Several studies have demonstrated direct reprogramming of mouse and human fibroblasts into immature neural stem or precursor cells, either by transient expression of pluripotency genes or by application of different combinations of neural transcription factors. Interestingly, in all of these studies SOX2 was introduced alone or in combination with other transcription factors. This review discusses the different combinations of ectopic transcription factors used to generate neural stem/precursor cells from somatic cells, with particular emphasis on SOX2 and its potential to act as a master regulator for reprogramming to a neural precursor state.
Keywords: Neural induction, Neural stem cell, Neural differentiation, Reprogramming, Stem/progenitor cell, Transcription factors
Abstract
Since induced pluripotent stem cells were first generated from mouse embryonic fibroblasts in 2006, somatic cell reprogramming has become a powerful and valuable tool in many fields of biomedical research, with the potential to lead to the development of in vitro disease models, cell-based drug screening platforms, and ultimately novel cell therapies. Recent research has now demonstrated the direct conversion of fibroblasts into stem, precursor, or mature cell types that are committed in their fate within a specific lineage, such as hematopoietic precursors or mature neurons. This has been achieved by ectopic expression of defined, tissue-specific transcription factors. Several studies have demonstrated direct reprogramming of mouse and human fibroblasts into immature neural stem or precursor cells, either by transient expression of the four pluripotency genes OCT3/4, KLF4, SOX2, and C-MYC or by application of different combinations of up to 11 neural transcription factors. Interestingly, in all of these studies SOX2 was introduced alone or in combination with other transcription factors. In this review we discuss the different combinations of ectopic transcription factors used to generate neural stem/precursor cells from somatic cells, with particular emphasis on SOX2 and its potential to act as a master regulator for reprogramming to a neural precursor state.
Introduction
The groundbreaking discovery in 2006 that forced expression of only four genes, namely OCT4, KLF4, SOX2, and C-MYC, was sufficient to reprogram fibroblasts back to a pluripotent embryonic stem cell-like state established the exciting field of somatic cell reprogramming [1, 2]. Other studies followed, with the aim of reducing the number of transcription factors required, and showed that the generation of induced pluripotent stem cells (iPSCs) was possible without the oncogene C-MYC [3, 4], using combinations of OCT4/KLF4 [5] or OCT4/SOX2 [6], and under specific culture conditions by transduction of the single transcription factor OCT4 alone [7].
The idea of transferring genes for the purpose of cell fate transformation was actually first implemented by Weintraub and colleagues [8, 9] with the conversion of fibroblast cells to myoblasts by activation of MYOD. Further development of reprogramming technologies led to the direct generation of various induced cell types, bypassing the pluripotent cell status by either adjusting the culture conditions or using lineage-specific transcription factors.
Neurons were the first cells demonstrated to be directly converted from fibroblasts by forced expression of the neural lineage-specific transcription factors ASCL1, BRN2, and MYT1L [10]. Direct generation of several other cell types, such as hepatocytes [11] and hematopoietic progenitor cells [12], followed these initial findings. Two studies also demonstrated the direct generation of induced cardiomyocytes from fibroblasts applying substantially different reprogramming strategies [13, 14]. Efe et al. [13] induced transient low-level expression of the four putative pluripotent genes OCT4, KLF4, SOX2, and C-MYC in mouse embryonic fibroblasts, presumably resulting in nonpluripotent-intermediate cells. These cells were further transformed into induced cardiomyocytes by application of cardiogenic media conditions. In contrast, Ieda et al. [14] narrowed 14 cardiac-developmental transcription factors down to GATA4, MAF2C, and TBX5 and demonstrated the induction of cardiomyocytes from mouse cardiac and dermal fibroblasts by viral triple transduction of the factors. Interestingly, the initial focus on a high number of cardiac transcription factors was justified by the authors with the fact that there is no single “master transcription factor” in cardiomyocytes that may be sufficient to induce reprogramming.
Several studies have recently demonstrated direct induction of neural stem/precursor cells using a range of pluripotent and neural transcription factors; interestingly, all studies showing direct reprogramming of fibroblasts to neural stem or precursor cells directly use ectopic SOX2 expression [15–23]. SOX2 is a key transcription factor expressed in pluripotent embryonic stem cells, and is widely expressed in early neuroectoderm and neural progenitor cells during development, and in neural stem cells in the adult brain [24]. SOX2 expression is tightly regulated, with twofold increases enough to alter self-renewal and induce differentiation of embryonic stem cells (ESCs) [25]. In this review we discuss the different transcription factor combinations that have been applied to directly convert somatic cells into neural stem/precursor cells, with special focus on SOX2 and its role as a possible master regulator in this context.
