Abstract
Intravenous recombinant tissue plasminogen activator (r-tPA) was approved for use in acute ischemic stroke in the United States in 1996. Approximately 2% to 5% of patients with acute ischemic stroke receive r-tPA. Complications related to intravenous r-tPA include symptomatic intracranial hemorrhage, major systemic hemorrhage, and angioedema in approximately 6%, 2%, and 5% of patients, respectively. Risk factors for symptomatic hemorrhage include age, male gender, obesity, increased stroke severity, diabetes, hyperglycemia, uncontrolled hypertension, combination antiplatelet use, large areas of early ischemic change, atrial fibrillation, congestive heart failure, and leukoariosis. A risk factor for angioedema is the use of angiotensin-converting enzyme inhibitor. Risk assessment scores, novel imaging strategies, and telemedicine may offer methods of optimizing the risk–benefit ratio.
Keywords: thrombolysis, stroke, safety
Introduction
Since its approval for use in the United States by the Food and Drug Administrator (FDA) in 1996, intravenous (IV) recombinant tissue plasminogen activator (r-tPA) has become the impetus for improved acute stroke treatment across the world. Despite the FDA approval and verified benefits of IV r-tPA, only 2% to 5% of all acute ischemic strokes in the Unites States are treated with IV r-tPA, largely due to a delay in presentation.1,2 Based on the results of the European Cooperative Acute Stroke Study (ECASS) III study, the American Stroke Association has advised extending the IV r-tPA treatment window to 4.5 hours in carefully selected patients.3 Intra-arterial (IA) thrombolysis with r-tPA and mechanical thrombectomy has become common in acute stroke centers, although the risk–benefit ratio has yet to be established.4–7 Of all thrombolysis-related complications, intracerebral hemorrhage causes the most significant morbidity and mortality (Figure 1 ).4,8 Systemic hemorrhages and other r-tPA-related complications also occur and require careful attention as well. Adherence to treatment guidelines and knowledge of the potential risk factors for hemorrhage and other complications improve the safety and effectiveness of acute stroke treatment.
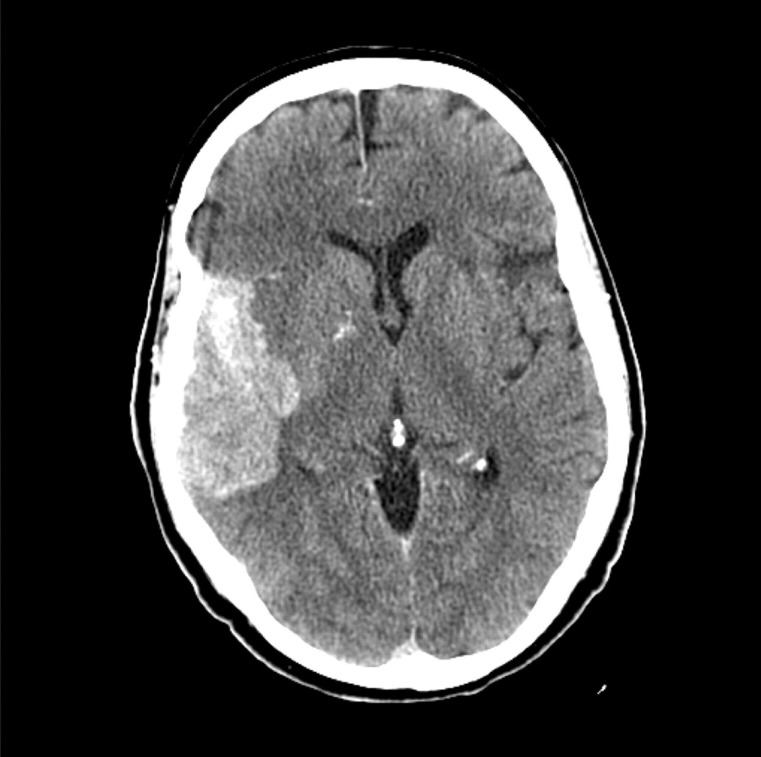
Figure 1.
Hemorrhage on head CT following intra-arterial thrombolysis. CT indicates computed tomography.
Risk Factors for Symptomatic Intracerebral Hemorrhage and Poor Outcomes
In the National Institute of Neurological Disorders and Stroke (NINDS) trials, symptomatic intracerebral hemorrhage (sICH) was defined as a computed tomography (CT)-documented hemorrhage within 36 hours of treatment, which was temporally related to deterioration in the patient's clinical condition in the judgment of the clinical investigator.9 Symptomatic ICH occurred in 6.4% of r-tPA-treated patients versus 0.6% of placebo-treated patients treated within 3 hours using this definition.9 The ECASS III trial defined sICH as a CT- or magnetic resonance imaging (MRI)-documented hemorrhage associated with clinical deterioration defined as an increase in the National Institutes of Heart Stroke Scale (NIHSS) score of 4 points or more or led to death and was determined to be the predominant cause of neurological deterioration.10 In the ECASS III trial, sICH occurred in 2.4% of r-tPA-treated and 0.2% of placebo-treated patients.10 Subsequent studies have confirmed that the sICH rate after IV r-tPA is approximately 6%, even when performed outside randomized controlled trials.11 The risk of sICH after IA thrombolysis with or without IV r-tPA is estimated at 10%.4,12 Furthermore, patients with sICH have mortality rates4 up to 83%; in a survey of emergency physicians, risk of sICH was the most common reason for not wanting to give r-tPA even in an ideal setting.13 We will review several patient characteristics that alter the probability of developing sICH and outcomes after thrombolysis.
