Repair in the peripheral nervous system (PNS) is a complex process that requires the active de-differentiation of Schwann cells to an immature repair state following injury; this de-differentiation is dependent upon the activity of the p38 and ERK1/2 mitogen-activated protein kinases, as well as the transcription factor cJun. Understanding the mechanisms of repair raises the possibility of both boosting repair after PNS trauma and even, possibly, blocking the inappropriate demyelination seen in some disorders of the peripheral nervous system.
Summary
Repair in the peripheral nervous system (PNS) depends upon the plasticity of the myelinating cells, Schwann cells, and their ability to dedifferentiate, direct axonal regrowth, remyelinate, and allow functional recovery. The ability of such an exquisitely specialized myelinating cell to revert to an immature dedifferentiated cell that can direct repair is remarkable, making Schwann cells one of the very few regenerative cell types in our bodies. However, the idea that the PNS always repairs after injury, in contrast to the central nervous system, is not true. Repair in patients after nerve trauma can be incredibly variable, depending on the site and type of injury, and only a relatively small number of axons may fully regrow and reinnervate their targets. Recent research has shown that it is an active process that drives Schwann cells back to an immature state after injury and that this requires activity of the p38 and extracellular-regulated kinase 1/2 mitogen-activated protein kinases, as well as the transcription factor cJun. Analysis of the events after peripheral nerve transection has shown how signaling from nerve fibroblasts forms Schwann cells into cords in the newly generated nerve bridge, via Sox2 induction, to allow the regenerating axons to cross the gap. Understanding these pathways and identifying additional mechanisms involved in these processes raises the possibility of both boosting repair after PNS trauma and even, possibly, blocking the inappropriate demyelination seen in some disorders of the peripheral nervous system.
Introduction
Schwann cells are the glial cells of the peripheral nervous system (PNS) and are made up of the myelinating Schwann cells that myelinate large-diameter axons and nonmyelinating Schwann cells that envelop and support small diameter sensory axons. In addition to their ability to form the complex structures of myelin vital for rapid saltatory conduction, Schwann cells have impressive regenerative properties, permitting functional repair of the PNS following injury, which make them almost unique within our bodies. In this review, we will discuss the recent advances that have identified some of the mechanisms underlying these regenerative abilities.
During development of the PNS, Schwann cells differentiate into highly specialized myelinating and nonmyelinating cells, yet even in adult animals, when the nerve is damaged they maintain the ability to revert back to a nondifferentiated, proliferative phenotype. This injury-induced cell plasticity has recently been proposed as a transdifferentiation that generates a specialized repair cell, also termed a Büngner cell, which can be distinguished from the Schwann cells found in the developing nerve. These repair cells guide regrowth of the injured axons and eventually remyelinate them to allow functional recovery of the damaged nerve [1]. They also promote breakdown of the blood-nerve barrier and the recruitment of macrophages to the site of injury to clear myelin debris. The molecular mechanisms that govern the regenerative properties of the Schwann cell are not fully understood; however, recent studies using transgenic mouse technology have identified molecular components involved in the process. It is now clear that adult Schwann cell plasticity is regulated by a complex array of signaling pathways and transcription factors that are activated within Schwann cells in response to injury. In this review, we will look at recent advances made in the field, which identify the extracellular-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK) pathways, and transcription factors cJun and Sox2 as regulators of Schwann cell plasticity and PNS repair. We will also discuss possible therapeutic strategies targeting these molecular components for improving peripheral nerve regeneration and repair.
Roles of the MAPK Pathways in Regulating Schwann Cell Plasticity and the Injury Response
Mechanical insult to the peripheral nerve initiates a cascade of molecular events in the distal nerve stump that results in myelin degeneration followed by dedifferentiation and proliferation of the Schwann cells. The Schwann cell injury response is accompanied by rapid and sustained activation of the ERK1/2 and p38 MAPK pathways within the Schwann cells of the distal stump [2–5]. Using both gain-of-function and loss-of-function strategies in vivo, recent studies have described the essential roles of the MAPK pathways in mediating the Schwann cell injury response.