Transcription Factors Used for Direct Reprogramming of Induced Neural Stem/Precursor Cells
We and others have recently demonstrated that mouse and human fibroblasts can be converted into neural stem/precursor cells by somatic cell reprogramming technology [15–21]. Interestingly, induced neural stem/precursor cells have been generated using the same two principal approaches as previously described for the induction of cardiomyocytes: either transient expression of the four pluripotent factors OCT4, KLF4, SOX2, and C-MYC [15, 16] or forced expression of neural-specific transcription factors [17–21].
In 2011, Kim et al. [15] performed temporal overexpression of OCT4, KLF4, SOX2, and C-MYC in mouse embryonic fibroblasts by lentiviral transduction and observed the formation of neural stem cell colonies expressing PAX6 and the neural stem cell-rosette marker promyelocytic leukemia zinc finger within 4–6 days. Further experiments showed that the induced neural stem cells did not derive from transient pluripotent intermediates and were directly induced from somatic fibroblasts. Following 1–2 weeks of spontaneous differentiation, the induced neural stem cells generated multiple neuronal subtypes and glial cells. The potential for the pluripotent transcription factors to directly generate neural stem cells from mouse embryonic fibroblasts was further confirmed by generation of induced neural stem cells using retroviral transduction of SOX2, KLF4, and C-MYC in combination with transient OCT4 expression [16]. Using this approach, induced cells uniformly displayed morphological and molecular features of neural stem cells, such as the expression of NESTIN, PAX6, and OLIG2, and exhibited a genome-wide transcriptional profile similar to that of brain-derived neural stem cells. Moreover, these cells could be expanded for more than 50 passages and demonstrated tripotency.
Using an alternative strategy by which to directly induce neural precursor cells, Lujan et al. [19] narrowed a pool of 11 neural-specific transcription factors down to a triple combination of SOX2, BRN2, and FOXG1 [19]. Lentiviral transduction of SOX2, BRN2, and FOXG1 was sufficient to convert mouse embryonic fibroblasts into induced neural precursor cells with the potential to further differentiate into neurons, astrocytes, and oligodendrocytes. Interestingly, the combination of SOX2 and FOXG1 resulted in the formation of induced neural precursor cells that gave rise to astrocytes and functional neurons. However, whereas the transcription factors FOXG1 and BRN2 alone were also capable of generating neural precursor cells that could produce neurons, astrocytes, and oligodendrocytes, the neurons were less mature than with the addition of SOX2. This suggests a requirement for SOX2 to promote effective reprogramming of fibroblast cells and maturation of neurons within the neural lineage.
Another study identified an overlapping combination of five transcription factors, namely SOX2, BRN2, TLX (NR2E1), C-MYC, and BMI1, to be capable of reprogramming adult mouse fibroblast into neural precursor cells [20]. The resulting induced neural precursor cells possessed properties similar to those of primary neural precursor cells, including proliferation, self-renewal, and differentiation. Although omitting single transcription factors from this combination revealed that ectopic expression of C-MYC and BMI1 was crucial for proliferation and self-renewal in the five factor-induced neural precursor cells, all of the combinations tested by Tian et al. [20] included SOX2 indicating the requirement of SOX2 in direct neural precursor cell reprogramming.
Han et al. [17] investigated a range of 11 stem cell and neural-specific transcription factors for their potential to reprogram mouse embryonic fibroblasts into induced neural stem cells. Generation of stable neural stem cell clusters was achieved with a combination of three pluripotent factors, SOX2, KLF4, and C-MYC, and two additional factors, BRN4 and E47. Interestingly, although these induced neural stem cells were able to differentiate into neurons and astrocytes in vitro, oligodendrocytic differentiation was compromised. The study further investigated changes in cell morphology 2–3 weeks following transduction, either from different transcription factor combinations or single transcription factors alone [17]. Neural stem cell cluster formation was observed only when at least four transcription factors were applied. In contrast, transduction of three, two, or one transcription factor, including SOX2 alone, did not result in neural stem cell formation.