Age
Several analyses have determined that a patient’s age may influence the outcome and risk of hemorrhage after r-tPA.8,14,15 Advancing age is associated with an increased number of comorbidities and worse outcomes regardless of r-tPA-related complications.16 In the NINDS trial, those over the age of 80 years had 2.87 times the likelihood of developing sICH.14 This group of patients was excluded from the ECASS III trial.10 In the ECASS III trial, age greater than 65 years was a risk factor for sICH, however the overall clinical outcomes were not affected.17 Another study16 of patients receiving IV r-tPA showed no difference in the rates of sICH or parenchymal hematoma formation between those less than and greater than age 80. To date, studies have not shown age alone to negate the beneficial effects of r-tPA and should not be used to exclude patients from treatment within the 3-hour treatment window. Symptomatic intracerebral hemorrhage rates11 among those under the age of 60 years receiving IV r-tPA is particularly low (2%).
Gender and Weight
Male gender and weight have been independently associated with increased rates of ICH.18 In the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) multivariate analysis for predictors of sICH, the odds ratio (OR) for ICH related to weight was 1.13 to 1.32, depending on the definition of sICH.18 In the same analysis, male gender was associated with higher mortality rates and is concordant with other studies.15,18
Stroke Severity
Severe strokes, defined by higher NIHSS scores, have been associated with worse outcomes, regardless of the administration of treatment. However, stroke severity has also been identified as an independent predictor of sICH after IV or IA thrombolysis (OR 1.8).18 Larger infarcts are generally associated with higher NIHSS scores; the larger the volume of damaged tissue, the greater the risk of hemorrhage.8,15,19 Conversely, minor strokes or imaging negative infarcts have low hemorrhage rates of 0% to 3%.15,20 Nevertheless, strokes with high NIHSS scores or those with an evidence of large early infarct changes still benefit from IV r-tPA within 3 hours (OR for favorable outcome 3.4 and 4.3).9
Diabetes and Hyperglycemia
Several studies have revealed an independent association of baseline hyperglycemia or history of diabetes and risk of sICH.8,14,17,21 In an analysis of IA r-tPA-treated patients in the Prolyse in Acute Cerebral Thromboembolism Trial (PROACT) II trial, 36% of patients with hyperglycemia (serum glucose >200) had intracerebral hemorrhage (relative risk 4.2).4 The NINDS study had a similar rate of ICH in patients with hyperglycemia; and in a confirmatory study in Houston the rate of sICH in those with hyperglycemia or diabetes treated with IV r-tPA within 3 hours was 25% (OR 2.26).21 Various other studies have confirmed hyperglycemia or diabetes to be a risk of either sICH or poor outcomes.8,15,18,22
Blood Pressure
About 75% of patients present with elevated blood pressure (greater than 140 mm Hg systolic) at the time of ischemic stroke.23 Anticipating the possibility of uncontrolled hypertension at presentation as a risk of hemorrhage, the NINDS r-tPA trial developed strict blood pressure parameters for before and after the administration of r-tPA.9,24 Subsequent studies have confirmed significantly higher rates of sICH with elevated pretreatment and posttreatment blood pressure readings.15,18,22 The rate of sICH with r-tPA administration and uncontrolled hypertension at presentation is 26% compared to 12% in those without uncontrolled hypertension (OR 2.59).25 High systolic blood pressure during the 24 hours following r-tPA administration has also been associated with sICH.26 Whether clinical outcomes are also affected by markedly elevated blood pressures following IV r-tPA is less clear. Pending further data, it is advisable to adhere to the blood pressure parameters set forth by the NINDS r-tPA trial with a pretreatment blood pressure of less than 185/110 mm Hg and posttreatment blood pressure of less than 180/105 mm Hg.9,24
Combination Antiplatelet Use
Approximately 30% of patients have taken an aspirin prior to hospitalization and treatment with r-tPA.27 Some small, retrospective studies, 2 with 100 patients or less, suggest that aspirin use prior to r-tPA may increase the rate of sICH and poor outcomes while other studies, including an ECASS III analysis with 418 patients and a systematic review with 3613 patients, suggest rates are unaffected.17,19,22,27,28 In one analysis of 965 IV r-tPA-treated patients, combination therapy with aspirin and clopidogrel was associated with a significantly elevated risk of sICH (OR 9.29), however there was no relationship between antiplatelet use and outcome.19,29 Dual antiplatelet therapy has a higher risk of sICH than single antiplatelet therapy or no antiplatelet use.19 While combination antiplatelet use with aspirin and clopidogrel is possibly associated with increased rates of sICH, outcomes do not seem to be affected and is not an exclusion criterion for IV r-tPA.
