Using India as a model system, this study reviews the role of key agencies and processes involved in the field of stem cell-based clinical research. This study identifies areas that need attention and provides solutions from other established and functioning models in the world to streamline and unify the regulatory and ethics approval processes for cell-based therapies; it also provides recommendations to check the growth and functioning of clinics offering unproven treatments.
Keywords: Cellular therapy, Clinical trials, Stem cells, Clinical translation
Abstract
Increasingly, a number of rapidly developing countries, including India, China, Brazil, and others, are becoming global hot spots for the development of regenerative medicine applications, including stem cell-based therapies. Identifying and overcoming regulatory and translational research challenges and promoting scientific and ethical clinical trials with cells will help curb the growth of stem cell tourism for unproven therapies. It will also enable academic investigators, local regulators, and national and international biotechnology and biopharmaceutical companies to accelerate stem cell-based clinical research that could lead to effective innovative treatments in these regions. Using India as a model system and obtaining input from regulators, clinicians, academics, and industry representatives across the stem cell field in India, we reviewed the role of key agencies and processes involved in this field. We have identified areas that need attention and here provide solutions from other established and functioning models in the world to streamline and unify the regulatory and ethics approval processes for cell-based therapies. We also make recommendations to check the growth and functioning of clinics offering unproven treatments. Addressing these issues will remove considerable hurdles to both local and international investigators, accelerate the pace of research and development, and create a quality environment for reliable products to emerge. By doing so, these countries would have taken one important step to move to the forefront of stem cell-based therapeutics.
Introduction
As in other rapidly developing countries, India's biotechnology research and development efforts are accelerating quickly. Many hospitals and research and biotechnology companies are leading efforts in stem cell research and development. A search for “stem cells” in the Clinical Trials Registry-India (CTRI) already reveals 29 stem cell-based clinical trials (as of November 19, 2012). In fact, India has been among the pioneers in developing a time- and cost-effective treatment for ocular surface regeneration [1] using limbal progenitor cells to treat corneal disease.
Despite this rapid progress, challenges persist in translating India's stem cell research and development efforts into clinical trials, including hurdles to regulatory processes and approval criteria, standardization of cell therapy product production and release criteria, and harmonization of regulations between agencies and ethics committees. Here, we explore the challenges and recommend ways to overcome them.
Current Regulatory Framework and Cell Therapy Status in India
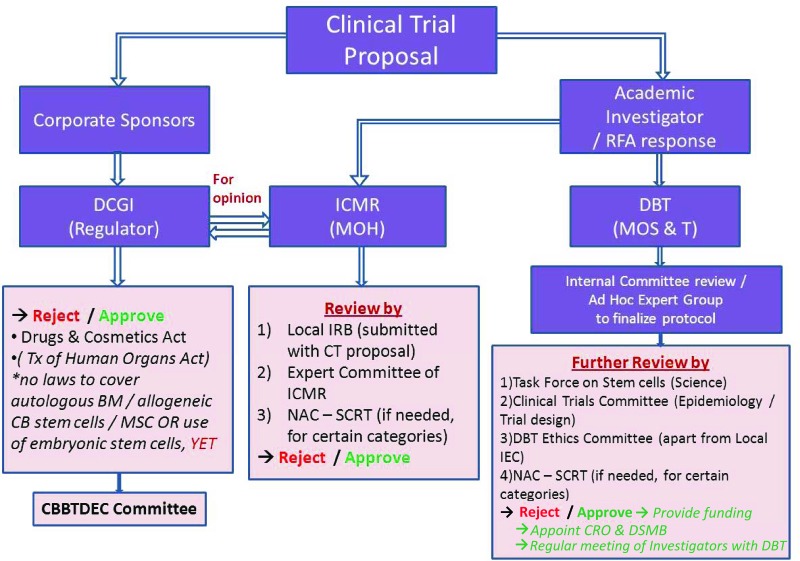
Several government agencies and bodies regulate ethics, provide funding, and give approval for stem cell-based clinical trials in India (Fig. 1). The regulatory route for approval is determined by the type of sponsor, whether the processing of cells is classified as minimal, moderate, or major according to the Guidelines for Stem Cell Research and Therapy [2], and whether prior international approval exists. These multilayered requirements can be confusing, but they are part of existing systems and thus familiar to the Indian pharmaceutical and biotechnology industry.