However, a recent study by Ring et al. [21] demonstrated induction of neural stem cells from both mouse embryonic and human fetal fibroblasts by single-factor transduction of SOX2 within 6 weeks, when cells were cultured on a mitotically inactive feeder layer [21]. They observed that both mouse- and human-derived neural stem cells could be extensively passaged and represented a homogeneous tripotent population. Ring et al. [21] proposed that under conditions conducive to neural stem cell expansion, including the presence of growth factors and proper surface substrates, overexpression of SOX2 alone is sufficient to reprogram fibroblasts into multipotent neural stem cells.
Interestingly, in contrast to mesodermal fibroblast cells, terminally differentiated mesodermal Sertoli cells derived from postnatal mice have also demonstrated the ability to be reprogrammed into induced neuronal stem/progenitor cells, even when SOX2 is excluded. This may be related to the differences in the starting cell populations [22]. Sheng et al. [22] used a pool of nine transcription factors from the basic helix-loop-helix and homeodomain families, ASCL1, NGN2, HES1, ID1, PAX6, BRN2, C-MYC, KLF4, and SOX2. The induced neuronal stem/progenitor cells showed gene expression patterns similar to those of normal embryonic neural stem cells by microarray and were capable of self-renewal and differentiating into functional neurons both in vitro and in vivo. In contrast to all other papers described in this review, when Sheng et al. [22] tested eight-factor combinations by removal of each individual factor, they found that most of the combinations did not give rise to induced neuronal stem/progenitor cell colonies and that the only dispensable factor was SOX2. Differentiation and functional analysis, however, was performed on the SOX2-inclusive induced neuronal stem/progenitor cells. These were shown to express neural stem cell and progenitor markers, including NESTIN, PAX6, SOX2, OLIG2, and DCX, and were tripotent. Importantly, in the eight-factor combination, SOX2 was found to be endogenously expressed, even though it was left out of the pool of reprogramming factors.
Extending these previous studies, our own work has demonstrated that combined ectopic expression of SOX2 and PAX6 using nonviral delivery is capable of converting adult human fibroblasts into neural precursor cells [18]. This represented the first study to demonstrate direct reprogramming of adult human fibroblasts into induced neural precursor cells. SOX2 and PAX6 were delivered to adult human fibroblasts either as full-length proteins or by transient plasmid DNA transfection. This resulted in the generation of induced neural precursor cells expressing a range of neural stem and proneural genes. Upon differentiation, induced neural precursor cells gave rise to neurons exhibiting typical neuronal morphologies and expressing multiple neuronal markers, including tyrosine hydroxylase and GAD65/67. Importantly, induced neural precursor cell-derived neurons demonstrated electrophysiological properties of functionally mature neurons with the capacity to generate action potentials. In addition, induced neural precursor cell are capable of differentiating into glial fibrillary acidic protein-expressing astrocytes. However, oligodendrocytic differentiation was not detected. Interestingly, others have observed similar difficulties in the differentiation of neural precursor cells into oligodendrocytes following the use of neural-specific transcription factors [17, 19].
Currently most papers show “completeness ” of direct reprogramming of fibroblasts into neural stem or precursor cells through differentiation and electrophysiology studies, and these studies are essential to show that functional, mature neuronal cells can be generated from reprogrammed cells. However, to characterize induced neural stem cells (iNSCs) more completely, the epigenetic landscape needs to be investigated. Epigenetic memory from starting cell types has been observed in the iPSC field, which was linked to limited differentiation potential [26]. MicroRNA profiles have also been found to be altered in reprogrammed cells. A comparison of microRNA expression in iNSCs/induced neural precursor cells with that in endogenous NPCs would also be very interesting [26]. Thus, there exists a need for further investigation to determine more complete transcriptomes, DNA methylation patterns, and modifications of histones, especially for key fibroblast and neural genes involved in reprogramming.
Is SOX2 the Master Controller?
