS in 1906, and most cases subsequently reported followed an aggressive course leading to death within 1 year. Radiographically, the lesions demonstrate significant mass effect and edema and may overlap with findings in tumefactive MS or ADEM. Pathologically, there may be severe myelin and axon destruction with prominent tissue necrosis and macrophage infiltration.21 Marburg variant may be best thought of as an extreme end of the demyelinating disease spectrum and distinguishing it from other fulminant variants may be difficult in the clinical setting.
Balo Concentric Sclerosis
Most reported cases of Balo concentric sclerosis follow a monophasic course with progression to substantial disability within several months. The classic findings are seen pathologically as layers of demyelination alternating with a rim of preserved myelin, often described as “onion bulbs” due to the appearance.21 Radiographically, this is demonstrated by rims of T2 hyperintensity surrounded by T2 hypointensity, reflecting the alternating layers of myelin preservation.14,35 Cases of Balo concentric sclerosis have been included in larger series of tumefactive demyelination.27,28
Myelinoclastic Diffuse Sclerosis (Schilder Disease)
The initial presentation of this disorder was described in 1912 and has been reported rarely in children and adults. Characteristic radiographic findings are the presence of 1 or 2 large, bilaterally symmetric white matter lesions with predominant involvement of the centrum semiovale and frequent contrast enhancement.37,38 The CSF findings include pleocytosis or increased IgG production.37 Many experts have abandoned the concept of Schilder disease due to contamination of early series with cases of adrenoleukodystrophy.
Prognosis
Outcome after a fulminant demyelinating attack is also generally favorable. Most patients regain independence and employment.27 Patients with tumefactive disease seemingly have a lower Expanded Disability Status Scale score indicative of less disability after 10 years when matched to a population-based MS cohort adjusted for disease duration, suggesting a more favorable long-term prognosis in fulminant cases.28 Recurrence is variable, ranging from 10% to 70% after a median follow-up of 1 to 4 years.27,28,39 A higher T2 lesion load is associated with an increased risk of the development of MS and may prompt early initiation of immunomodulatory treatment.28A monophasic presentation without clinical or radiographic criteria to satisfy the diagnosis of MS could be managed as low risk.
NMO and NMO-Spectrum Disorders
Neuromyelitis optica is an autoimmune demyelinating disease associated with autoantibodies to the aquaporin 4 water channel, which results in severe attacks of both optic neuritis and transverse myelitis. Over 80% of the patients develop a relapsing course.40 The NMO-spectrum disorders include recurrent myelitis and recurrent optic neuritis as well as Asian opticospinal MS and cases with prominent brain involvement. Symptomatic brain lesions were initially regarded to be inconsistent with a diagnosis of NMO, but it is now widely accepted that brain lesions are commonly present in NMO and in some instances may be the initial manifestation of the disease.41 The availability of serum NMO IgG antibody testing has allowed better identification of the spectrum of this disorder. The reported specificity of 91% and sensitivity of 73% make this a useful test for discriminating from fulminant MS and ADEM presentations.42