Early Ischemic Changes on CT
In addition to ruling out hemorrhage, tumors, and prior infarcts, the baseline CT may provide useful information regarding the risk and benefit ratio related to r-tPA administration. Several CT characteristics have been found to correlate with the development of sICH and/or poor outcomes, however reanalysis of the NINDS trial failed to show this correlation.15,19,22,26,30 Early ischemic changes (EICs) include hypodensity, loss of gray–white differentiation, and cerebral edema. Additional characteristics that significantly alter rates of sICH and poor outcome include a hyperdense artery sign and EICs involving >one third of the middle cerebral artery (MCA) territory.15,26 EICs involving > one third of the MCA territory are associated with ICH, OR of 9.38, versus 3.17 if <one third of the MCA is involved and is an exclusion criterion for IV r-tPA in the 3- to 4.5-hour window.15 Grading scales, such as the Alberta Stroke Programme Early CT Score (ASPECTS), may also be helpful in quantifying EICs (Figure 2 ).19 A score of 7 or less is associated with an OR of 4.6 for sICH.19 Despite the risk of sICH, EICs alone should not be a reason to exclude a patient from treatment within the 3-hour window30,31; however, a clearly defined hypodensity of any size should raise suspicion as to the true timing of ischemic stroke onset, and treatment should be approached cautiously.
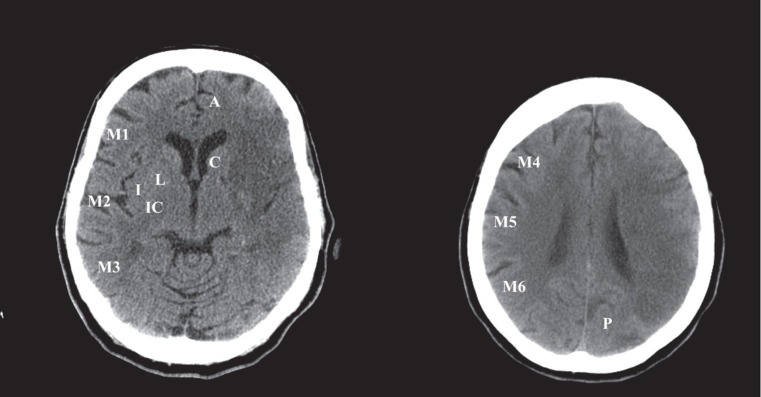
Figure 2.
ASPECTS system. ASPECTS is a semiquantitative method where 1 point is assigned for each normal area (maximum score of 10). The areas scored are depicted on the left-hand side of the scans and the caudate nucleus (C). The anterior circulation and posterior circulation, depicted on the right-hand side of the scan are not scored. The total score for this scan is 3 (1 point each for caudate, M4, and M5). A indicates anterior circulation; P, posterior circulation; C, caudate head; L, lentiform nucleus; I, insular ribbon; M1, anterior MCA cortex; M2, MCA cortex lateral to insular ribbon; M3, posterior cortex; M4, anterior MCA territory 2 cm superior to M1; M5, lateral MCA territory 2 cm superior to M2; M6, posterior MCA territory 2 cm superior to M3. ASPECTS indicates the Alberta Stroke Programme Early CT Score; CT, computed tomography; MCA, middle cerebral artery.
Risk of Symptomatic ICH and Outcomes Related to Time
Successful treatment of acute ischemic stroke with IV r-tPA is highly time dependent with efficacy quickly diminishing with time.32 It has been successfully proven that thrombolysis with IV r-tPA can be beneficial up to 4.5 hours and is not associated with increased rates of sICH, when adopting the parameters defined in the NINDS and ECASS III trials.9,17 In contrast, sICH appears to increase starting in the 5- to 6-hour time frame.33 Therefore, acute stroke patients should be treated as early as possible after meeting the inclusion and exclusion criteria to receive maximum benefit.
Other Factors
Several other factors have been associated with a potentially increased risk of sICH and poor outcomes after r-tPA administration. Atrial fibrillation and congestive heart failure are among the cardiac risk factors for poor outcome and development of sICH.15,18,22 Lower platelet counts have also been associated with an increased risk of sICH, and a platelet count less than 100 000 is an exclusion criterion for IV-r-tPA.15 Hyperlipidemia or use of lipid-lowering medications appears to have an association with increased sICH rates, raising the question of statin-associated hemorrhages.25,26 Cervical artery dissection does not appear to be associated with increased rates of sICH or poor outcomes after thrombolysis.34 Preexisting leukoaraiosis, or chronic white matter ischemic disease, as defined by CT, has been associated with an increased risk of both sICH and poor outcomes.35,36 The presence of these features alone is not sufficient to exclude a patient from treatment with IV r-tPA.37
Treatment of Intracerebral Hemorrhage After Thrombolysis
Given the amount of time spent evaluating potential risk factors for sICH, we should spend some time discussing what to do when presented with a postthrombolysis intracerebral hemorrhage. The first step is to quickly identify the patient with sICH, which is defined as any significant neurological change or an NIHSS increase of 4 points or greater accompanied by a CT or MRI showing ICH.9,10 Once sICH is suspected, infusion of thrombolytics should be discontinued until ICH is excluded, and once diagnosed, transfusion of cryoprecipitate containing factor VIII and platelets (6-8 units) should be given to rapidly correct the fibrinolytic state produced by r-tPA.38 The use of other agents, including prothrombin complex concentrate (PCC), fibrinogen, or fresh frozen plasma with or without recombinant factor VII, has been investigated with unclear benefits in spontaneous ICH and usefulness in postthrombolysis ICH is unknown.38,39 Guidelines for blood pressure and surgical management for spontaneous ICH exist, however are lacking in regard to the post-thrombolysis ICH.38,39 A reasonable minimum blood pressure should be maintained to preserve cerebral perfusion (cerebral perfusion pressure ≥60 mm Hg), however aggressive blood pressure reduction, with the use of intermittent or continuous IV medication, should be attempted to limit hematoma expansion.38,39 Current guidelines for spontaneous ICH suggest a target blood pressure of 160/90 mm Hg and surgical intervention for cerebellar hemorrhages with brain stem compression or development of hydrocephalous. Any surgical intervention should be done only after adequate reversal of the fibrinolytic effects of r-tPA.38,39 Patients should be monitored in a neurointensive care unit with intracranial pressure (ICP) monitoring and intubation when appropriate and available.38,39
Protocol Deviations and Potential Consequences
Randomized controlled trial protocols define the target population believed to benefit by a treatment and supply reasonable safety parameters to guide that proposed treatment. The consequence of deviating from that protocol is not always known. With regard to treatment of acute stroke with r-tPA, some consequences to protocol deviations have been found. Protocol deviations occur in approximately 16% to 32.6% of r-tPA-treated patients and occur regardless of the clinical setting.11,40,41 Deviations from protocol may involve inclusion and exclusion criteria, posttreatment protocols of blood pressure management, and use of antiplatelet/anticoagulant medications. The inclusion and exclusion criteria for IV r-tPA treatment of acute ischemic stroke are listed in the Table 1 .10,24,42 Several studies have found a correlation between protocol deviations and subsequent complications. Nevertheless, many of the items in the NINDS protocol were arbitrarily defined and should still be considered within the context of individual cases and best physician judgment.