The high mobility group-box transcription factor SOX2 is one of the four pluripotency genes used to first demonstrate the capability to generate iPSCs from somatic cells [2] and has been used in alternative combinations to induce pluripotency [6, 27]. SOX2 has also been used to induce neural stem or precursor cells. Indeed, to date all studies showing direct reprogramming of fibroblasts to neural stem or precursor cells directly use ectopic SOX2 expression (Table 1). SOX2 is one of three members of the SOXB1 subgroup and has been shown to be a key transcription factor expressed in pluripotent embryonic stem cells [28]. In the transcriptional network of human embryonic stem cells (hESCs), SOX2, NANOG, and OCT4 together control pluripotency and self-renewal, and are crucial for maintaining cells in an undifferentiated state [29–31]. Several studies indicate that SOX2 levels in hESCs are tightly regulated and that small changes in expression levels can cause a significant change in differentiation behavior [30, 32, 33]. Using mouse ESCs, Thomson et al. [34] demonstrated that in addition to maintaining pluripotency, SOX2 and OCT4 also orchestrate germ layer fate selection. OCT4 suppresses neural ectodermal differentiation and promotes mesendodermal differentiation, whereas SOX2 inhibits mesendodermal differentiation and promotes neural ectodermal differentiation. Differentiation toward a neural precursor fate therefore involves maintenance of SOX2 expression in conjunction with OCT4 downregulation (Fig. 1).
Table 1.
Summary of the reprogramming factors used for the induction of neural stem and precursor cells
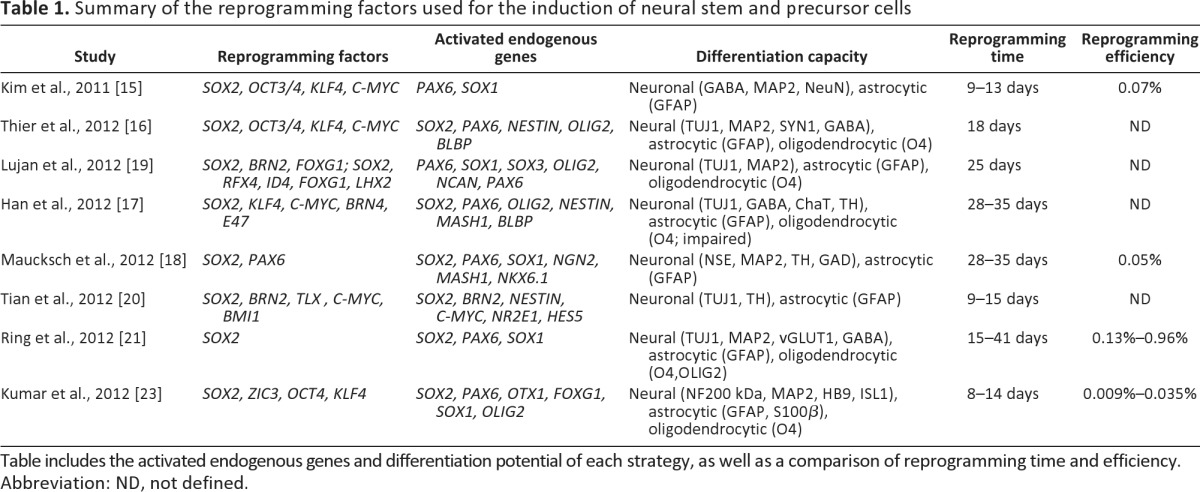
Table includes the activated endogenous genes and differentiation potential of each strategy, as well as a comparison of reprogramming time and efficiency.
Abbreviation: ND, not defined.
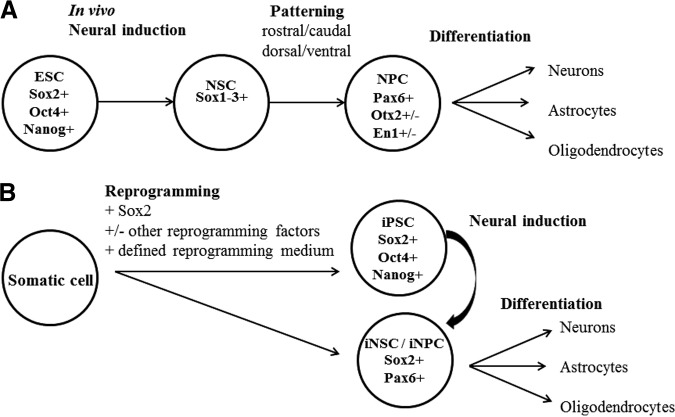
Figure 1.