Clinical Features
The 2006 revised diagnostic criteria for NMO are listed in Table 1. Attacks may be severe and result in devastating unilateral or bilateral vision loss, paraplegia, tetraplegia, a pronounced sensory level, or neurogenic respiratory failure.40,43Loss of deep tendon reflexes implies a worse prognosis.40
Brain lesions in NMO may cause severe impairment and may also fulfill radiographic criteria for MS.43Patients may present with syndromes of recurrent nausea and vomiting, hiccups, brainstem dysfunction, encephalopathy, and endocrine disorders as manifestations of NMO pathology.44,45
Radiographic Features
Characteristic spinal cord lesions are longitudinally extensive and may involve the entire transverse diameter of the spinal cord (Figure 3A). Despite this fulminant appearance, lesions may regress after the attack, therefore evaluating MRI at the onset of symptoms is important when considering the diagnosis.46 Optic nerve lesions may demonstrate contrast-enhancement and edema. Brain MRI abnormalities associated with NMO are illustrated in Figure 3 and include periaqueductal lesions, bilateral longitudinally extensive corticospinal tract lesions from the internal capsule to the pons, dorsal medullary lesions extending into the upper cervical cord, abnormal signal of both optic nerves extending into the chiasm or optic radiations, thalamic/hypothalamic involvement, large confluent lesions of the cerebral hemispheres, findings to suggest posterior reversible encephalopathy syndrome, and nonspecific subcortical white matter disease.43–48
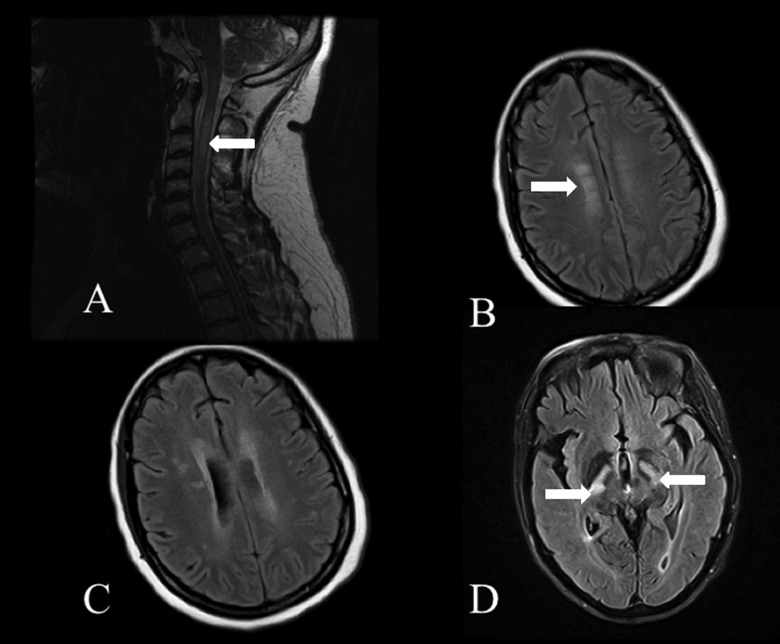
Figure 3.
Neuromyelitis optica (NMO). A, Longitudinally extensive T2 hyperintensity extending throughout the central cervical spinal cord (arrow). B, FLAIR hyperintensity in the subcortical and pericallosal white matter. C, Periependymal FLAIR hyperintensities along the lateral ventricles. D, Longitudinally extensive FLAIR hyperintensities in the corticospinal tracts (arrows) extending from the internal capsule into the pons.
Ancillary Testing
The CSF findings in NMO may include pleocytosis and an elevation in protein. Oligoclonal banding may be present in up to 37% of the cases, but is generally transient.43 Patients with NMO also have a higher prevalence of systemic autoimmunity.40
Pathological findings in NMO acutely demonstrate immunoglobulin and complement deposition that occurs in a vasculocentric pattern, corresponding to the location of aquaporin 4 within astrocytes.49,50 This promotes extensive macrophage infiltration, edema, and loss of myelin and axons with resulting necrosis and cavitation of the gray and white matter.50
Prognosis
Recovery after an NMO relapse is generally incomplete and overall prognosis is worse than MS. After 5 years of disease, 50% of the patients with relapsing NMO were blind in one or both eyes or required assistance for ambulation.40 Factors associated with a poor prognosis included a higher number of relapses in the first 2 years and severity of the initial attack.51 The presence of brain lesions in NMO has been associated with a higher relapse rate.52 Most deaths are attributed to acute neurogenic respiratory failure.51
Acute Treatment of IDD
Standard acute treatment for severe relapses of MS, ADEM/AHLE, fulminant MS variants, and NMO/NMO-spectrum disorders is approached in a similar manner. Intravenous corticosteroids and PLEX are the most commonly administered acute treatments, and dosing parameters are outlined in Figure 4.
