Abstract
Spontaneous, nontraumatic intracerebral hemorrhage (ICH) is defined as bleeding within the brain parenchyma. Intracranial hemorrhage includes bleeding within the cranial vault and encompasses ICH, subdural hematoma, epidural bleeds, and subarachnoid hemorrhage (SAH). This review will focus only on ICH. This stroke subtype accounts for about 10% of all strokes. The hematoma locations are deep or ganglionic, lobar, cerebellar, and brain stem in descending order of frequency. Intracerebral hemorrhage occurs twice as common as SAH and is equally as deadly. Risk factors for ICH include hypertension, cerebral amyloid angiopathy, advanced age, antithrombotic therapy and history of cerebrovascular disease. The clinical presentation is “stroke like” with sudden onset of focal neurological deficits. Noncontrast head computerized tomography (CT) scan is the standard diagnostic tool. However, newer neuroimaging techniques have improved the diagnostic yield in terms of underlying pathophysiology and may aid in prognosis. Intracerebral hemorrhage is a neurological emergency. Medical care begins with stabilization of airway, breathing function, and circulation (ABCs), followed by specific measures aimed to decrease secondary neurological damage and to prevent both medical and neurological complications. Reversal of coagulopathy when present is of the essence. Blood pressure management can be key and continues as an area of debate and ongoing research. Surgical evacuation of ICH is of unproven benefit though a subset of well-selected patients may have improved outcomes. Ventriculostomy and intracranial pressure (ICP) monitoring are interventions also used in this patient population. To date, hemostatic medications and neuroprotectants have failed to result in clinical improvement. A multidisciplinary approach is recommended, with participation of vascular neurology, vascular neurosurgery, critical care, and rehabilitation medicine as the main players.
Keywords: intracerebral hemorrhage, diagnosis, treatment, prognosis, surgery
Introduction
Intracerebral hemorrhage (ICH) is defined as bleeding in the brain parenchyma, whereas intracranial hemorrhage refers to any bleeding within the cranial vault. While traumatic ICH is by far the most common type of ICH, this review will focus on spontaneous, nontraumatic ICH, implying bleeding that occurs without trauma or known bleeding causes such as an arteriovenous malformation, cerebral aneurysm, or tumor.
Intracerebral hemorrhage is defined by its location within the brain parenchyma, with “deep” ICH being located within the basal ganglia and internal capsule (35%-70%), brain stem (5%-10%), and cerebellum (5%-10%). In contrast, “lobar” ICH (15%-30%) refers to hemorrhages located in cortical–subcortical areas and follows a “lobar” pattern across one or less often multiple lobes of the brain. Deep ICH accounts for about two third of spontaneous ICH cases, and lobar ICH accounts for the remaining one third1,2 (Figures 1 and 2 ).
Figure 1.
Intracerebral hemorrhage (ICH) classification and frequency based on hematoma location. Deep, 35% to 70%; lobar, 15% to 30%; cerebellum, 5% to 10%; and brain stem, 5% to 10%.
Figure 2.
Ganglionic or deep (A) versus lobar (B) ICH. A, Supratentorial, intracerebral (intraparenchymal) hemorrhage which originated within the right thalamus and extends into the right lateral ventricle. Etiology hypertension. B, Supratentorial right hemisphere large right lobar hematoma with mass effect, cerebral edema, and midline shift. Heterogeneous hematoma suggestive of blood in different stages. Etiology cerebral amyloid angiopathy. ICH indicates intracerebral hemorrhage.
Epidemiology and Risk Factors
Intracerebral hemorrhage is twice as common as aneurysmal subarachnoid hemorrhage (SAH).3 The incidence of ICH is 12 to 15 cases per 100 000 individuals4 or about 40 000 cases per year in the United States.5 Incidence varies among populations,1 Japanese have the highest incidence (60 of 100 000),6 followed by African Americans (twice the incidence). This correlates with the population’s incidence of hypertension (HTN). Risk factors for ICH are shown in Table 1 .
Table 1.
Risk Factors for Intracerebral Hemorrhage (ICH)
| Hypertension3 |
| Cerebral amyloid angiopathy8,64–66 |
| Advanced age13 |
| Anticoagulation intensity13 |
| Leukoaraiosis or white matter disease97 |
| Prior stroke or ICH75–77 |
| Hematologic abnormalities14 |
| Chronic kidney disease25 |
| Trauma and falls |
| Aneurysm/vascular malformations |
| Alcohol consumption26 |
| Drug abuse27,28 |
| Low cholesterol34 |
Hypertension is by far the most common attributable risk factor3; it accelerates age-related “wear and tear” of cerebral arterioles at branch points.7 Cerebral amyloid angiopathy (CAA), a condition that increases with age, is the second most common risk factor. Cerebral amyloid angiopathy is an important cause of lobar ICH, especially in the elderly individuals.8,9 This condition results from amyloid protein deposition in cortical arterioles; such deposition is rare in the basal ganglia and brain stem (usual locations of HTN-related ICH and unusual locations of CAA-related ICH). Apolipoprotein E (ApoE) genotype plays an important role in the pathogenesis of CAA, but it is neither sensitive nor specific for the primary diagnosis of this condition. Recurrent lobar CAA-related ICH is relatively common.10–12
Age is also an important risk factor for ICH; the overall odds of suffering an ICH is highest at and after the age of 85.13
Hematologic abnormalities are associated with up to 8% of all ICH; these include anticoagulant-induced coagulopathy, use of antiplatelet agents, congenital and acquired factor deficiencies, thrombocytopenic/thrombocytopathic disorders, and lymphoproliferative disorders.14
Warfarin anticoagulation increases the risk of ICH 2 to 5 times, and the risk is directly related to anticoagulation intensity.15 Nevertheless, most warfarin-associated ICH cases occur during times when warfarin is in the therapeutic range (international normalized ratio [INR] 2.0-3.0).16,17
Dabigatran, an oral direct thrombin inhibitor, has recently been approved by the Food and Drug Administration ([FDA] October 2010)18 for the prevention of stroke and blood clots in patients with abnormal heart rhythm (atrial fibrillation). In a randomized trial (dabigatran vs warfarin in patients with atrial fibrillation, RELY),19–21 it was found that dabigatran was not inferior to adjusted dose warfarin for the prevention of cardioembolic stroke in the setting of atrial fibrillation, and it had less major bleeding complications than warfarin. There is no known antidote to reverse the anticoagulant effect of dabigatran.22
Leukoaraiosis or white matter ischemic disease (WMD) is a risk factor for ICH, probably because of its association with arterial HTN.23 The presence of WMD in ICH patients is associated with larger hematoma volume and hematoma expansion.24
Chronic kidney disease is independently associated with both ischemic and hemorrhagic stroke, though implications for prevention are still to be determined.25
Excessive use of alcohol increases the risk of ICH; the postulated mechanisms are coagulopathy and increased systolic blood pressure.26
Drug abuse is also important when dealing with spontaneous ICH, and it is an important risk factor in adolescents and young adults.27,28 Use of amphetamine, cocaine, heroin, and ecstasy has been linked to ICH. The presumed mechanisms include increase in blood pressure and/or cardiac arrhythmias with subsequent brain embolism as well as vasculitis, vasculopathy, and the reversible cerebral vasoconstriction syndrome (RCVS).29–31 Among cocaine users, ischemic stroke, and TIA related to large vessel atherosclerosis are the most common neurovascular events in former users, whereas ICH is more common in acute/current users.32 In a series of 96 cocaine users, neither vasospasm nor vasculitis was a common cause of stroke in this cohort.32
A less well-established risk factor is low serum cholesterol. The Heart Protection Study (HPS)33 and the High-dose Atorvastatin after Stroke of Transient Ischemic Attack (SPARCLE)34 trials reported higher rates of ICH in participants assigned to statin treatment. In addition, higher rates of ICH were noted in participants with lower cholesterol levels. Inference should be cautious, however, as a meta-analysis of “nonstroke” lipid-lowering trials failed to show similar results.35 Hence, increased ICH associated with statin therapy and cholesterol reduction may be limited to patients with vascular disease of the brain rather than those with coronary artery disease.