Schematic demonstrating basic cell types and gene expression generated by Sox2-mediated reprogramming of fibroblasts compared with in vivo lineage of embryonic stem cells. (A): In vivo ESCs undergo neural induction where pluripotency genes OCT4 and NANOG are downregulated and NSC markers SOX1, SOX2, and SOX3 are expressed. The neural tube becomes regionalized by expression of patterning genes, including PAX6, OTX2, and EN1, in a rostral/caudal and dorsal/ventral manner. Domains are further subdivided by heterogeneous expression of transcription factors, for example ACHL1, OLIG2, NGN2, and DLX2, in the forebrain. NPCs go on to generate astrocytes, oligodendrocytes, and multiple neuronal types depending on the region of origin of the cell. (B): Direct reprogramming using Sox2 combined with other transcription factors in a defined reprogramming medium can generate iNSCs/iNPCs with gene expression patterns that are similar to those of in vivo-derived NSCs or NPCs while no longer expressing fibroblast genes. Induced NSCs/NPCs can be differentiated into cells expressing markers for astrocytes, oligodendrocytes, and neurons of multiple subtypes. Abbreviations: ESC, embryonic stem cell; iNPC, induced neural precursor cell; iNSC, induced neural stem cell; iPSC, induced pluripotent stem cell; NPC, neural precursor cell; NSC, neural stem cell.
SOX2 also prevents exiting of the cell cycle and differentiation of neural stem or precursor cells to a mature fate [35, 36]. Therefore, inhibition of SOX2 activity in neural stem or precursor cells is required for cells to exit mitosis and undergo differentiation [36] (Fig. 1). However, a complete knockout of SOX2 leads to lethality at an embryonic stage, whereas a knockdown results in reduced numbers of neural stem or precursor cells in the developing brain and neurodegeneration in the adult brain, suggesting a crucial role for SOX2 in the maintenance of neurons in selected brain areas [37–39].
Although limited information about SOX2-controlled gene networks is available, genome-wide binding studies of members of the SOX family of genes found SOX2 binding in ESCs preselected for neural-lineage genes to be expressed in NPCs, such as SOX3. SOX3-bound genes in NPCs were also later bound by SOX11 in differentiating neurons. Bergsland et al. [40] found that the early SOX proteins were preparing cells for a neuronal lineage by occupying neuronal enhancers and epigenetically predisposing genes for activation during neurogenesis. Thus, sequential coordination of neuronal differentiation from stem cells could be initiated by SOX2. This indicates the potential SOX2 may also have to act as a master regulator of reprogramming in fibroblasts. It may preselect genes to be activated down the neural lineage and alter chromatin structure and epigenetic state so as to be permissive for neural differentiation [24, 40].
Of the SOX proteins, SOX21 was found to increase within 3 hours of increased SOX2 expression, and overexpression of SOX21 could induce differentiation of ESCs toward a neuroectodermal fate. Microarray analysis also found that SOX21 specifically increased neuroectodermal lineage genes, including TAPA-1, ATBF1, NEUROD1, MASH1, HES1, HES6, and ID2, while decreasing NANOG, SALL4, and SOX2 proteins. SOX21 had previously been found to antagonize SOX1, SOX2, and SOX3 to promote neurogenesis in the chick embryo [41]. These studies indicate that SOX21 is an important gene in initiating downstream neuronal signaling from the SOX2-controlled gene network and may also be implicated in the SOX2-directed reprogramming described in this review.
Gene profiles of induced neural stem or precursor cells can also give an indication of what downstream transcriptional targets are induced by ectopic SOX2 expression. A comparison of neural precursor cells induced by transduction of FOXG1, RFX4, ID4, and LHX2 either with or without SOX2 revealed that endogenous expression of the neural precursor genes SOX1, SOX3, OLIG2, NCAN, and PAX6 was observed in the presence of SOX2 but not when SOX2 was omitted [19]. Furthermore, Lujan et al. [19] demonstrated that although ectopic expression of FOXG1 and BRN2 generated tripotent neural precursor cells, the addition of SOX2 was required for the generation of functionally mature neurons.