The ERK1/2 Pathway
ERK1/2 is activated in the distal stump within a few minutes after injury to peripheral nerves [2, 6]. Inhibition of this kinase activity using a pharmacological inhibitor blocked injury-induced Schwann cell dedifferentiation and delayed downregulation of the myelin proteins [2, 7]. The importance of this pathway was shown in elegant experiments using a tamoxifen-inducible transgenic mouse model in which Raf-1, an ERK1/2 activator, was ectopically expressed in adult Schwann cells. The study by Napoli et al. [7] demonstrated that the ectopic ERK1/2 activation was sufficient to trigger myelin breakdown, dedifferentiation, and proliferation of Schwann cells in the absence of nerve injury. Furthermore, the study also showed that the Schwann cell-specific ERK1/2 activation was sufficient to induce other responses associated with nerve injury and repair, including breakdown of the blood-nerve barrier and recruitment of inflammatory cells into the nerve [7]. This study strongly indicates that the Schwann cell ERK1/2 activation serves as the signal that initiates the Schwann cell injury response and places the Schwann cell as the key orchestrator of the repair process in the adult PNS. It also suggests a key role of the ERK1/2 pathway in regulating adult Schwann cell plasticity.
The in vivo injury-responsive signal that activates ERK1/2 in Schwann cells remains unknown. One of the possible upstream activators is the ErbB2 tyrosine kinase receptor, the activity of which is closely correlated with Ras/Raf/ERK pathway activation in Schwann cells. Supporting this, it has been shown that ErbB2 is activated within distal Schwann cells soon after injury and inhibition of the receptor activity blocks the initial Schwann cell injury response, including myelin breakdown and proliferation [6]. In an interesting parallel to this, activation of ErbB2 and signaling through ERK1/2 has also been shown to be a mechanism by which Mycobacterium leprae, the bacterium that causes leprosy in humans, induces Schwann cell demyelination and dedifferentiation [8].
Another possible upstream activator of ERK1/2 is the Notch receptor on the Schwann cell surface. Signaling through the Notch pathway in Schwann cells has been linked to the breakdown of myelin after PNS injury in vivo, and Notch activity has also been shown to increase activity of ERK1/2, as well as p38 MAPK in Schwann cells [9]. The relative contributions of ErbB2 or Notch signaling to MAPK activation and function following injury, and how they regulate Schwann cell plasticity, remain to be determined.
The p38 MAPK Pathway
Activity of p38 MAPK increases in Schwann cells of the distal stump after nerve injury. A recent study has shown that inhibition of the injury-induced kinase activation preserves the distal myelin despite the ongoing axon degeneration after sciatic nerve transection. Inhibition of p38 MAPK activity also attenuates dedifferentiation of denervated Schwann cells in culture, whereas ectopic activation is sufficient to drive Schwann cell dedifferentiation in vitro [3].
Therefore, it seems clear that both ERK1/2 and p38 MAPK pathways play similar roles in promoting Schwann cell plasticity, yet it is unclear whether the two pathways share a common injury-responsive signal for their activation within the same pathway or function in parallel to promote the Schwann cell injury response. In cultured Schwann cells, neuregulin 1, a ligand for ErbB receptors, has been shown to activate both pathways [3, 10].