Cerebral reperfusion syndrome is a rare but serious complication of carotid revascularization, seen both after endarterectomy (CEA) and angioplasty/stenting (CAS); it is a uncommon cause of ICH. It is due to increased cerebral blood flow (compared to pre-revascularization levels) in combination with impaired cerebrovascular autoregulation. It may occur up to several weeks after revascularization but usually in the first few days. The highest risk period is at 12 hours post CAS and at 6 days post CEA. Deterioration of consciousness, confusion, and headache are the typical presenting symptoms. Risk factors for this syndrome include long-standing elevated blood pressure, diabetes, advanced age, contralateral carotid occlusion, recent contralateral carotid revascularization within 3 months, postoperative HTN, and use of anticoagulants. Treatment strategies are directed toward regulation of blood pressure and limitation of rises in cerebral perfusion.36
Pathophysiology
Nontraumatic bleeding into the brain parenchyma results from rupture of small penetrating arteries. In deep hematomas, this has been attributed to degenerative changes in the vessel wall associated with advancing age, chronic HTN, diabetes, and other vascular risk factors. Charcot-Bouchard microaneurysms and lipohyalinosis of small arterioles have been suggested as mechanisms.7 In some circumstances, degenerative changes of these arterioles may be associated with lacunar stroke and in other cases they may lead to ICH.7 Cause and effect are not always clear-cut because ischemic and hemorrhagic stroke are both age- and HTN-related. Rigorous pathological studies in the era of modern imaging with computerized tomography (CT) and magnetic resonance imaging (MRI) are lacking. When ICH does occur, most bleeding occurs at or near the bifurcation of affected arterioles.5
In lobar hematomas related to CAA, the underlying mechanism is a combination of vascular amyloid deposition and vessel wall breakdown. Affected vessels are capillaries, arterioles, and small-sized arteries, primarily in the cerebral cortex. Initially congophilic material accumulates between the media and the adventitia layers; subsequently, vascular amyloid extends throughout the media to replace the smooth muscle layer. Ultimately amyloid deposits lead to vasculopathic changes, including microaneurysms, concentric splitting of the vessel wall, chronic perivascular inflammation, and fibrinoid necrosis.37,38
Intracerebral hemorrhage is not a monophasic event that stops promptly. The hematoma continues to expand for up to 6 hours in noncoagulopathic ICH and up to 24 hours in coagulopathic ICH.16,39,40 Perihematomal edema peaks at 72 hours and usually persists for 5 days, although it has been reported for up to 2 weeks.41
Secondary neuronal injury occurs as a result of hematoma-triggered edema. Both vasogenic and cytotoxic edema lead to disruption of the blood–brain barrier, sodium-pump failure, and neuronal death.42
Controversy exists as to whether there are areas of ischemic tissue at risk around an ICH analogous to an ischemic penumbra. There is direct experimental evidence of ischemic tissue surrounding the hematoma,43,44 and radiologic support is also available.45–47 On the other hand, several clinical studies have not demonstrated the presence of perihematomal penumbra.48–51 This phenomenon seems to be present in some patients but not all. The challenge is to identify which patients have a penumbra area at risk and which do not.52 The challenge is not trivial as those with perihematomal ischemia seem to have poorer clinical outcomes.53
Clinical Presentation and Diagnosis
Patients with ICH usually experience stroke-like symptoms with an abrupt or sudden clinical onset, accompanied with focal neurological deficits. Large hematomas usually lead to a decreased level of consciousness as a result of increased intracranial pressure (ICP).5 It is clinically difficult to distinguish an ICH from an ischemic stroke at the bedside, but headache, nausea, vomiting, and depressed level of consciousness should raise the suspicion for a hemorrhagic event compared to ischemic stroke. Systemic blood pressure tends to be higher in ICH than in acute ischemic stroke.54 In about 25% of patients who are initially alert, deterioration in the level of consciousness occurs in the first 24 hours.
The medical history should include presenting symptoms, associated activities at onset, vascular risk factors (HTN, diabetes, dyslipidemia, chronic kidney disease, and smoking), history of both ischemic and hemorrhagic stroke, trauma, preexisting vascular malformations/aneurysms, neoplasm, use of alcohol, prescription medications (with special emphasis on anticoagulants and antithrombotics), recreational drugs, history of coagulopathy, or other conditions that predispose to bleeding (eg liver disease).55 Physical examination should include vital signs, level of consciousness (eg Glasgow Coma Scale [GCS], or Mayo Clinic FOUR score56), and severity of neurological deficit (National Institutes of Health Stroke Scale [NIHSS]). Routine laboratory studies should include complete blood count (CBC) with platelets, electrolytes and renal function, coagulation studies (prothrombin time [PT], partial thromboplastin time [PTT], and INR), toxicology screen, and pregnancy test in women of childbearing age.55
Neuroimaging studies are needed to make the diagnosis and elucidate the etiology of ICH. Computerized tomography is considered the gold standard. On CT, blood appears hyperdense relative to brain and similar to bone or contrast. Intracerebral bleeding is evident on noncontrast CT immediately after onset of symptoms. Hematomas can appear isodense in cases of severe anemia; fluid–fluid levels can be seen in hematomas related to coagulopathy. Small cerebellar or brain stem hematomas can be missed, and thin (<5 mm) posterior fossa cuts should be obtained on CT if clinical suspicion exists. Alternatively, MRI can be performed when considering posterior fossa disease. Computerized tomography and MRI perform equally well identifying acute ICH. Computerized tomography may be superior, demonstrating intraventricular and subarachnoid involvement, whereas MRI is better in detecting the underlying structural lesions (ie, neoplasms and vascular malformations). Time, cost, and patient tolerance preclude emergent MRI in most cases.57
The appearance of ICH on MRI depends primarily on the age of the hematoma and the type of MR sequence (ie, T1 or T2 weighted). As the hematoma ages, hemoglobin (Hb) goes through the following stages in the initial 7 days: oxy-Hb, deoxy-Hb, and met-Hb.58 The signal intensity on MR depends on the specific form of Hb present (Table 2 ).