Figure 1.
Current clinical trial approval process in India. Abbreviations: BM, bone marrow; CB, cord blood; CBBTDEC, Cell Biology Based Therapeutic Drug Evaluation Committee; CRO, Contract Research Organization; CT, Cell Therapy; DBT, Department of Biotechnology (India); DCGI, Drug Controller General of India; DSMB, Data Safety and Monitoring Board; ICMR, Indian Council of Medical Research; IRB, institutional review board; MOH, Ministry of Health; MOS & T, Ministry of Science and Technology; MSC, mesenchymal stromal cell; NAC-SCRT, National Apex Committee for Stem Cell Research and Therapy; RFA, Request for Application; Tx, therapy.
Applications for clinical trials that seek market authorization, usually from corporate sponsors, are submitted to the Drug Controller General of India (DCGI) (as for any new drug application) with data including preclinical studies, toxicity, and manufacturing information as per schedule Y of the Drugs and Cosmetics Act (1940) and the Drugs and Cosmetics Rules (1945). The DCGI has a committee, the Cell Biology Based Therapeutic Drug Evaluation Committee (CBBTDEC), that provides recommendations to approve or reject an application under the Drugs and Cosmetics Act (1940) and Rules (1945), and according to the national Guidelines for Stem Cell Research and Therapy [2].
Additionally, prior to DCGI submission, approval by local ethics committees is required, earlier by the institutional ethics committee (IEC) but now by the more specialized Institutional Committee for Stem Cell Research (IC-SCR). Clinical trials using embryonic or other pluripotent stem cells that come under the restricted areas of stem cell research as per the national guidelines require review and approval of the National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT; http://www.icmr.nic.in/icmrnews/NAC.htm). All investigators and institutes, public and private, conducting stem cell clinical trials in the country need to be registered with NAC-SCRT through IC-SCR.
Investigator-initiated clinical trials (often sponsored by various government funding agencies) that are not seeking market authorization still require scientific and ethics approval at the institutional level and are also reviewed by the appropriate committees appointed by the funding agency concerned. If the investigator or institute intends to seek market authorization, it must also obtain DCGI approval. To date, no academic sponsor has undertaken commercialization of a stem cell therapeutic product, and thus there is no precedent.
Market authorization requires approval at two levels. Although approval for new drugs falls under the jurisdiction of the Central Drugs Standards Control Organization (CDSCO) [3], it is the state governments through the State Drugs Standards Control Organization (SDSCO) that provide actual licenses for sales, manufacturing, and distribution sites; conduct inspections; and control drug recalls. Furthermore, the CDSCO has zonal offices across the nation, which in turn liaise with individual SDSCOs. Additionally, the DCGI is separately responsible for approval of licenses of blood and blood products, i.v. fluids, vaccines, and sera. Currently, no stem cell-based products have received market authorization in India.
Under current guidelines, clinical trials using autologous, minimally manipulated stem cell products can be approved by the IC-SCR. Current interpretation of the definition classifies autologous cells that are processed within a few hours of harvesting as minimally manipulated; no distinction is made between homologous and non-homologous use. If autologous cells require more than minimal manipulation or if allogeneic stem cells are used, current guidelines call for NAC-SCRT approval, in addition to local ethics approval; if cells are to be commercialized, then a formal submission to DCGI is also required. Existing guidelines are therefore subject to interpretation in terms of how mesenchymal stromal cells (MSCs), human embryonic stem cells (hESCs), and induced pluripotent stem cells (iPSCs) should be regulated.
A search of the CTRI database for the string “stem cells” produces 10 records of open, recruiting trials, and all 10 of these list either autologous or allogeneic MSCs as the therapeutic product. Of the 14 completed stem cell trials, 12 used allogeneic or autologous MSCs, clearly indicating the priority given to this form of cell-based therapy in India, likely because of their non-controversial source, ease of access, and growing evidence of their anti-inflammatory and immunomodulatory properties.