Table 1.
Inclusion and Exclusion Criteria for Stroke Thrombolysis
| NINDS IV r-tPA inclusion criteria |
| Age greater than 18 |
| Clinical diagnosis of ischemic stroke with clearly defined time of onset |
| Time of onset <3 hours A deficit measurable on the NIHSS A baseline CT showing no evidence of intracranial hemorrhage |
| NINDS IV r-tPA exclusion criteria |
| Systolic BP >185 mm Hg or diastolic BP >110 mm Hg |
| Aggressive measures required to lower blood pressure |
| Ischemic stroke or serious head trauma within 3 months |
| Myocardial infarction within 3 month GI or GU hemorrhage within 21 days |
| Major surgery within 14 days |
| Arterial puncture at a noncompressible site within 7 days |
| Prior intracranial hemorrhage Symptoms suggestive of subarachnoid hemorrhage |
| Rapidly improving symptoms Minor symptoms Seizure at onset Heparin therapy within 48 hours with elevated aPTT Use of anticoagulant within 48 hours with PT >15 seconds or INR >1.7 Platelet < 100 000 Glucose <50 mg/dL or >400 mg/dL Additional ECASS III exclusion criteria for 3- to 4.5-hour window Age greater than 80 Use of anticoagulant regardless of INR NIHSS greater than 25 CT early infarct signs less than one third of the MCA territory |
Abbreviation: IV r-tPA, intravenous recombinant tissue plasminogen activator; NIHSS, the National Institutes of Heart Stroke Scale; BP, blood pressure; CT, computed tomography; GI, gastrointestinal; MCA, middle cerebral artery; GU, genitourinary; NINDS, National Institute of Neurological Disorders and Stroke; ECASS, European Cooperative Acute Stroke Study.
In a large analysis of the safety of r-tPA use in clinical practice, as protocol deviation rates increased, so did the mortality rates.11 The center with the highest protocol deviation percentage (66.7%) in this study had the highest mortality rate (25.4%).11 Similar correlations have been found with protocol deviations and rates of sICH.25,41 In one study, the rate of sICH was 38% in those with protocol deviations versus only 5% in those without, and all hemorrhages occurred in 75% of those with protocol deviations versus 10% of those without.41 Some of the protocol deviations11,25,41 that have been associated with symptomatic intracranial hemorrhage include stroke or head trauma within 3 months, prior intracranial hemorrhage, and use of anticoagulants with an international normalized ratio (INR) >1.7. Deviations specifically pertaining to blood pressure parameters resulted in similar findings.15,18,22,25,26 Certain protocol deviations, such as unruptured aneurysm, arteriovenous malformation, and neoplasm, have an uncertain relationship to sICH.43,44 Additionally, many patients not treated with IV r-tPA due to “rapidly improving or minor deficit,” develop subsequent worsening and may indeed have benefited from r-tPA.43 Despite current levels of protocol deviations, treatment of acute stroke patients with thrombolysis has been shown to be as safe in clinical practice as in controlled trials with or without the use of acute stroke teams.11,40,41,45
Dabigatran Use and Thrombolysis
A novel challenge is the treatment of a patient who presents acutely with stroke and is taking dabigatran etexilate for atrial fibrillation. In the exclusion criteria for the NINDS trial and ECASS III, patients were excluded if presentation was within 3 hours and were using warfarin with prothrombin time (PT) >15 seconds or INR >1.7, or if they presented between 3 and 4½ hours and were using warfarin, regardless of INR. At the time these trials were designed, direct thrombin inhibitors were not available or being tested. During the RELY-ABLE trial, a 46-year-old woman who had been assigned to treatment with dabigatran presented with a dissection of the right common and internal carotid arteries and left hemiplegia.46 Her NIHSS was 19, INR 1.2, activated partial thromboplastin time (aPTT) 34.8 seconds, and fibrinogen level 506 mg/dL. She was treated with r-tPA just under 4.5 hours after symptom onset and 7 hours after the last ingestion of dabigatran. At 24 hours, her NIHSS improved to 12 and her follow-up head CT showed an infarction limited to the right putamen without evidence of hemorrhage.