Table 2.
Magnetic Resonance Appearance of Intracerebral Hemorrhagea
| Stage | Age | Hemoglobin | T1-MRI | T2-MRI |
|---|---|---|---|---|
| Hyperacute | <24 h | Intracellular oxy-Hb | Isointense | Slightly hyperintense |
| Acute | 1-3 d | Intracellular deoxy-Hb | Slightly hypointense | Very hypointense |
| Early subacute | >3 d | Intracellular met-Hb | Very hyperintense | Very hypointense |
| Late subacute | >7 d | Extracellular met-Hb | Very hyperintense | Very hyperintense |
| Chronic center | >14 d | Extracellular hemichromes | Isointense | Slightly hyperintense |
| Chronic rim | >14 d | Intracellular hemosiderin | Slightly hypointense | Very hypointense |
Abbreviations: H, hours; d, days; Hb, hemoglobin; MRI, magnetic resonance imaging.
a Adapted from Bradley WG.58
A “spot” sign on postcontrast CT is a small enhancing foci within the hematoma, related to vascular leak at the point of enhancement; the presence of the “spot” sign seems to independently predict hematoma enlargement.59
Between 1 and 6 weeks, hematomas become isodense with the brain parenchyma and are referred to as “subacute hematomas.”60 Unless rebleeding occurs, chronic hematomas (older than 6 weeks) are hypodense on CT. Residual radiologic findings after ICH include low attenuation, slit-like lesions, and calcifications. About 25% to 30% of ICH survivors have no residual abnormalities on CT.60
Estimating the volume of the hematoma is important as larger hematomas have a poorer prognosis.61 Intracerebral hematoma volume (cc or cm3) can be obtained by the “ABC/2” formula62 for spherical or ellipsoid ICH. A is the maximum ICH diameter (in cm) estimated visually; B is the maximum ICH diameter perpendicular to A (in cm), and C is the total number of CT slices with the ICH seen in the vertical plane multiplied by the CT slice thickness (typically 5 mm or 0.5 cm). A, B, and C numbers are then multiplied together and divided by 2.62
The information obtained from CT scan, along with the patients' age and medical history, (especially history of HTN) determines who needs further investigations to identify potential underlying structural pathology (ie, arterial-venous malformation (AVM), aneurysms, and tumors). Magnetic resonance can show flow voids in AVM, contrast enhancement in tumors, and diffuse microbleeds (MBs) in CAA. If the first MR scan is negative with an atypical hematoma location, a follow-up MRI study should be obtained in 4 to 6 weeks once the hematoma has been reabsorbed, as pathologic vessels or tumors can be missed in the presence of acute blood63 (Figure 3 ).
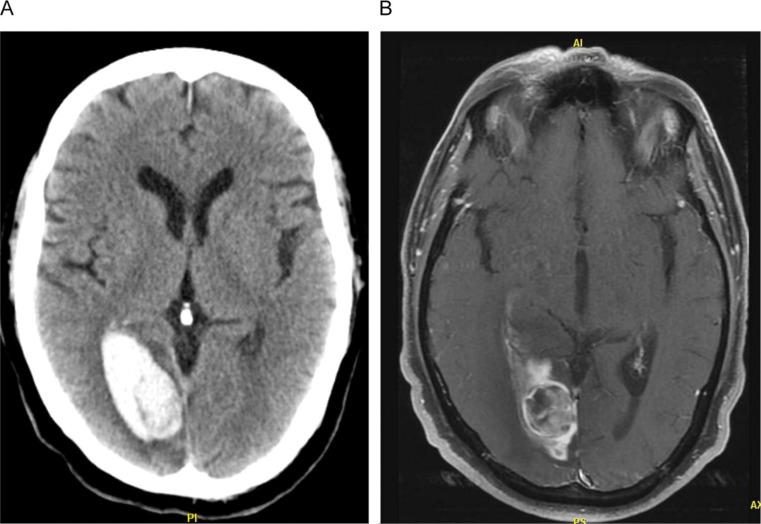
Figure 3.
Atypical ICH case (hemorrhage and re-hemorrhage, lobar location) of a 57-year-old male presenting with complaints of headache with no history of hypertension. On examination, complete left homonymous hemianopsia was found. Computerized tomography showed a lobar hematoma in the right occipital lobe (A). Initial MR and conventional angiography (data not shown) failed to reveal neoplastic process, radiologic signs of CAA, or vascular malformation, respectively. Owing to the atypical radiologic presentation, a follow-up MRI was obtained 10 weeks later, showing a new rounded lesion surrounded by extensive edema in the right occipital lobe (B). Biopsy confirmed the diagnosis of melanoma. ICH indicates intracerebral hemorrhage; MRI, magnetic resonance imaging; CAA, cerebral amyloid angiopathy.
Microbleeds or microhemorrhages are small areas (<10 mm) of ferritin and hemosiderin deposition seen as signal drop (profoundly hypointense) on T2 gradient echo (GRE) MR and are considered part of the spectrum of small vessel disease when located in the basal ganglia, brain stem and cerebellum, or a possible “footprint” of CAA when located in a lobar distribution64–66 (Figure 4 ).