Figure 4.
Treatment of fulminant demyelinating disease.
Corticosteroids and PLEX
The recommended dose of intravenous methylprednisolone is 1000 mg/day for a total of 3 to 5 days. Some experts recommend an additional oral corticosteroid taper over 2 to 6 weeks, extrapolating from the protocol used in the Optic Neuritis Treatment Trial; however, we do not routinely prescribe this.27 Side effects of high-dose short-term corticosteroid therapy may include hypertension, hyperglycemia, mood disturbance, insomnia, and gastritis.
In cases that do not respond to steroids, PLEX has shown benefit.53 This recommendation is based on Class II Level of evidence in fulminant demyelinating attacks.54 A randomized, double-blinded placebo-controlled trial of PLEX in cases of steroid-refractory IDD showed a 42% response rate to PLEX.53 Early initiation of PLEX and preservation of the deep tendon reflexes have been associated with a better response.55 Most patients demonstrate improvement within a median of 4 days; however, a small subset of patients may improve after 60 days.55 Those with mass effect and edema on the initial MRI were more likely to respond favorably.55 These factors argue for early, aggressive treatment. A higher PLEX response rate has been associated with pathologically confirmed immunopattern II lesions that are characterized by complement and antibody-mediated demyelination. This is the most common pattern identified in early MS plaques.21,56 In cases of suspected NMO, the presence or absence of serum NMO IgG antibody does not predict PLEX response, and seronegative patients may also benefit from the treatment.55
PLEX requires central venous access. Adverse effects associated with PLEX may include paresthesias, muscle cramps, line infection, bleeding, hypotension, and anaphylaxis. The risk of a systemic reaction including flushing, hypotension, and gastrointestinal disturbance is increased in patients with concomitant use of ace inhibitors; therefore, these agents should be discontinued at least 24 hours prior to the exchange.
Intravenous Immunoglobulin
There are studies suggesting benefit of intravenous immunoglobulin (IVIG) in ADEM and MS.57–59 Dosing is 0.4 g/kg per day over 5 days. Although there is more data to support the use of corticosteroids and PLEX, IVIG may be an alternative in cases with contraindications to these agents or during pregnancy.
Immunomodulatory and Immunosuppressive Therapies
Chronic therapy is not indicated after a monophasic ADEM episode. Immunomodulatory therapy is indicated for chronic therapy in relapsing MS or those deemed at a high risk of subsequent relapses. Early immunosuppressive therapy should be considered in fulminant MS cases if deficits are severe or progressing despite steroids and PLEX. There are case reports in the literature of response to immunosuppression with cyclophosphamide and mitoxantrone.60–62
Interferon β is not indicated in NMO and may actually worsen the disease course.63 In these cases, chronic immunosuppression is often accomplished with the use of azathioprine, mycophenolate mofetil, rituximab, or chronic oral corticosteroids.43,64–66 A detailed review of chronic therapy in IDD is beyond the scope of this review.