At recommended therapeutic doses, dabigatran etexilate prolongs the aPTT, activated clotting time (ACT), ecarin clotting time (ECT), and thrombin time (TT). According to the manufacturer’s Web site, the onset of effect of dabigatran is within 1 hour of dosing and the anticoagulant effects parallel plasma concentrations. Twelve hours after a dose, approximately 50% of the drug is eliminated and 75% is excreted within 24 hours. International normalized ratio measurement is not a reliable indicator of drug activity. At 150 mg twice daily, less than 10% of patients have aPTTs greater than 65 seconds (or 2 times control) at 12 hours after dosing. The correlation between dabigatran plasma concentrations and aPTT is nonlinear and linear with ECT and TT. If rapid testing is not available, the time of the last dose may be useful in clinical decision making.
Risk of Systemic Hemorrhage
Serious systemic hemorrhage occurred in approximately 1.6% of IV r-tPA-treated patients in the NINDS r-tPA trials.9,24 Those considered to be at the highest risk of significant complications related to systemic hemorrhage were excluded from the trial, which included history of myocardial infarction within 1 month, gastrointestinal or urinary tract hemorrhage within 21 days, major surgery within 14 days, and arterial puncture at a noncompressible site within 7 days.9,41
While some hemorrhages are manageable with transfusion and surgical correction, others may be more serious and result in higher mortality rates.11,41,43 Several cases of fatal cardiac tamponade have been reported in the off-label use of thrombolysis after myocardial infarction or cardiac surgery.43 Urinary tract hemorrhages may lead to urinary obstruction, and gastrointestinal hemorrhages may lead to hemodynamic compromise in the stroke patient who may require augmented cerebral perfusion. A review of exclusion criteria with attention to prior hemorrhage or surgery is a component of the pretreatment assessment. When possible, a rapid discussion with the patient’s primary physician or surgeon is beneficial in assessing the potential risk of complications associated with the patient’s premorbid condition.
Interpretation of Risk Factors for Clinical Decision Making
Discussion of the risks and benefits of thrombolysis with patients and families should be undertaken when such a discussion can be done expeditiously. The patient and family should feel that they have made an informed decision based on the best available information; however, when they are not able to consent, informed consent is not required and treatment should not be delayed. Intravenous r-tPA is considered an emergent therapy for acute stroke and does not universally require informed consent, particularly in situations when a patient is unable to communicate and waiting for family to arrive or be contacted might delay therapy. Use of simplified benefit and risk ratios such as number needed to benefit of 6 and number needed to harm of 36, may be useful in expressing the effect of r-tPA on outcomes.8 Use of absolute risks are also helpful, such as a 6.4% risk of sICH, a 1.6% risk of serious systemic hemorrhage, and a 30% to 50% greater chance of improvement to no or minimal disability at 3 months.9 Visual aids have improved and may also be beneficial.47 Two risk assessment tools, the Hemorrhage After Thrombolysis (HAT) score and the Stroke-Thrombolytic Predictive Instrument (TPI), have been published but have not been externally validated or approved for clinical decision making.48,49 The HAT score is determined by adding points in 3 categories, 1 point for diabetes or glucose >200 mg/dL, 1 point for NIHSS 15 to 20, or 2 if ≤20, and 1 point for an easily detectable hypodensity <1/3 the MCA or 2 points if ≥1/3 the MCA.48 Increasing scores resulted in increased rates of ICH, with a score of >3 resulting in a 44% sICH rate and a 33% rate of hemorrhage with final fatal outcome.48 The Stroke-TPI tool takes into account 7 variables to predict various outcomes with and without IV r-tPA, however requires a computer or handheld device.49 Adherence to the guidelines for thrombolysis is recommended and subsequent decisions should be based on informed consent and compassionate reasoning. Further observation and study may allow a more defined and formal protocol for risk assessment based on premorbid risk factors.
Thrombolysis and Angioedema
Orolingual angioedema following alteplase treatment can be bilateral or unilateral and is generally mild. If unilateral, then the tongue swelling is typically contralateral to the affected hemisphere.50 Reported rates50,51 of angioedema range between 1.3% and 5.1%. The risk of orolingual angioedema is increased with the use of angiotensin-converting enzyme inhibitor medications (relative risk 13.6) and frontal and insular strokes (relative risk 6.4).51 The firstline treatment for angioedema after the use of alteplase has not been determined, and therefore, standard treatment for anaphylaxis is recommended, including corticosteroids, antihistamines, and intubation. Further analysis regarding the mechanism underlying the angioedema may help determine the best treatment.