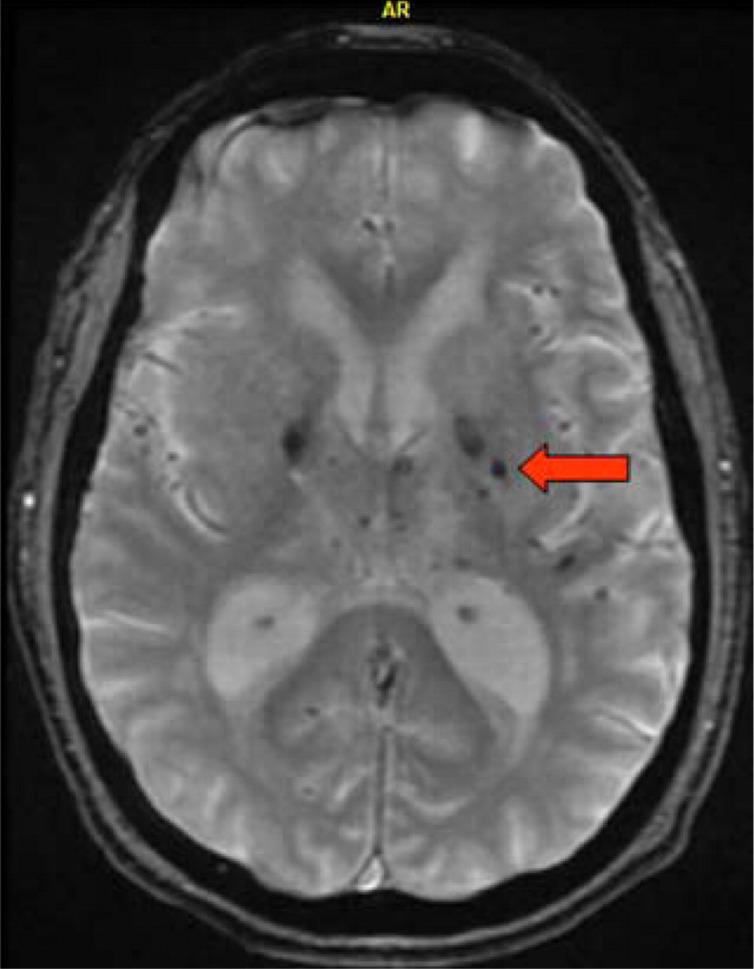
Figure 4.
Microbleeds gradient echo MRI showing multiple dark (hypointense) hemosiderin deposit within the basal ganglia bilaterally, as sometimes seen with chronic uncontrolled hypertension. Arrow pointing at microbleed located in the left glubus pallidus. MRI indicates magnetic resonance imaging.
Conventional catheter angiography is indicated in patients with SAH, ICH with abnormal calcifications, blood in atypical locations or presentation (eg, SAH in patients with headache or nonhypertensive participants with ganglionic hematomas), or in young patients with no obvious cause for ICH.67
Reversible cerebral vasoconstriction syndrome is a spectrum of disorders (ie, postpartum angiopathy, migranous vasospasm, and drug-induced arteritis) characterized by prolonged but reversible vasoconstriction associated with thunderclap headache. RCVS has been reported to occur in various clinical settings (ie postpartum and pregnancy, head trauma, and postcarotid endarterectomy) and in association with several pharmacologic agents (ie, selective serotonin reuptake inhibitors, triptans, tacrolimus, cyclophosphamyde, intravenous immunoglobulin, and bromocriptine, among others). Major ischemic or hemorrhagic stroke and even SAH have been described. Transient HTN is common. Although there are no validated criteria for the diagnosis of RCVS, the patient ideally should have all the following features: conventional catheter angiography, CT angiography (CTA), or MR angiography (MRA)-proven multifocal segmental cerebral vasoconstriction (Figure 5 ); normal or near normal cerebral spinal fluid ([CSF] normal glucose, <10 leukocytes, and protein <80 mg/dL); thunderclap headache with or without additional neurological signs or symptoms; reversibility of vasospasm within 12 weeks; no evidence of aneurysmal SAH. Vasculitis, atherosclerosis, and vasospasm from SAH should be considered in the differential.30 To date, the treatment of RCVS is based on anecdotal data as no clinical trials have been conducted. Identification and discontinuation of potential triggering agents is key (medications listed above). Alleviation of symptoms and rapid reversibility of vascular abnormalities has been achieved with calcium-channel blockers (administered orally, intravenously, and intra-arterially), brief courses of glucocorticoids, magnesium sulfate, and also by brief observation and supportive treatment (rest, hydration, and analgesic use). Stroke as an outcome, though, has been reported to be as high as 54%,68 and hence observation and supportive treatment alone are not an appropriate approach. Empirical therapy is not justified for patients presenting with thunderclap headache who have not had vascular imaging; once cerebral vasoconstriction is documented, treatment should be promptly initiated. Nimodipine and verapamil are considered first-line therapy.69 These agents should be used cautiously because of the risk of watershed infarction in the setting of cerebral vasoconstriction and systemic hypotension.
Figure 5.
Diffuse areas or segmental arterial vasospasm (arrows) seen on MR angiography, affecting anterior and posterior circulation. MR indicates magnetic resonance.
Prognosis
Intracerebral hemorrhage mortality is about 40% at 30 days, making ICH one of the most deadly acute medical events, similar to SAH in acute mortality.70 At 1 year, the mortality is 50%.61,71 Half of the deaths take place in the first 48 to 72 hours and are related to neurological complications (ie, mass effect, increased ICP, and/or herniation); deaths occurring after the first month are usually the result of medical complications (ie, pulmonary embolism, aspiration pneumonia, sepsis, and gastrointestinal bleeding). Many deaths also occur in the setting of withdrawal of support due to presumed poor prognosis.57
In the acute setting, predictors of 30-day mortality include hematoma size, hematoma expansion, older patient age, coma, intraventricular hemorrhage (IVH), and infratentorial location.61
These and other clinical characteristics have been used to develop a number of models to predict mortality and functional outcome. Features retained in most models include clinical deficit (determined by GCS and NIHSS scores), hematoma volume and location, the presence of IVH, and patient age.57 Current methods of early prognostication, though, are likely biased by failure to account for the influence of withdrawal of support and early do-not-resuscitate (DNR) orders. Aggressive full care immediately after ICH onset and postponement of new DNR orders for at least 48 hours is recommended by the most recent American Heart Association (AHA) guidelines.57
The ICH score by Hemphill72 (Table 3 ) is a simple and easy-to-use clinical grading scale to risk stratify patients with ICH at presentation. The score ranges from 0 to 6. Mortality for those with the lowest Hemphill score is 0, and for those with scores of 5 or higher mortality is 100%. For those who survive a spontaneous ICH, the annual risk of recurrence has been estimated to be in the 2% range, annually. The risk of any vascular event has been estimated to be approximately 6% and the risk of vascular death approximately 3%. Advanced age is strongly predictive of these 3 outcomes.71
Table 3.
Intracerebral Hemorrhage Score72
| Component | Points | |
|---|---|---|
| GCS score | 3-4 | 2 |
| 5-12 | 1 | |
| 13-15 | 0 | |
| ICH volume (cm3) | ≥30 | 1 |
| <30 | 0 | |
| IVH | Yes | 1 |
| No | 0 | |
| Infratentorial origin | Yes | 1 |
| No | 0 | |
| Age | ≥80 | 1 |
| <80 | 0 |
Abbreviations: ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; and IVH, intraventricular hemorrhage.