Supportive Care
Supportive care is aimed at reducing the incidence of pneumonia, deep venous thrombosis, decubitus ulcers, and gastric ulceration as in most illnesses requiring an extended hospital stay. Mechanical ventilation may be required for several months.20 Symptomatic intracranial hypertension is managed in a standard fashion using additional corticosteroids, mannitol, and hyperventilation. In some cases, barbiturate coma or induced hypothermia may be indicated.24 In cases that fail to respond to medical management, hemicraniectomy should be offered on the basis of the overall favorable prognosis of these conditions and positive response to therapy.22–24,67–69
Summary
Fulminant demyelinating diseases demonstrate significant overlap in presenting clinical and radiographic features. Discriminating the various subtypes has important prognostic and treatment implications. ADEM represents a monophasic illness, generally with substantial neurologic recovery. The MS variants and NMO-spectrum disorders can mimic ADEM but commonly recur and may require prolonged immunomodulatory or immunosuppressive treatment. In NMO, attacks are often more severe with less neurologic recovery. Discriminating these disorders is not always possible at first presentation, but should not delay treatment of the acute episode. If fulminant demyelinating disease does not respond to initial therapy, early immunosuppression should be strongly considered.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673–1680 [DOI] [PubMed] [Google Scholar]
- 2. Marchioni E, Ravaglia S, Piccolo G, et al. Postinfectious inflammatory disorders: subgroups based on prospective follow-up. Neurology. 2005;65(7):1057–1065 [DOI] [PubMed] [Google Scholar]
- 3. Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56(10):1308–1312 [DOI] [PubMed] [Google Scholar]
- 4. Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407–2422 [DOI] [PubMed] [Google Scholar]
- 5. Leake JA, Albani S, Kao AS, et al. , Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical, and laboratory features. Pediatr Infect Dis J. 2004;23(8):756–764 [DOI] [PubMed] [Google Scholar]
- 6. Alper G, Heyman R, Wang L. Multiple sclerosis and acute disseminated encephalomyelitis diagnosed in children after long-term follow-up: comparison of presenting features. Dev Med Child Neurol. 2009;51(6):480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wender M. Acute disseminated encephalomyelitis (ADEM). J Neuroimmunol. 2011;231(1-2):92–99 [DOI] [PubMed] [Google Scholar]
- 8. Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231 [DOI] [PubMed] [Google Scholar]
- 9. De Seze J, Debouverie M, Zephir H, et al. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol. 2007;64(10):1426–1432 [DOI] [PubMed] [Google Scholar]
- 10. Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56(10):1313–1318 [DOI] [PubMed] [Google Scholar]
- 11. Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 suppl 2):S7–S12 [DOI] [PubMed] [Google Scholar]
- 12. Young NP, Weinshenker BG, Parisi JE, et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133(Pt 2):333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honkaniemi J, Dastibar P, Kahara V, Haapasalo H. Delayed MR imaging changes in acute disseminated encephalomyelitis. Am J Neuroradiol. 2001;22(6):1117–1124 [PMC free article] [PubMed] [Google Scholar]
- 14. Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimag Clin N Am. 2008;18(1):149–161 [DOI] [PubMed] [Google Scholar]
- 15. Soneville R, Demeret S, Klein I, et al. Acute disseminated encephalomyelitis in the intensive care unit: clinical features and outcome of 20 adults. Intensive Care Med. 2008;34(3):528–532 [DOI] [PubMed] [Google Scholar]
- 16. Balasubramanya KS, Kovoor JM, Jayakumar PN, et al. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology. 2007;49(2):177–183 [DOI] [PubMed] [Google Scholar]
- 17. Callen DJ, Shroff MM, Branson HM, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72(11):968–973 [DOI] [PubMed] [Google Scholar]
- 18. Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol. 2011;10(12):1065–1073 [DOI] [PubMed] [Google Scholar]