Postthrombolysis Reperfusion Injury
“Reperfusion injury” has been postulated to occur due to tissue injury after recanalization of a blood vessel after r-tPA, IA treatment, or carotid endarterectomy. Clinical deterioration following stroke can occur in patients with reocclusion of the artery recanalized by r-tPA or hyperperfusion of the tissue due to recanalization. Hyperperfusion is a common occurrence; in one study 40% of patients had hyperperfusion within hours and 50% within 1 week. Late hyperperfusion leads to a larger infarct volume, while early hyperperfusion does not have clinical significance.52
Telemedicine and Stroke
Video teleconferencing is a modality that can be utilized in the emergency room setting to provide subspecialty care to a high number of patients. Video teleconferencing consists of an audiovisual system that allows the neurologist to examine the patient, while simultaneously allowing the patient and emergency room physician to visualize and communicate with the neurologist. The radiological images can also be viewed either through the video teleconferencing system or through remote computer access.53
Examiners can perform the NIHSS through the video teleconferencing system, which has been shown to be reliable when tested by telemedicine neurologists and bedside examiners. Ataxia, facial palsy, and dysarthria were the least reliable portions of the examination between examiners.53 Stroke mimics can be determined by the subspecialty expertise, and treatment can be provided to eligible patients. More patients are treated if a video teleconferencing system is available than if a neurologist is not present.53 Compared to telephone consultation, video teleconferencing is more accurate diagnostically (correct treatment decisions in 98% of video teleconferencing patients versus 82% in telephone consultations) but shows no difference in 90-day functional outcomes, mortality, and intracerebral hemorrhage.54
Future Directions
Imaging-Guided Thrombolysis
A noncontrast CT scan is the most commonly used screening tool for detecting acute stroke mimics (such as mass lesion and intracerebral hemorrhage) and for early signs of stroke. However, MRI is superior to a noncontrast CT scan to diagnose an acute stroke, with a sensitivity of 77%, specificity of 96%, and accuracy of 86% compared to 16%, 97%, and 55%, respectively, for CT.55 Many facilities use CT perfusion combined with CT angiogram imaging due to the ease of acquisition and time limitations. Computed tomography perfusion with CT angiogram can accurately determine the site of occlusion (sensitivity of 95% and specificity 100%), as well as infarct size (sensitivity 80% and specificity 97%).56 Many studies use either MRI perfusion-weighted images (PWIs)/diffusion-weighted image (DWIs) or CT perfusion.
Comparison of PWIs and DWIs with MRI can help determine the area of ischemia and an area at risk of ischemia, that is the penumbra. Ischemia is posited to be reflected in the DWIs, while the penumbra is posited to be reflected in the PWIs. Intravenous r-tPA reduces final infarct size in patients with a mismatch between the diffusion and perfusion images.57 The DEFUSE study did not use MRI diffusion/perfusion mismatch to determine patient selection for r-tPA, had no control group, and treated all patients with r-tPA. This study correlated the outcomes with the diffusion/perfusion mismatch found on imaging. A 20% mismatch in size of the perfusion to diffusion-weighted abnormalities was considered to be tissue at risk of ischemia. Patients were treated with r-tPA 3 to 6 hours after symptom onset, if NIHSS was greater than 5. Patients with early reperfusion benefited when a mismatch was found (OR 8.7). If no mismatch was seen, patients did not benefit from treatment.58 The incidence of sICH was 9.5% for all patients. 57,58
The DIAS and DEDAS studies selected patients up to 9 hours after symptom onset for treatment with an experimental medication (desmoteplase) based upon imaging characteristics. A diffusion/perfusion mismatch of 20% was required prior to treatment.59,60 The studies showed benefit with treating patients with desmoteplase, but were small, with only 104 patients randomized in DIAS and 37 in DEDAS. The DIAS-2 study had the largest number of patients (n = 186) and used a similar mismatch criteria using CT or MRI perfusion but did not show a benefit of desmoteplase treatment.61
Treatment of Patients With Unknown Symptom Onset Time
Many patients present with unknown times of onset, and researchers have postulated if MRI can be used to identify patients who are thrombolysis candidates. A retrospective study compared the so-called wake-up strokes patients with unknown times of onset, who received intravenous thrombolysis, and those who presented in the 0- to 3-hour window. In the treated patients, 60.9% received full-dose IV r-tPA, 30.4 % received IA therapy, and 8.7% received a combination of IV and IA therapy. Only 34.8% of patients who had an unknown time of onset and received treatement had a CT perfusion or MRI DWIs/PWIs performed. Wake-up strokes who were treated with thrombolysis had an excellent (14% vs 6%, P = .06) or favorable (28% vs 13%, P = .006) outcome more often than those who were untreated. Mortality was significantly higher (15% vs 0%), despite having a hemorrhage rate of 4.3% in patients treated with an unknown time of onset. The mortality rate may be due to selection bias; severe wake-up strokes were treated (NIHSS 16 vs 10).62
A multicenter study evaluating patients with unclear time of onset strokes was presented at the 2011 International Stroke Conference as an abstract.63 Magnetic resonance imaging perfusion–diffusion mismatch of more than 20% and less than one third of the MCA distribution affected by stroke on T2 or fluid-attenuated inversion-recovery (FLAIR) imaging was required. Intravenous thrombolysis with r-tPA with or without IA urokinase could be given within 3 hours of symptom identification or IA urokinase within 6 hours of symptom onset. There was no control arm and all comparisons were made with historical data. Stenting or mechanical devices could also be utilized. Symptomatic ICH was defined as any parenchymal hemorrhage within 48 hours, causing either neurological decline or an increase in the NIHSS by 4 or more points. The primary end point was a modified Rankin score of 2 or less at 3 months. A total of 430 patients were screened and 83 (19.3%) received reperfusion therapy. Median age was 67 years and median NIHSS was 14. Symptomatic hemorrhage occurred in 8 patients (9.8%), 5 of whom had neurological decline and 3 of whom had a 4 point or more increase in NIHSS. The primary end point was seen in 37 patients (44.6%), and a modified Rankin score of 0 to 1 was seen in 24 patients (28.9%). Given the nearly 10% rate of symptomatic hemorrhage and lack of control arm for comparison of good clinical outcomes, large randomized trials are needed prior to adopting this practice.