The risk of ICH recurrence is driven by the underlying etiology11,12,73,74; the most consistently identified risk factor for recurrent ICH is a lobar location of the initial bleed (ie, lobar ICH). Lobar bleeds (often caused by CAA) carry higher risk of re-bleeding than deep or ganglionic hematomas.12 Apolipoprotein E genotype might be clinically useful in assessing patient’s risk of recurrent hemorrhage after a lobar ICH.11
Hypertension is currently the most important modifiable risk factor for the prevention of ICH recurrence. After the acute period, a goal target blood pressure of <140/90 (or <130/80 if diabetic or with chronic kidney disease) is desirable.57
Prior ischemic infarct increases the relative risk of ICH by 5- to 20-fold.75–77 Prior ICH is a risk factor for stroke overall, with a risk of about 1.2% per year for occurrence of either recurrent ICH or ischemic stroke.77
Treatment
There is indirect evidence that aggressive medical management and specialist care improve outcome in patients with ICH. In-hospital mortality is lower for patients treated in a neurological intensive care unit.78
Hemostatic Therapy
The presence of coagulopathy (congenital or acquired) worsens the prognosis of ICH by increasing the rate of hematoma expansion and the duration of that expansion.79 Reversal of coagulopathy is of the essence, and specific antidotes should be used depending on the clinical scenario.16,80–82 Anticoagulant agents are discontinued immediately. In the case of warfarin, vitamin K, 10 mg, is administered slowly, intravenously, followed by either fresh frozen plasma (FFP) along with prothrombin complex concentrates (PCCs) or in some selected cases recombinant factor VIIa. The PCC dosage is calculated according to body weight, degree of INR prolongation, and desired level of correction; typical dosages are 25 to 50 IU/kg.83,84 Coagulopathy reversal is of the essence16 (Table 4 ).
Table 4.
Reversing Anticoagulation in Warfarin-Associated Intracerebral Hemorrhagea
| Intervention | Time to Anticoagulation Reversal | Comments |
|---|---|---|
| Vitamin K | 6-24 h | Risk of anaphylaxis with IV injection. Warfarin resistance for up to 1 week |
| FFP | 3-6 h for infusion, 12-32 h for reversal | Volume can be prohibitive |
| PCC | 10 min-1 h infusion. 15 min to reversal | Limited availability, potentially prothrombotic |
| Factor VIIa | 15 min after after bolus infusion | Short half-life, cost, prothrombotic |
Abbreviations: H, hours; min, minutes; IV, intravenous; FFP, fresh frozen plasma; PCCs, prothrombin complex concentrates.
a Adapted from Aguilar et al.16
For noncoagulopathic ICH, the use of procoagulant agents like recombinant factor VIIa (rFVIIa) demonstrated efficacy in reducing hematoma growth in a phase II trial85 but failed to demonstrate clinical improvement over placebo in a subsequent phase III trial.86 Based on these data, we do not advise rFVIIa use outside a clinical trial, unless it is for ICH associated to hemophilia with factor inhibitors, which is the drug’s FDA label indication (http://factorviia.com/pi.pdf).
Blood Pressure
The management of acute HTN in the setting of ICH is a medical emergency. However, the exact therapeutic blood pressure target remains one of the considerable controversies in the context of incomplete evidence.55 Two contradicting theories, yet unproven, illustrate this uncertainty. The first theory is that there is a region of perihematomal “penumbra” or brain tissue at risk that can become ischemic if the blood pressure is reduced precipitously.51 The second theory is that acute HTN actually causes worsening hematoma growth.87
Recent consensus guidelines57 call for a goal systolic blood pressure (SBP) of <160 mm Hg or a mean arterial pressure (MAP) below 110 mm Hg. If SBP >180, or MAP >130, and increased ICP are suspected on clinical grounds, ICP monitoring is recommended. Intravenous, short-acting medications used to treat acute HTN in patients with ICH include labetalol (bolus dose 5-20 mg every 15 minutes and infusion 2 mg/min), nicardipine (infusion 5-15 mg/h), esmolol (bolus 250 μg/kg, infusion 25-300 μg/kg/min), enalapril (1.25-5 mg every 6 hours, with 0.625 mg IV as initial dose), hydralazine (5-10 mg every 30 minutes, or infusion 1.5-5 μg/kg per min), nitroprusside (infusion 0.1-10 μg/kg per min), and nitroglycerin (infusion 20-400 μg/min).55
The 2010 AHA management guidelines consider “probably safe” acute lowering of SBP to 140 mm Hg.57 This recommendation is new since the prior 2007 guidelines.55
A phase I feasibility and safety clinical trial (ATACH)88 of intravenous nicardipine for acute HTN management in the setting of ICH has completed recruitment (n = 60). Preliminary results suggest that acute lowering of BP (to SBP 110-140 mm Hg) in the setting of acute ICH is safe. However, the ATACH I study was underpowered to detect the benefits in clinical outcomes or attenuated ICH growth. Accordingly, the ATACH-II trial is under way, evaluating nicardipine to treat acute HTN in the setting of ICH. End points include clinical outcomes and ICH growth. The INTERACT pilot trial89 is another study that investigated intensive blood pressure control (SBP, 140 mm Hg or less) compared to more liberal blood pressure (SBP, 180 mm Hg). The data suggested that the intensive blood pressure reduction group had reduced hematoma growth and that the intensive blood pressure reduction was well tolerated. However, the trial had limitations, namely relatively small hematomas which were primarily those of the deep or ganglionic type. Further validation of INTERACT data is needed.
The INTERACT 2 trial is ongoing and is expected to confirm (or not) the earlier study; 663 of the planned 2800 participants have been enrolled. INTERACT 2 will hopefully provide further insight into “how low one can safely go” in the management of blood pressure in acute ICH-infected patients (www.strokecenter.org).
Intracranial Pressure
The intracranial volume is fixed in adults due to the skull’s rigidity. Conditions that cause increasing volume (eg hematoma) eventually create a rise in ICP. In addition, when a new content or volume is introduced into the intracranial vault (eg, blood), there is compensatory decrease in the volume of one of the other intracranial constituents (cerebrospinal fluid, vascular volume, or brain parenchyma) until there is no further compliance. At that point, ICP rises rapidly in response to relatively small changes in volume. Cerebral perfusion pressure (CPP) is directly dependent on ICP, as it is determined by the difference between MAP and ICP (CPP = MAP − ICP). Intracranial pressure is measured using devices inserted into the skull, typically at the bedside. There are 2 types of devices: ventricular catheter systems (IVC) and parenchymal catheter systems. Intraventricular catheter systems also allow CSF drainage, which can help reduce ICP. Indirect data suggest higher risk of hemorrhage and infection with IVC compared to parenchymal catheter systems. Because of limited data regarding ICP in ICH, current management principles are based on traumatic brain injury guidelines.57
Treatment of elevated ICP begins with general measures such as elevation of the head of the bed to 30°, analgesia, and sedation. More aggressive therapies to lower ICP include osmotic diuresis (mannitol and hypertonic saline), CSF drainage via ventriculostomy, neuromuscular blockade, and transient hyperventilation. These later interventions require concomitant ICP and BP monitoring to reach a goal CPP >60 mm Hg and may be combined with surgical decompression.55,57
The AHA guidelines recommend ICP monitoring and treatment of patients with ICH with a GCS <8, those with clinical evidence of transtentorial herniation, or those with significant IVH or hydrocephalus.