- 19. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ketelslegers IA, Visser IER, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2010;17(4):441–448 [DOI] [PubMed] [Google Scholar]
- 21. Hu W, Lucchinetti CF. The pathological spectrum of CNS inflammatory demyelinating diseases. Semin Immunopathol. 2009;31(4):439–453 [DOI] [PubMed] [Google Scholar]
- 22. Von Stuckrad-Barre S, Klippel E, Foerch C, Lang JM, du Mesnil de Rochemont R, Sitzer M. Hemicraniectomy as a successful treatment of mass effect in aute disseminated encephalomyelitis. Neurology. 2003;61(3):420–421 [DOI] [PubMed] [Google Scholar]
- 23. Refai D, Lee MC, Goldenberg FD, Frank JI. Decompressive hemicraniectomy for acute disseminated encephalomyelitis. case report. Neurosurgery. 2005;56(4):E872. [DOI] [PubMed] [Google Scholar]
- 24. Takata T, Hirakawa M, Sakurai M, Kanazawa I. Fulminant form of acute disseminated encephalomyelitis: successful treatment with hypothermia. J Neurol Sci. 1999;165(1):94–97 [DOI] [PubMed] [Google Scholar]
- 25. Ryan LJ, Bowman R, Zantek ND, et al. Use of therapeutic plasma exchange in the management of acute hemorrhagic leukoencephalitis: a case report and review of the literature. Hemapheresis. 2007;47(6):981–986 [DOI] [PubMed] [Google Scholar]
- 26. Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985-2000. Neurology. 2003;61(10):1373–1377 [DOI] [PubMed] [Google Scholar]
- 27. Wattamwar PR, Baheti NN, Kesavadas C, Nair M, Radhakrishnan A. Evolution and long-term outcome in patients presenting with large demyelinating lesions as their first clinical event. J Neurol Sci. 2010;297(1-2):29–35 [DOI] [PubMed] [Google Scholar]
- 28. Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131(Pt 7):1759–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masdeu JC, Moreira J, Trasi S, et al. The open ring. A new imaging sign in demyelinating disease. J Neuroimaging. 1996;6(2)104–107 [DOI] [PubMed] [Google Scholar]
- 30. Comi G. Multiple sclerosis: pseudotumoral forms. Neurol Sci. 2004;25(suppl 4):S374–S379 [DOI] [PubMed] [Google Scholar]
- 31. Krivickas LS, Hochberg FH, Freeman S. Chronic inflammatory demyelinating polyradiculoneuropathy with tumefactive central demyelination. Muscle Nerve. 2006;33(2):283–288 [DOI] [PubMed] [Google Scholar]
- 32. Kiriyama T, Kataoka H, Taoka T, et al. Characteristic neuroimaging in patients with tumefactive demyelinating lesions exceeding 30mm. J Neuroimaging. 2011;21(2):e69–e77 [DOI] [PubMed] [Google Scholar]
- 33. Masdeu JC, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology. 2000;54(17):1427–1433 [DOI] [PubMed] [Google Scholar]
- 34. Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology. 2004;62(2):226–233 [DOI] [PubMed] [Google Scholar]
- 35. Seewann A, Enzinger C, Filippi M, et al. MRI characteristics of atypical idiopathic inflammatory demyelinating lesions of the brain: a review of reported findings. J Neurol. 2008;255(1):1–10 [DOI] [PubMed] [Google Scholar]
- 36. Miller RC, Lachance DH, Lucchinetti CF, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2006;66(4):1178–1186 [DOI] [PubMed] [Google Scholar]
- 37. Poser CM, Goutieres F, Carpentier MA, Aicardi J. Schilder’s myelinoclastic diffuse sclerosis. Pediatrics. 1986;77(1):107–112 [PubMed] [Google Scholar]
- 38. Mehler MF, Rabinowich L. Inflammatory myelinoclastic diffuse sclerosis (Schilder's disease): neuroradiologic findings. AJNR. 1989;10(1):176–180 [PMC free article] [PubMed] [Google Scholar]
- 39. Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33(1):18–27 [DOI] [PubMed] [Google Scholar]
- 40. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815 [DOI] [PubMed] [Google Scholar]
- 41. Kim W, Kim SH, Lee SH, et al. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011;17(9):1107–1112 [DOI] [PubMed] [Google Scholar]
- 42. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112 [DOI] [PubMed] [Google Scholar]
- 43. Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019–1032 [DOI] [PubMed] [Google Scholar]
- 44. Cabrera-Gomez J, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2011;19(6):812–819 [DOI] [PubMed] [Google Scholar]