The MAPK Regulation of Myelination
In light of their roles in promoting Schwann cell dedifferentiation, perhaps it is not surprising that both ERK1/2 and p38 MAPK pathways have been shown to function as negative regulators of Schwann cell differentiation and myelination. Inhibition of ERK1/2 or p38 MAPK enhances Schwann cell myelination in culture; conversely, ectopic activation of p38 MAPK blocks cyclic AMP-induced Schwann cell differentiation and myelin gene expression in vitro [3, 10]. In the inducible transgenic mouse model mentioned above, withdrawal of tamoxifen following ERK1/2-driven Schwann cell demyelination and dedifferentiation allows the Schwann cells to redifferentiate and remyelinate, indicating that downregulation of the ectopic ERK1/2 activity is necessary to initiate Schwann cell myelination. However, ERK1/2 may not always function as a negative regulator of myelination. Recent in vivo studies have shown that ablation of ERK1 and ERK2 in embryonic Schwann cells causes myelination defects, and increasing the Schwann cell ERK1/2 activity during development causes hypermyelination in the PNS [11–13]. These findings suggest that ERK1/2 and possibly the p38 MAPK pathway(s) may play different roles during developmental myelination and remyelination. Although the kinase activities are required for embryonic Schwann cell differentiation and myelination, they are inhibitory during remyelination in the adult PNS. It is also possible that the strength and duration of the kinase activities determine the response of the Schwann cell. The short-term transient activation of the MAPK pathways during development is required during Schwann cell differentiation, whereas the strong sustained signaling following nerve injury triggers the dedifferentiation response of Schwann cells and inhibits remyelination. Certainly there is support for a signal dosage-dependent mechanism that regulates Schwann cell myelination. In Schwann cell-dorsal root ganglion (DRG) cocultures, low doses of neuregulin 1 promote myelination, whereas at high concentrations it inhibits myelination and induces Schwann cell myelin breakdown [10].
A temporal requirement of p38 MAPK during Schwann cell myelination has been suggested as well. In Schwann cell-DRG neuron cocultures, although inhibition of p38 MAPK activity at the time of initiating myelination blocks the subsequent myelin formation, a delayed addition of the inhibitor once myelination has begun actually promotes the process [3, 14]. The effect of the p38 MAPK block during the early stages was shown to be associated with impaired basal lamina formation, a process that is a prerequisite for myelination. Once the basal lamina is generated and myelination is proceeding, inhibition of p38 MAPK promotes the subsequent process of myelination [3].
The Transcription Factor cJun Promotes the Generation of a Repair-Competent Schwann Cell or Büngner Cell After Injury
It has been known for some time that the AP-1 transcription factor cJun is upregulated after injury in both myelinating and nonmyelinating Schwann cells that are removed from axonal contact. Although cJun has been shown to play a significant role in the regeneration of neurons [15], it was not until recently that the role of cJun in Schwann cells was assessed in Wallerian degeneration and functional repair of peripheral nerves after injury. Conditional deletion of cJun in the Schwann cell lineage does not seemingly affect development or myelination of peripheral nerves [1, 16]. However, a striking phenotype is seen in Schwann cells in adult nerves following injury. Schwann cells lacking cJun fail to downregulate myelin proteins and take on the repair cell state after losing contact with axons. Analysis of cJun-regulated genes show that neurotrophins such as glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor, and Artemin are all reduced in cJun-null nerves after injury. Loss of the neurotrophin expression is thought to lead to increased death of sensory neurons and to a decrease in reinnervation and functional recovery after nerve crush [1]. Genetic ablation of Ret, the receptor for GDNF and Artemin, in neurons produced regeneration defects similar to those observed with cJun loss in Schwann cells after injury. Correspondingly, administration of recombinant GDNF and Artemin into nerves containing cJun-null Schwann cells partially restores the regeneration defects seen in these nerves after PNS injury [17]. The catastrophic lack of repair in cJun-null nerves has led to the idea that the dedifferentiation of Schwann cells after injury leads to the appearance of specialized Schwann cells, termed Büngner cells, which depend upon cJun for their generation [1]. These cells differ in their transcriptional profile from immature Schwann cells prior to myelination and function specifically to produce both the environment and neurotrophic support necessary for effective axonal regeneration and repair of the adult nerve.