Intra-Arterial Therapy After IV r-tPA
The safety of IA therapy after intravenous thrombolysis has not been proven. An ongoing study, the Interventional Management of Stroke (IMS) III Trial, is evaluating patients with an NIHSS of greater than or equal to 10, who have received r-tPA within 3 hours of stroke symptom onset and IA treatment. Patients in the study group will receive a lower dose (0.6 mg/kg, maximum dose of 60 mg) of IV r-tPA, while the control population receives the standard dose. Intra-arterial r-tPA, mechanical thrombolysis with the mechanical embolus removal in cerebral ischemia (MERCI) retrieval device, and/or low-intensity ultrasound at the site of the occlusion are the IA therapies utilized in this study. A Modified Rankin Scale of 2 or less at 3 months, mortality at 3 months, and sICH at 30 hours are the primary end points. Secondary end points include other functional assessments at 3 months, recanalization rates at 24 hours, ascertainment of ICH subtypes, and cost-effectiveness of treatment. The study will be closed after the recruitment of 900 patients. Until the results of this study are published, physicians must use clinical judgment to justify the use of IA treatment after IV r-tPA.5
Conclusion
Thrombolysis for acute ischemic stroke has had a significant positive improvement in the lives of patients who receive it. Use of the medication in the appropriate setting maximizes benefit while reducing risk. Knowledge of risk factors is important when estimating the risk–benefit ratio. Novel tools such as risk assessment scores, advanced imaging strategies, and telemedicine may further optimize the risk–benefit ratio.
Footnotes
This review article has not been submitted for publication elsewhere. All authors have contributed substantively to the drafting of the manuscript and critical revision for important intellectual content and the final approval of the version to be published.
The author(s) declared a potential conflict of interest (e.g. a financial relationship with the commercial organizations or products discussed in this article) as follows: Drs Miller and Simpson have nothing to report. Dr Silver has received consulting fees from Abbott Vascular and has served as an expert for stroke medical malpractice defense.
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
- 1. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56(8):1015–1020 [DOI] [PubMed] [Google Scholar]
- 2. Deng YZ, Reeves MJ, Jacobs BS, et al. IV tissue plasminogen activator use in acute stroke: experience from a statewide registry. Neurology. 2006;66(3):306–312 [DOI] [PubMed] [Google Scholar]
- 3. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57(9):1603–1610 [DOI] [PubMed] [Google Scholar]
- 5. Khatri P, Hill MD, Palesch YY, et al. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3(2):130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212 [DOI] [PubMed] [Google Scholar]
- 7. Penumbra pivotal stroke trial investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–2768 [DOI] [PubMed] [Google Scholar]
- 8. Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38(8):2279–2283 [DOI] [PubMed] [Google Scholar]
- 9. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group Stroke. November 1997;28(11):2109–2118 [DOI] [PubMed] [Google Scholar]
- 10. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329 [DOI] [PubMed] [Google Scholar]
- 11. Graham GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke. 2003;34(12):2847–2850 [DOI] [PubMed] [Google Scholar]
- 12. Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8(9):802–809 [DOI] [PubMed] [Google Scholar]
- 13. Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46(1):56–60 [DOI] [PubMed] [Google Scholar]
- 14. Longstreth WT, Jr, Katz R, Tirschwell DL, Cushman M, Psaty BM. Intravenous tissue plasminogen activator and stroke in the elderly. Am J Emerg Med. 2010;28(3):359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002;105(14):1679–1685 [DOI] [PubMed] [Google Scholar]
- 16. Berrouschot J, Rother J, Glahn J, Kucinski T, Fiehler J, Thomalla G. Outcome and severe hemorrhagic complications of intravenous thrombolysis with tissue plasminogen activator in very old (> or =80 years) stroke patients. Stroke. 2005;36(11):2421–2425 [DOI] [PubMed] [Google Scholar]
- 17. Bluhmki E, Chamorro A, Davalos A, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8(12):1095–1102 [DOI] [PubMed] [Google Scholar]
- 18. Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke. 2008;39(12):3316–3322 [DOI] [PubMed] [Google Scholar]
- 19. Cucchiara B, Kasner SE, Tanne D, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40(9):3067–3072 [DOI] [PubMed] [Google Scholar]
- 20. Chernyshev OY, Martin-Schild S, Albright KC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demchuk AM, Morgenstern LB, Krieger DW, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30(1):34–39 [DOI] [PubMed] [Google Scholar]
- 22. Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10 [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez-Garcia JL, Botia E, de La Sierra A, Villanueva MA, Gonzalez-Spinola J. Significance of elevated blood pressure and its management on the short-term outcome of patients with acute ischemic stroke. Am J Hypertens. 2005;18(3):379–384 [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. December 14 1995;333(24):1581–1587 [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Frey JL, Flaster M, et al. Pre-tissue plasminogen activator blood pressure levels and risk of symptomatic intracerebral hemorrhage. Stroke. November 2009;40(11):3631–3634 [DOI] [PubMed] [Google Scholar]