Given the burgeoning growth of cell-based clinical investigations, there is a pressing need to simplify the system to enable better translation to clinical practice. We highlight the need for clarification in the following areas: (a) a uniform and streamlined regulatory approval process, (b) cell manufacturing and quality control issues, and (c) stem cell tourism.
Uniform and Streamlined Regulatory Approval Process
Criteria for Therapeutic Product Versus Tissue
We recommend that all cell-based therapies that are considered unproven (i.e., hematopoietic stem cell transplants for blood diseases, limbal stem cell transplants for corneal diseases) be uniformly regulated, similar to European Union (EU) directives and/or the U.S. Code of Federal Regulations (CFR), by an authorized agency such as DCGI via the CBBTDEC. In Europe, the EU Annex I to Directive 2001/83/EC defines human somatic cell therapy medicinal products [4] and mandates clinical trials under the EU Clinical Trials Directive [5]; this directive describes requirements for the approval process by the Committee for Advanced Therapies within the European Medicines Agency (EMA).
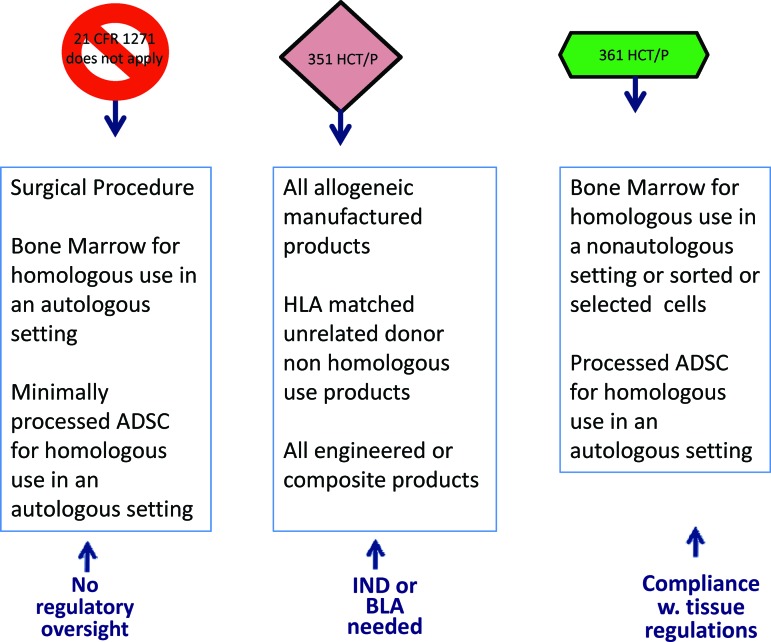
In North America, both the U.S. Food and Drug Administration (FDA) and Health Canada stratify somatic cell products based on whether they are minimally manipulated, used in a homologous fashion, combined with other drugs and/or devices, and have systemic/metabolic effects. Thus, products such as bone marrow, blood products, skin, and cornea fall under the Tissue categorization and are required to follow Cells, Tissues and Organs regulations in Canada [6] and Section 361 of the Public Health Services (PHS) Act in the United States. Part 1271 of 21 CFR provides U.S. regulations on cells, tissues, and cellular and tissue-based products and guidelines for conformity to good tissue practices (GTPs) [7]. In the United States, surgical procedures are exempt from compliance with 21 CFR Part 1271, including homologous use of bone marrow or minimally processed adipose-derived stem cells in an autologous setting (Fig. 2). Products that do not meet the above-specified criteria are regulated under section 351 of the PHS Act in the United States as an investigational product and therefore require formal review through an investigational new drug (IND) application or a clinical trial application (CTA) (Canada).
Figure 2.
Food and Drug Administration stratification of somatic cell therapy products based on extent of manipulation and intended use. Abbreviations: ADSC, adipose-derived stem cell; BLA, Biologics License Application; CFR, Code of Federal Regulations; HCT/P, human cell and tissue products; HLA, human leukocyte antigen; IND, investigational new drug; w., with.