The Transcription Factor Sox2 and PNS Repair
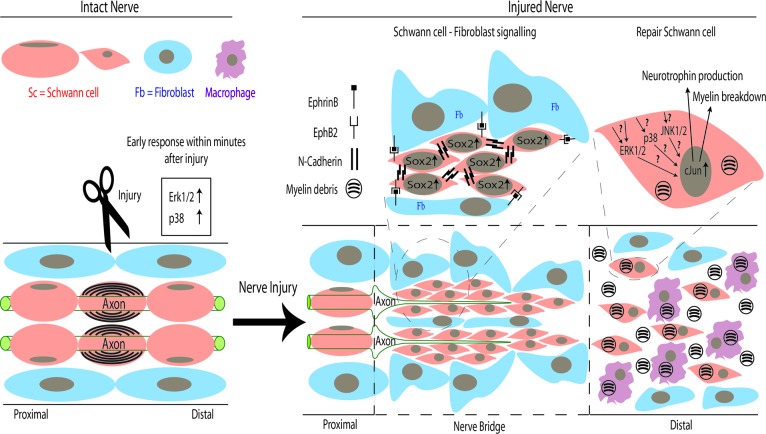
Sox2 is one of the group of transcription factors that induce pluripotent stem cells from adult somatic cells [18]. During development, Sox2 is expressed in immature Schwann cells and the expression decreases as the Schwann cell begin to differentiate and form myelin. In adult peripheral nerves, Sox2 expression is reinduced in the Schwann cell following nerve injury [16, 19]. It is unknown whether the injury-induced Sox2 expression plays a role in promoting adult Schwann cell plasticity. However, a recent study has proposed an alternative role of Sox2 in Schwann cells that is necessary for the PNS repair after nerve transection. Unlike nerve crush injury, after which the gross structure of the nerve and the Schwann cell basal lamina are maintained, thus allowing axons to regenerate across the site of injury, nerve transection poses much more of a problem because of the complete disconnection, which retracts the ends of the severed nerve (Fig. 1).
Figure 1.
Schematic of repair processes following peripheral nerve transection. In intact mature nerve, differentiated Schwann cells myelinate the large-diameter axons. Following transection injury, ERK1/2 and p38 mitogen-activated protein kinase signaling rapidly increases within Schwann cells distal to the site of injury and causes them to proliferate and take on an undifferentiated “repair” or Büngner cell phenotype. In the distal stump of the nerve, this transdifferentiation of Schwann cells is dependent upon the transcription factor cJun, which directs both the breakdown of myelin and the production of neurotrophins, such as glial-derived neurotrophic factor, brain-derived neurotrophic factor, and Artemin [1]. Following nerve transection, Schwann cells and nerve fibroblasts proliferate and migrate from proximal and distal nerve stumps to form a nerve bridge. Within this bridge, EphrinB-EphB2 signaling between fibroblasts and Schwann cells results in elevation of Sox2, which causes relocalization of N-cadherin to the surface of the Schwann cells. The resulting sorting of Schwann cells into cords in the bridge allows regenerating axons to cross the nerve bridge and reach the distal stump of the nerve [20]. Abbreviations: Erk1/2, extracellular-regulated kinase 1/2; Fb, fibroblast; Sc, Schwann cell.
In order to repair, a “nerve bridge” must be formed across the gap to guide growth of the proximal axons into the distal stump. Parrinello et al. have shown that this process is mediated by EphrinB-EphB2 signaling between Schwann cells and nerve fibroblast that initiates Sox2-dependent Schwann cell sorting, via relocalization of N-Cadherin, and collective migration out of the transected nerve stumps [20]. This finding highlights the importance of Sox2 in promoting the nerve repair function of adult Schwann cells (Fig. 1).