Unbiased proteomic screens of SOX2 binding partners in mouse ESCs found 60–70 proteins that associate with SOX2, many of which are related to self-renewal and pluripotency [25]. These proteins included other transcription factors (including KLF4, OCT4, NANOG, SALL4, and SOX21), repressor proteins, DNA repair and replication machinery, subunits of chromatin remodeling complexes, and RNA binding elements. Many SOX2-interacting proteins were found to interact with other pluripotency-associated factors. When profiling of ESCs was expanded to examine chromatin immunoprecipitation (ChIP)-chip binding of SOX2, OCT4, and NANOG together, between 350 and 600 genes were found that were bound by all three factors [25, 28]. In many cases the proteins bound promoter sequences on genes that regulated their own expression. In fact, SOX2 was found to bind to the regulatory regions of more than 50% of SOX2-regulatory proteins, indicating tight regulation over the SOX2 controlled signaling network. Furthermore, 40% of SOX2-regulatory genes were also bound by OCT4. Substantial overlap in the target genes for SOX2, OCT4, and NANOG could also explain how SOX2 overexpression alone could potentially compensate for the lack of these other factors in reprogramming protocols.
Conclusion
Current reprogramming technologies established to induce neural stem or precursor cells from murine and human fibroblast cells require the ectopic expression of SOX2 (Fig. 1). This leads us to suggest that SOX2 has a critical role in direct reprogramming of fibroblasts into a neural lineage, potentially as a “master regulator.” SOX2 expression has been found to prime pluripotent stem cells epigenetically toward the neural lineage, and it is essential for neural development. Combined evidence from direct reprogramming studies strongly indicates that SOX2 is important both for inducing a neural stem/precursor profile and for conferring full neuronal differentiation potential onto induced neural stem or precursor cells. However, comparative gene expression profiling will be necessary to characterize the corresponding cell stage of differently induced neural stem/precursor cells within the reprogramming process. This will help to determine the specific role of SOX2 and additional transcription factors in direct reprogramming, and may eventually expose gene combinations capable of generating precisely defined neural stem/precursor cells.
Author Contributions
C.M., K.S.J., and B.C.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.M. has compensated employment from Ethris GmbH (Germany).
References
- 1.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa M. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Meissner A, Cassady JP, et al. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Desponts C, Do JT, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang Q, Yin X, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1 2 fibroblasts to myoblasts. Cell. 1986;47:649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 9.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 10.Vierbuchen T, Ostermeier AP, Zhiping P, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 12.Szabo E, Rampalli S, Risueno RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 13.Efe JA, Hilcove S, Kim J, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 14.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Efe JA, Zhu S, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thier M, Worsdorfer P, Lakes YB, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Han DW, Tapia N, Hermann A, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Maucksch C, Firmin E, Butler-Munro C, et al. Non-viral generation of neural precursor-like cells from adult human fibroblasts. J Stem Cells Regen Med. 2012;8:162–170. doi: 10.46582/jsrm.0803009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lujan E, Chanda S, Ahlenius H, et al. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian C, Ambroz RJ, Sun L, et al. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12:126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng C, Zheng Q, Wu J, et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Declercq J, Eggermont K, et al. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J Mol Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- 24.Wegner M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 2011;25:2423–2428. doi: 10.1101/gad.181487.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzino A. Concise Review: The Sox2-Oct4 connection: Critical players in a much larger interdependent network integrated at multiple levels. Stem Cells. 2013;31:1033–1039. doi: 10.1002/stem.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp B, Plath K. Reprogramming to pluripotency: Stepwise resetting of the epigenetic landscape. Cell Res. 2011;21:486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 28.Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong HL, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- 30.Adachi K, Suemori H, Yasuda SY, et al. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15:455–469. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- 31.Masui S, Nakatake Y, Toyooka Y, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 32.Zhao SL, Nichols J, Smith AG, et al. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol Cell Neurosci. 2004;27:332–342. doi: 10.1016/j.mcn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kopp JL, Ormsbee BD, Desler M, et al. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- 34.Thomson M, Liu SJ, Zou LN, et al. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bylund M, Andersson E, Novitch BG, et al. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 36.Graham V, Khudyakov J, Ellis P, et al. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 37.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferri ALM, Cavallaro M, Braida D, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi S, Masui S, Niwa H, et al. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 2008;582:2811–2815. doi: 10.1016/j.febslet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Bergsland M, Ramsköld D, Zaouter C, et al. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallanna SK, Ormsbee BD, Iacovino M, et al. Proteomic analysis of Sox2-associated proteins during early stages of mouse embryonic stem cell differentiation identifies Sox21 as a novel regulator of stem cell fate. Stem Cells. 2010;28:1715–1727. doi: 10.1002/stem.494. [DOI] [PMC free article] [PubMed] [Google Scholar]