Anticonvulsant Therapy
Early seizures occur in about 4% of ICH cases and in about 8% within 30 days.55,90 Lobar hematomas carry a higher risk of seizures than deep ICH.55,90 Clinical seizures in the setting of ICH should be treated with appropriate antiepileptic drugs. Initial medication choices include benzodiazepines, followed by fosphenytoin or phenytoin. The evidence to support routine prophylactic anticonvulsants in all patients with deep ICH is lacking but it is optional for lobar hematoma, given higher risk of seizures.55,57,90 Continuous electroencephalograph (EEG) monitoring should be considered for patients with ICH having depressed level of consciousness out of proportion to the degree of brain injury.57
Deep Vein Thrombosis Prophylaxis
Intermittent pneumatic sequential compression devices (SCDs) are indicated for patients with ICH for deep vein thrombosis (DVT) prophylaxis, assuming there is no history of DVT or leg fracture to contraindicate use. Once the intracranial bleeding has stopped and after 3 to 4 days from onset of the ICH, low-dose molecular weight heparin or unfractionated heparin may be used for DVT prevention in patients with hemiplegia.55,57
Neuroprotection
The CHANT trial failed to show an impact on mortality or outcomes in patients with ICH treated with NXY-059, despite good safety and tolerability.91 Further neuroprotective trials for ICH are needed.
Surgical Intervention
For most patients with ICH, the usefulness of surgery is uncertain.57 The role of surgical hematoma evacuation remains controversial55,90,92–94 for deep or ganglionic hematomas. In general, patients with profound coma (GSC < 4) are considered poor surgical candidates with dismal prognosis regardless of treatment modality. Deep and small hematomas (<10 cm3) are rarely evacuated based on prior trials.55,95 However, surgical ICH evacuation is indicated as early as possible for patients with posterior fossa hemorrhages larger than 3 cm in maximal diameter, who are deteriorating clinically, or who have brain stem compression or hydrocephalus from ventricular obstruction.55 The STICH trial95 showed no benefit of surgery over medical management in 1033 participants with primarily deep ICH across 83 centers. In subgroup analysis, patients with superficial hematomas (<1 cm from the cortex) seemed more likely to have a favorable outcome when managed surgically compared to deep ICH. This particular group of participants is the target population of the ongoing STICH II trail; 315 participants have been recruited out of 600 (www.strokecenter.org).
The usefulness of minimally invasive techniques to evacuate hematomas remains uncertain. Endoscopic evacuation93 and aspiration along with thrombolysis94 have been studied. The MISTIE trial, under way, is evaluating minimally invasive surgery along with clot lysis. Preliminary results appear promising,92 but the final results are not yet available. Preliminary results of the CLEAR IVH96 trial suggest that low-dose recombinant tissue plasminogen activator (rt-PA) can be safely administered to stable patients with IVH via IVC to help expedite IVH absorption rates. The follow-up multicenter CLEAR IVH III randomized trial is studying patients with relatively small ICH (<30 cc) with IVH blood occluding the third or lateral ventricles. Patients are treated using either 1 mg intraventicular tPA every 8 hours or placebo via IVC until there is significant IVH clearance, meeting a safety end point (re-hemorrhage) or 5 days (http://www.strokecenter.org/trials). The end points of this phase III study are clinical functional outcomes.
Conclusion
Intracerebral hemorrhage diagnosis and treatment have evolved over the past decade, in the setting of increasing knowledge about risk factors, pathophysiology, and management. Neuroimaging has advanced the field. Microbleeds detected by MRI may help predict underlying pathophysiology and help determine prognosis. Wider appreciation of hematoma growth and the utility of contrast-enhanced CT “spot sign” have increased the value of CT. Primary management of ICH involves rapid clinical evaluation, correction of any coagulation defects, admission to an ICU setting, and careful control of blood pressure. More specific blood pressure targets are being evaluated within the ATACH II and INTERACT 2 trials with the hopes of further reducing morbidity and mortality. The role of surgical hematoma evacuation is uncertain and likely not beneficial for most of the patients with ICH. Studies addressing minimally invasive surgical management for ICH and IVH are underway. The studies may answer whether these techniques offer more than medical management for patients with ICH. Further trials are needed to determine the best measures to halt early ICH growth, minimize cerebral edema, and attenuate the toxic effects of blood products.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Flaherty ML, Woo D, Haverbusch M, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36(5):934–937 [DOI] [PubMed] [Google Scholar]
- 2. Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871–895 [DOI] [PubMed] [Google Scholar]
- 3. Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30(4):905–915 [DOI] [PubMed] [Google Scholar]
- 4. Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin. 2000;18(2):419–438 [DOI] [PubMed] [Google Scholar]
- 5. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. [see comment]. N Engl J Med. 2001;344(19):1450–1460 [DOI] [PubMed] [Google Scholar]
- 6. Inagawa T, Ohbayashi N, Takechi A, Shibukawa M, Yahara K. Primary intracerebral hemorrhage in Izumo City, Japan: incidence rates and outcome in relation to the site of hemorrhage. Neurosurgery. 2003;53(6):1283–1297; discussion 1297-1288 [DOI] [PubMed] [Google Scholar]
- 7. Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J NeuropatholExp Neurol. 1971;30(3):536–550 [DOI] [PubMed] [Google Scholar]
- 8. Feldmann E, Tornabene J. Diagnosis and treatment of cerebral amyloid angiopathy. Clin Geriatr Med. 1991;7(3):617–630 [PubMed] [Google Scholar]
- 9. Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci. 1993;116(2):135–141 [DOI] [PubMed] [Google Scholar]
- 10. Neau JP, Ingrand P, Couderq C, et al. Recurrent intracerebral hemorrhage. Neurology. 1997;49(1):106–113 [DOI] [PubMed] [Google Scholar]
- 11. O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342(4):240–245 [DOI] [PubMed] [Google Scholar]
- 12. Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke. 1995;26(7):1189–1192 [DOI] [PubMed] [Google Scholar]