- 45. Popescu BF, Lennon VA, Parisi JE, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76(14):1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol. 2008;10(1):55–66 [DOI] [PubMed] [Google Scholar]
- 47. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63(3):390–396 [DOI] [PubMed] [Google Scholar]
- 48. Magna SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72(8):712–717 [DOI] [PubMed] [Google Scholar]
- 49. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;127(Pt 7):1450–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roemer SF, Parisi JE, Lennon VA, et al. Pattern specific loss of aquaporin 4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205 [DOI] [PubMed] [Google Scholar]
- 51. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60(5):848–853 [DOI] [PubMed] [Google Scholar]
- 52. Matsushita T, Isobe N, Piao H, et al. Reappraisal of brain MRI features in patients with multiple sclerosis and neuromyelitis optica according to anti-aquaporin-4 antibody status. J Neurol Sci. 2010;291(1-2):37–43 [DOI] [PubMed] [Google Scholar]
- 53. Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–886 [DOI] [PubMed] [Google Scholar]
- 54. Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2011;76(3):294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Magana SM, Keegan M, Weinshenker BG, et al. , Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68(7):870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keegan M, Konig F, McClelland R, et al. Relationship between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366(9485):579–582 [DOI] [PubMed] [Google Scholar]
- 57. Sahlas DJ, Miller SP, Guerin M, Veilleux M, Francis G. Treatment of acute disseminated encephalomyelitis with intravenous immunoglobulin. Neurology. 2000;54(6):1370–1372 [DOI] [PubMed] [Google Scholar]
- 58. Marchioni E, Marinou-Aktipi K, Uggetti C, et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249(1):100–104 [DOI] [PubMed] [Google Scholar]
- 59. Pradhan S, Gupta RP, Shashank S, Pandey N. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis. J Neurol Sci. 1999;165(1):56–61 [DOI] [PubMed] [Google Scholar]
- 60. Turatti M, Gajofatto A, Rossi F, Vedovello M, Benedetti MD. Long survival and clinical stability in Marburg’s variant multiple sclerosis. Neurol Sci. 2010;31(6):807–811 [DOI] [PubMed] [Google Scholar]
- 61. Nozaki K, Abou-Fayassal N. High dose cyclophosphamide treatment in Marburg variant multiple sclerosis: A case report. J Neurol Sci. 2010;296(1-2):121–123 [DOI] [PubMed] [Google Scholar]
- 62. Jeffery DR, Lefkowitz DS, Crittenden JP. Treatment of Marburg variant multiple sclerosis with mitoxantrone. J Neuroimaging. 2004;14(1):58–62 [PubMed] [Google Scholar]
- 63. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Does interferon beta treatment exacerbate neuromyelitis optic spectrum disorder? Mult Scler. 2012;18(10):1480–1483 [DOI] [PubMed] [Google Scholar]
- 64. Costanzi C, Matiello M, Lucchinetti CF, et al. Azathioprine: tolerability, efficancy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77(7):659–666 [DOI] [PubMed] [Google Scholar]
- 65. Jacob A, Matiello M, Weinshenker B, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66(9):1128–1133 [DOI] [PubMed] [Google Scholar]
- 66. Jacob A, Weinshenker B, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65(11):1443–1448 [DOI] [PubMed] [Google Scholar]
- 67. Ragel BT, Fassett DR, Baringer JR, Browd SR, Dailey AT. Decompressive hemicraniectomy for tumefactive demyelination with transtentorial herniation: observation. Surg Neurol. 2006;65(6):582–583 [DOI] [PubMed] [Google Scholar]
- 68. Censori B, Agostinis C, Partziguian T, Gazzaniga G, Biroli F, Mamoli A. Large demyelinating brain lesion mimicking a herniating tumour. Neurol Sci. 2001;22(4):325–329 [DOI] [PubMed] [Google Scholar]
- 69. Gonzalez Sanchez JJ, Ensenyat NJ, de Notaris M, Arboix JR, García CG, Rodríguez EF. A case of malignant monophasic multiple sclerosis (Marburg’s disease type) successfully treated with decompressive hemicraniectomy. J Neurol Neurosurg Psychiatry. 2010;81(9):1056–1057 [DOI] [PubMed] [Google Scholar]