- Derex L, Hermier M, Adeleine P, et al. Clinical and imaging predictors of intracerebral haemorrhage in stroke patients treated with intravenous tissue plasminogen activator. J Neurol Neurosurg Psychiatry. January 2005;76(1):70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado L, Millan M, de la Ossa NP, et al. Influence of antiplatelet pre-treatment on the risk of intracranial haemorrhage in acute ischaemic stroke after intravenous thrombolysis. Eur J Neurol. February;17(2):301–306 [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, Hakim A, Fang J, Sharma M. Pre admission antithrombotics are associated with improved outcomes following ischaemic stroke: a cohort from the Registry of the Canadian Stroke Network. Int J Stroke. October 2009;4(5):328–334 [DOI] [PubMed] [Google Scholar]
- Hermann A, Dzialowski I, Koch R, Gahn G. Combined anti-platelet therapy with aspirin and clopidogrel: risk factor for thrombolysis-related intracerebral hemorrhage in acute ischemic stroke? J Neurol Sci. September 15 2009;284 (1-2):155–157 [DOI] [PubMed] [Google Scholar]
- Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. December 12 2001;286(22):2830–2838 [DOI] [PubMed] [Google Scholar]
- Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke. October 2005;36(10):2110–2115 [DOI] [PubMed] [Google Scholar]
- Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. December 12 2000;55(11):1649–1655 [DOI] [PubMed] [Google Scholar]
- Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. April 2000;31(4):811–816 [DOI] [PubMed] [Google Scholar]
- Zinkstok S, Nederkoorn P, Vergouwen M. Safety of Thrombolysis in Patients with Acute Ischemic Stroke Caused by Cervical Artery Dissection-A Review. European Neurological Journal. 2010;2(1):65–72 [Google Scholar]
- Aries MJ, Uyttenboogaart M, Vroomen PC, De Keyser J, Luijckx GJ. tPA treatment for acute ischaemic stroke in patients with leukoaraiosis. Eur J Neurol. June 1;17(6):866–870 [DOI] [PubMed] [Google Scholar]
- Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology. March 27 2007;68(13):1020–1024 [DOI] [PubMed] [Google Scholar]
- Demchuk AM, Khan F, Hill MD, et al. Importance of leukoaraiosis on CT for tissue plasminogen activator decision making: evaluation of the NINDS rt-PA Stroke Study. Cerebrovasc Dis. 2008;26(2):120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. June 2007;38(6):2001–2023 [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Hemphill JC, 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. September;41(9):2108–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. March 1 2000;283(9):1145–1150 [DOI] [PubMed] [Google Scholar]
- Lopez-Yunez AM, Bruno A, Williams LS, Yilmaz E, Zurru C, Biller J. Protocol violations in community-based rTPA stroke treatment are associated with symptomatic intracerebral hemorrhage. Stroke. January 2001;32(1):12–16 [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. May 2007;38(5):1655–1711 [DOI] [PubMed] [Google Scholar]
- Aleu A, Mellado P, Lichy C, Kohrmann M, Schellinger PD. Hemorrhagic complications after off-label thrombolysis for ischemic stroke. Stroke. February 2007;38(2):417–422 [DOI] [PubMed] [Google Scholar]
- Kim JT, Park MS, Yoon W, Cho KH. Detection and Significance of Incidental Unruptured Cerebral Aneurysms in Patients Undergoing Intravenous Thrombolysis for Acute Ischemic Stroke . J Neuroimaging. December 9 [DOI] [PubMed] [Google Scholar]
- Scott PA, Frederiksen SM, Kalbfleisch JD, et al. Safety of intravenous thrombolytic use in four emergency departments without acute stroke teams. Acad Emerg Med. October;17(10):1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt A, De Raedt S, Nieboer K, De Keyser J, Brouns R. Intravenous thrombolysis with recombinant tissue plasminogen activator in a stroke patient treated with dabigatran. Cerebrovasc Dis. 2010;30(5):533–534 [DOI] [PubMed] [Google Scholar]
- Gadhia J, Starkman S, Ovbiagele B, Ali L, Liebeskind D, Saver JL. Assessment and improvement of figures to visually convey benefit and risk of stroke thrombolysis. Stroke. February;41(2):300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Safdar A, Mehdiratta M, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. October 28 2008;71(18):1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke. December 2006;37(12):2957–2962 [DOI] [PubMed] [Google Scholar]
- Rudolf J, Grond M, Schmulling S, Neveling M, Heiss W. Orolingual angioneurotic edema following therapy of acute ischemic stroke with alteplase. Neurology. August 22 2000;55(4):599–600 [DOI] [PubMed] [Google Scholar]
- Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. May 13 2003;60(9):1525–1527 [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, et al. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology. 2001;57(11):2015–2021 [DOI] [PubMed] [Google Scholar]
- Schwamm LH, Holloway RG, Amarenco P, et al. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke. July 2009;40(7):2616–2634 [DOI] [PubMed] [Google Scholar]
- Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. September 2008;7(9):787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75(2):177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. June 2007;61(6):533–543 [DOI] [PubMed] [Google Scholar]
- Davis S, Donnan G, Parsons M, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet neurology. 2008;7(4):299–309 [DOI] [PubMed] [Google Scholar]
- Albers G, Thijs V, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Annals of neurology. 2006;60(5):508–517 [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. January 2005;36(1):66–73 [DOI] [PubMed] [Google Scholar]
- Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. May 2006;37(5):1227–1231 [DOI] [PubMed] [Google Scholar]
- Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. February 2009;8(2):141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. March 2009;40(3):827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-W, Sohn S-I, Yu K-H, et al. Reperfusion Therapy in Acute Ischemic Stroke with Unclear Onset by MRI Evaluation (RESTORE). Stroke. 2011;42(3):e110 [Google Scholar]