- 13. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation [see comment] [summary for patients in. Ann Intern Med. 2004;141(10):I38; PMID: 15545670] Ann Intern Med. 2004;141(10): 745–752 [DOI] [PubMed] [Google Scholar]
- 14. del Zoppo GJ, Mori E. Hematologic causes of intracerebral hemorrhage and their treatment. Neurosurg Clin N Am. 1992;3(3):637–658 [PubMed] [Google Scholar]
- 15. Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26(8):1471–1477 [DOI] [PubMed] [Google Scholar]
- 16. Aguilar MI, Hart RG, Kase CS, et al. Treatment of Warfarin-associated intracerebral hemorrhage: litearure review and expert opinion. Mayo Clin Proc. 2007;82(1):82–92 [DOI] [PubMed] [Google Scholar]
- 17. Neau JP, Couderq C, Ingrand P, Blanchon P, Gil R. VGP Study Group Intracranial hemorrhage and oral anticoagulant treatment. Cerebrovasc Dis. 2001;11(3):195–200 [DOI] [PubMed] [Google Scholar]
- 18. Walsh S. FDA Approves Pradaxa to Prevent Stroke in People With Atrial Fibrillation. FDA;2010. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm [Google Scholar]
- 19. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157(5):805–810 [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975–983 [DOI] [PubMed] [Google Scholar]
- 21. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151 [DOI] [PubMed] [Google Scholar]
- 22. Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(suppl 1):9S–16S [DOI] [PubMed] [Google Scholar]
- 23. Inzitari D, Giordano GP, Ancona AL, Pracucci G, Mascalchi M, Amaducci L. Leukoaraiosis, intracerebral hemorrhage, and arterial hypertension. Stroke. 1990;21(10):1419–1423 [DOI] [PubMed] [Google Scholar]
- 24. Lou M, Al-Hazzani A, Goddeau RP, Jr, Novak V, Selim M. Relationship between white-matter hyperintensities and hematoma volume and growth in patients with intracerebral hemorrhage. Stroke. 2010;41(1):34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aguilar MI, O’Meara ES, Seliger S, et al. Albuminuria and the risk of incident stroke and stroke types in older adults. Neurology. 2010;75(15):1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorelick PB. Alcohol and stroke. Stroke. 1987;18(1):268–271 [DOI] [PubMed] [Google Scholar]
- 27. McEvoy AW, Kitchen ND, Thomas DG. Intracerebral haemorrhage and drug abuse in young adults. Br J Neurosurg. 2000;14(5):449–454 [DOI] [PubMed] [Google Scholar]
- 28. McEvoy AW, Kitchen ND, Thomas DG. Lesson of the week: intracerebral haemorrhage in young adults: the emerging importance of drug misuse. BMJ. 2000;320(7245): 1322–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aggarwal SK, Williams V, Levine SR, Cassin BJ, Garcia JH. Cocaine-associated intracranial hemorrhage: absence of vasculitis in 14 cases. Neurology. 1996;46(6):1741–1743 [DOI] [PubMed] [Google Scholar]
- 30. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–44 [DOI] [PubMed] [Google Scholar]
- 31. Nolte KB, Brass LM, Fletterick CF. Intracranial hemorrhage associated with cocaine abuse: a prospective autopsy study. Neurology. 1996;46(5):1291–1296 [DOI] [PubMed] [Google Scholar]
- 32. Toossi S, Hess CP, Hills NK, Josephson SA. Neurovascular complications of cocaine use at a tertiary stroke center. J Stroke Cerebrovasc Dis. 19(4):273–278 [DOI] [PubMed] [Google Scholar]
- 33. Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative G Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions [see comment]. Lancet. 2004;363(9411):757–767 [DOI] [PubMed] [Google Scholar]
- 34. Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack [see comment]. N Engl J Med. 2006;355(6):549–559 [DOI] [PubMed] [Google Scholar]
- 35. Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35(12): 2902–2909 [DOI] [PubMed] [Google Scholar]
- 36. Moulakakis KG, Mylonas SN, Sfyroeras GS, Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009;49(4):1060–1068 [DOI] [PubMed] [Google Scholar]
- 37. Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45(1):79–90 [PubMed] [Google Scholar]
- 38. Vinters HV, Natté R, Maat-Schieman ML, et al. Secondary microvascular degeneration in amyloid angiopathy of patients with hereditary cerebral hemorrhage with amyloidosis, Dutch type (HCHWA-D). Acta Neuropathol. 1998;95(3):235–244 [DOI] [PubMed] [Google Scholar]
- 39. Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27(10):1783–1787 [DOI] [PubMed] [Google Scholar]
- 40. Yasaka M, Minematsu K, Naritomi H, Sakata T, Yamaguchi T. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89(2):278–283 [PubMed] [Google Scholar]
- 41. Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30(6):1167–1173 [DOI] [PubMed] [Google Scholar]
- 42. Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88(6):1058–1065 [DOI] [PubMed] [Google Scholar]
- 43. Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: description of technique, ICP changes and neuropathological findings. Neurol Res. 1984;6(4):184–188 [DOI] [PubMed] [Google Scholar]
- 44. Mendelow AD, Bullock R, Teasdale GM, Graham DI, McCulloch J. Intracranial haemorrhage induced at arterial pressure in the rat. Part 2: short term changes in local cerebral blood flow measured by autoradiography. Neurol Res. 1984;6(4):189–193 [DOI] [PubMed] [Google Scholar]
- 45. Rosand J, Eskey C, Chang Y, Gonzalez RG, Greenberg SM, Koroshetz WJ. Dynamic single-section CT demonstrates reduced cerebral blood flow in acute intracerebral hemorrhage. Cerebrovas Dis. 2002;14(3-4):214–220 [DOI] [PubMed] [Google Scholar]
- 46. Siddique MS, Fernandes HM, Wooldridge TD, Fenwick JD, Slomka P, Mendelow AD. Reversible ischemia around intracerebral hemorrhage: a single-photon emission computerized tomography study. J Neurosurg. 2002;96(4):736–741 [DOI] [PubMed] [Google Scholar]
- 47. Uemura K, Shishido F, Higano S, et al. Positron emission tomography in patients with a primary intracerebral hematoma. Acta Radiol Suppl. 1986;369:426–428 [PubMed] [Google Scholar]
- 48. Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57(1):18–24 [DOI] [PubMed] [Google Scholar]
- 49. Videen TO, Dunford-Shore JE, Diringer MN, Powers WJ. Correction for partial volume effects in regional blood flow measurements adjacent to hematomas in humans with intracerebral hemorrhage: implementation and validation. J Comput Assist Tomogr. 1999;23(2):248–256 [DOI] [PubMed] [Google Scholar]
- 50. Zazulia AR, Diringer MN, Videen TO, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21(7):804–810 [DOI] [PubMed] [Google Scholar]
- 51. Schellinger PD, Fiebach JB, Hoffmann K, et al. Stroke MRI in Intracerebral hemorrhage: is there a perihemorrhagic penumbra?. Stroke. 2003;34(7):1674–1679 [DOI] [PubMed] [Google Scholar]