Conclusion
The regenerative potential of the adult peripheral nervous system is impressive, and recent work has clearly placed Schwann cells at the center of coordinating the cellular environment to clear myelin debris, encourage axonal regrowth, and allow functional repair. Certainly we now know more about some of the key players in this cell plasticity, but how these MAPKs and transcription factors such as cJun and Sox2 collaborate to regulate these distinct stages of PNS repair is still a mystery. To give one example, more than 4,000 genes are regulated in Schwann cells after peripheral nerve injury but only 172 of these are affected by loss of cJun [1]. Similarly, although ectopic activation of ERK1/2 induces cJun mRNA in Schwann cells, it also activates a host of other genes [7]. The key experiment to determine whether the effect of sustained ERK1/2 or p38 MAPK signaling that induces adult Schwann cell dedifferentiation is cJun-dependent remains to be carried out in order to determine how, if at all, these pathways interact. One additional area of research that has emerged recently are the new roles for axon guidance molecules, such as Netrin-1 and its receptors Uncoordinated 5H2 (Unc5H2) and Deleted in Colorectal Cancer (DCC), as well as the Slit-Robo system in regulating axon regeneration and Schwann cell migration after injury [21, 22]. How functions of these axon-guidance systems and the MAPK signaling along with the transcriptional changes in Schwann cells are coordinated to promote nerve repair is not yet clear.
The operational definition of a stem cell is a cell that can self-renew indefinitely and that gives rise to multiple, if not all, cell lineages within the developing embryo. Within the embryonic PNS, neural crest cells serve the purpose; they give rise to mesenchymal stem cells, neurons, and the glia, including the Schwann cells. Neural crest cells also generate boundary cap cells that later differentiate into PNS glial cells and neurons [23] and even into astrocytes, neurons, and oligodendrocyte cells when transplanted into the central nervous system [24].
Although such pluripotential cells do not appear to normally exist in adult nerve, Schwann cells exhibit a few stem cell-like properties. For instance, they appear to lack the replicative senescence seen in other cell types in vitro [25]. Furthermore, following injury to an adult nerve, Schwann cells transdifferentiate into specialized repair Schwann cells or Büngner cells in a cJun-dependent manner, that may differ from the immature Schwann cell prior to myelination [1]. The plasticity of adult Schwann cells after nerve injury has also been demonstrated in mice heterozygous for the tumor suppressor neurofibromin or NF1. In Nf1+/− mice, sciatic nerve transection induces the formation of melanocytes derived from the Schwann cells in the area of injury, supporting the previous idea of a bipotential glial-melanocyte precursor in the developing PNS [26]. Interestingly, however, generation of the Schwann cell-derived melanocytes was seen only after nerve transection and not in crush injuries, pointing to the effects of cellular microenvironment that are specific to a nerve cut injury [27].
Also relevant to the idea of Schwann cells exhibiting stem cell-like properties is the recent finding that the leprosy pathogen, M. leprae, can reprogram Schwann cells into a mesenchymal stem cell-like phenotype in vitro, seemingly through silencing of the key Schwann cell transcription factor Sox10 and induction of molecular profiles that are characteristic of mesenchymal stem cells. These Schwann cells are highly proliferative and migratory and can redifferentiate into mesenchymal cell types including skeletal and smooth muscle cells. They also possess immunomodulatory properties that attract macrophages that provide another route for spreading the M. leprae infection [28].
The studies discussed in this review have undoubtedly provided insights into understanding the mechanisms that promote Schwann cell plasticity and their potential to aid PNS repair in adult nerves. It has been estimated that only 10% of adult patients with nerve transection injury will recover full function even with the suturing together of the nerve ends. When the distal stump of nerves are left unsutured and denervated for long periods, they become largely unsupportive to regeneration [29–32]. In addition to this, misdirected growth of regenerating axons following injury can commonly lead to both sensory mislocalization and motor synkinesias in patients [33]. Increasing the repair potential of the Schwann cells by targeting the components of the MAPK pathways may prove useful for facilitating myelin clearance and axon regeneration. However, it should be cautioned that prolonged activation of the pathways may hinder the subsequent remyelination of the regenerated axons required for achieving complete functional recovery. Furthermore, sustained activation of the Ras/Raf/Erk pathway is linked to Schwann cell hyperplasia and development of peripheral nerve tumors [34–36]. Therefore, development of targeted therapeutic strategies that allow both temporal and quantitative regulation of the MAPK activation is necessary. This clearly represents a huge challenge to translate what we presently know to improving functional recovery not just in cases of trauma but in all diseases that cause dysfunction of the peripheral nervous system.
Author Contributions
H.A.K.: manuscript writing; T.M.: preparation of figure, manuscript writing; D.B.P.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrisingh MC, Perez-Nadales E, Parkinson DB, et al. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang DP, Kim J, Syed N, et al. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zrouri H, Le Goascogne C, Li WW, et al. The role of MAP kinases in rapid gene induction after lesioning of the rat sciatic nerve. Eur J Neurosci. 2004;20:1811–1818. doi: 10.1111/j.1460-9568.2004.03641.x. [DOI] [PubMed] [Google Scholar]
- 5.Myers RR, Sekiguchi Y, Kikuchi S, et al. Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp Neurol. 2003;184:606–614. doi: 10.1016/S0014-4886(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 6.Guertin AD, Zhang DP, Mak KS, et al. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napoli I, Noon LA, Ribeiro S, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 9.Woodhoo A, Alonso MB, Droggiti A, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed N, Reddy K, Yang DP, et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newbern JM, Li X, Shoemaker SE, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossmann KS, Wende H, Paul FE, et al. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci USA. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and Schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fragoso G, Robertson J, Athlan E, et al. Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp Neurol. 2003;183:34–46. doi: 10.1016/s0014-4886(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 15.Raivich G, Bohatschek M, Da Costa C, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson DB, Bhaskaran A, Arthur-Farraj P, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana X, Hristova M, Da Costa C, et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Le N, Nagarajan R, Wang JY, et al. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrinello S, Napoli I, Ribeiro S, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webber CA, Christie KJ, Cheng C, et al. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, Dcc and Unc5H2. Glia. 2011;59:1503–1517. doi: 10.1002/glia.21194. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Teng HL, Huang ZH. Repulsive migration of Schwann cells induced by Slit-2 through Ca(2+)-dependent RhoA-Myosin signaling. Glia. 2013;61:710–723. doi: 10.1002/glia.22464. [DOI] [PubMed] [Google Scholar]
- 23.Maro GS, Vermeren M, Voiculescu O, et al. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- 24.Zujovic V, Thibaud J, Bachelin C, et al. Boundary cap cells are peripheral nervous system stem cells that can be redirected into central nervous system lineages. Proc Natl Acad Sci USA. 2011;108:10714–10719. doi: 10.1073/pnas.1018687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathon NF, Malcolm DS, Harrisingh MC, et al. Lack of replicative senescence in normal rodent glia. Science. 2001;291:872–875. doi: 10.1126/science.1056782. [DOI] [PubMed] [Google Scholar]
- 26.Nataf V, Le Douarin NM. Induction of melanogenesis by tetradecanoylphorbol-13 acetate and endothelin 3 in embryonic avian peripheral nerve cultures. Pigment Cell Res. 2000;13:172–178. doi: 10.1034/j.1600-0749.2000.130309.x. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi TA, Huang Y, Sidani A, et al. A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. J Neurosci. 2002;22:9831–9840. doi: 10.1523/JNEUROSCI.22-22-09831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masaki T, Qu J, Cholewa-Waclaw J, et al. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst. 2012;17:1–18. doi: 10.1111/j.1529-8027.2012.00378.x. [DOI] [PubMed] [Google Scholar]
- 30.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J Neurosci. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlin LB. Techniques of peripheral nerve repair. Scand J Surg. 2008;97:310–316. doi: 10.1177/145749690809700407. [DOI] [PubMed] [Google Scholar]
- 33.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Parrinello S, Lloyd AC. Neurofibroma development in NF1: Insights into tumour initiation. Trends Cell Biol. 2009;19:395–403. doi: 10.1016/j.tcb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Ammoun S, Ristic N, Matthies C, et al. Targeting ERK1/2 activation and proliferation in human primary schwannoma cells with MEK1/2 inhibitor AZD6244. Neurobiol Dis. 2010;37:141–146. doi: 10.1016/j.nbd.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Ammoun S, Flaiz C, Ristic N, et al. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]