- 52. Tayal AH, Gupta R, Yonas H, et al. Quantitative perihematomal blood flow in spontaneous intracerebral hemorrhage predicts in-hospital functional outcome. Stroke. 2007;38(2):319–324 [DOI] [PubMed] [Google Scholar]
- 53. Kidwell CS, Saver JL, Mattiello J, et al. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology. 2001;57(9):1611–1617 [DOI] [PubMed] [Google Scholar]
- 54. Arboix A, Comes E, Garcia-Eroles L, et al. Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta Neurol Scand. 2002;105(4):282–288 [DOI] [PubMed] [Google Scholar]
- 55. Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007; 38(6):2001–2023 [DOI] [PubMed] [Google Scholar]
- 56. Iyer VN, Mandrekar JN, Danielson RD, Zubkov AY, Elmer JL, Wijdicks EF. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. 2009;84(8):694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morgenstern LB, Hemphill 3rd JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bradley Jr WG. MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26 [DOI] [PubMed] [Google Scholar]
- 59. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38(4):1257–1262 [DOI] [PubMed] [Google Scholar]
- 60. Kreel L, Kay R, Woo J, Wong HY, Nicholls MG. The radiological (CT) and clinical sequelae of primary intracerebral haemorrhage. Br J Radiol. 1991;64(768):1096–1100 [DOI] [PubMed] [Google Scholar]
- 61. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality [see comment]. Stroke. 1993;24(7):987–993 [DOI] [PubMed] [Google Scholar]
- 62. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305 [DOI] [PubMed] [Google Scholar]
- 63. Ronning P, Sorteberg W, Nakstad P, Russell D, Helseth E. Aspects of intracerebral hematomas—an update. Acta Neurol Scand. 2008;118(6):347–361 [DOI] [PubMed] [Google Scholar]
- 64. Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20(4):637–642 [PMC free article] [PubMed] [Google Scholar]
- 65. Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2000;31(11): 2665–2669 [DOI] [PubMed] [Google Scholar]
- 66. Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52(5):991–994 [DOI] [PubMed] [Google Scholar]
- 67. Aguilar MI, Demaerschalk BM. Intracerebral hemorrhage. Semin Neurol. 2007;27(4):376–384 [DOI] [PubMed] [Google Scholar]
- 68. Singhal AB. Cerebral vasoconstriction without subarachnoid blood: associated conditions, clinical and neuroimaging characteristics. Ann Neurol. 2002;52(3S):S58–S63 [Google Scholar]
- 69. Dodick DW. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists. Cephalalgia. 2003;23(3):163–165 [DOI] [PubMed] [Google Scholar]
- 70. Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326(11): 733–736 [DOI] [PubMed] [Google Scholar]
- 71. Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJ. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology. 2002;59(2):205–209 [DOI] [PubMed] [Google Scholar]
- 72. Hemphill 3rd JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage [see comment]. Stroke. 2001;32(4):891–897 [DOI] [PubMed] [Google Scholar]
- 73. Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31(1):123–127 [DOI] [PubMed] [Google Scholar]
- 74. Bae H, Jeong D, Doh J, Lee K, Yun I, Byun B. Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis. 1999;9(2):102–108 [DOI] [PubMed] [Google Scholar]
- 75. Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986;17(6):1078–1083 [DOI] [PubMed] [Google Scholar]
- 76. Okada H, Horibe H, Yoshiyuki O, Hayakawa N, Aoki N. A prospective study of cerebrovascular disease in Japanese rural communities, Akabane and Asahi. Part 1: evaluation of risk factors in the occurrence of cerebral hemorrhage and thrombosis. Stroke. 1976;7(6):599–607 [DOI] [PubMed] [Google Scholar]
- 77. Hanger HC, Wilkinson TJ, Fayez-Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol, Neurosurg Psychiatry. 2007;78(8):836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage [see comment]. Crit Care Med. 2001;29(3):635–640 [DOI] [PubMed] [Google Scholar]
- 79. Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63(6):1059–1064 [DOI] [PubMed] [Google Scholar]
- 80. Flaherty ML, Haverbusch M, Sekar P, et al. Location and outcome of anticoagulant-associated intracerebral hemorrhage. Neurocrit Care. 2006;5(3):197–201 [DOI] [PubMed] [Google Scholar]
- 81. Flaherty ML, Haverbusch M, Sekar P, et al. The Increasing burden of anticoagulant-associated intracerebral hemorrhage. Stroke. 2006;37(2):623 [Google Scholar]
- 82. Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68(2):116–121 [DOI] [PubMed] [Google Scholar]
- 83. Hellstern P. Production and composition of prothrombin complex concentrates: correlation between composition and therapeutic efficiency. Thromb Res. 1999;95(4 suppl 1):S7–S12 [DOI] [PubMed] [Google Scholar]
- 84. Hellstern P, Halbmayer WM, Kohler M, Seitz R, Muller-Berghaus G. Prothrombin complex concentrates: indications, contraindications, and risks: a task force summary. Thromb Res. 1999;95(4 suppl 1):S3–S6 [DOI] [PubMed] [Google Scholar]
- 85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777–785 [DOI] [PubMed] [Google Scholar]
- 86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage [comment]. N Engl J Med. 2008;358(20):2127–2137 [DOI] [PubMed] [Google Scholar]
- 87. Jauch EC, Lindsell CJ, Adeoye O, et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke. 2006;37(8):2061–2065 [DOI] [PubMed] [Google Scholar]
- 88. Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6(1):56–66 [DOI] [PubMed] [Google Scholar]
- 89. Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391–399 [DOI] [PubMed] [Google Scholar]
- 90. Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43(10):1175–1180 [DOI] [PubMed] [Google Scholar]
- 91. Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38(8):2262–2269 [DOI] [PubMed] [Google Scholar]
- 92. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147–151 [DOI] [PubMed] [Google Scholar]
- 93. Nishihara T, Nagata K, Tanaka S, et al. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. 2005;2(1):67–74 [DOI] [PubMed] [Google Scholar]
- 94. Vespa P, McArthur D, Miller C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2(3): 274–281 [DOI] [PubMed] [Google Scholar]
- 95. Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial [see comment]. Lancet. 2005;365(9457): 387–397 [DOI] [PubMed] [Google Scholar]
- 96. Hanley D. Final results of CLEAR IVH trial: clot lysis, safety, and functional outcomes. Cerebrovasc Dis. 2008;25(suppl 2):9 [Google Scholar]
- 97. Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53(6): 1319–1327 [DOI] [PubMed] [Google Scholar]