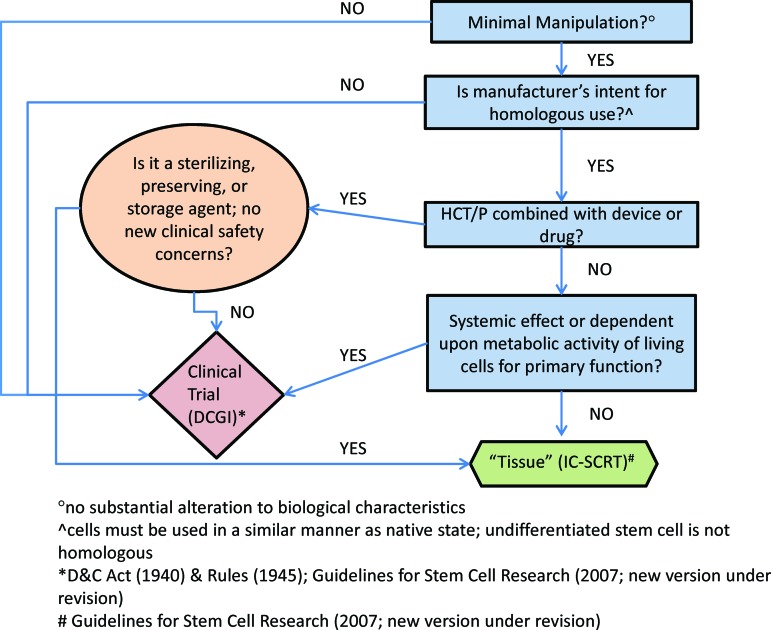
We have created a similar road map for sponsors in India, whether academic or industry, to ensure a consistent and uniform regulatory process (Fig. 3). Under this schematic, any product that undergoes more than minimal alteration of biological characteristics, whether autologous or allogeneic, is regulated as a therapeutic product requiring controlled clinical investigation. Regulatory approval is provided by DCGI via the CBBTDEC, and concurrent (not a priori) local ethics committee approval and, where required, NAC-SCRT (restricted use) approval are recommended. A product that is minimally altered, is homologous, and has no systemic effect or metabolic activity would be classified as a tissue and would be subject to GTP guidelines (Fig. 3).
Figure 3.
Suggested classification criteria for a Somatic Cell Therapy Product in India, based on the simplified stratification strategy used by the Food and Drug Administration. If a cell-based therapy meets four criteria, it is regulated as a tissue (green), similar to 361 HCT/P regulations in the United States. If a cell-based therapy fails to meet any of these four criteria, it is regulated as a drug (pink), similar to 351 HCT/P regulations in the United States. Abbreviations: D&C, Drugs and Cosmetics Act; DCGI, Drug Controller General of India; HCT/P, human cells and tissue products; IC-SCRT, Institutional Committee for Stem Cell Research and Therapy.
Time Limits for the Regulatory Review Process
Jurisdictions such as Canada and the United States have a 30-day turnaround process when an application is filed. European Union countries, following the Clinical Trials Directive (2001), have a maximum 60-day process for approvals [5], similar to the regulatory process in Australia.
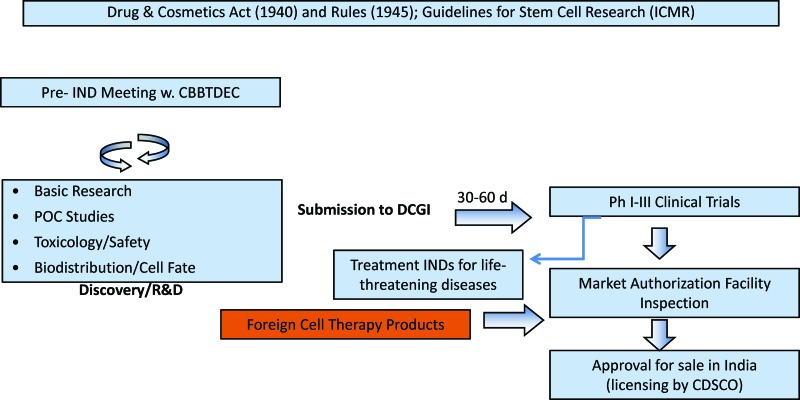
There are currently no set time limits to regulatory review in India, making it difficult for sponsors to plan submissions, initiate sites for clinical trials, and manufacture sufficient quantities of the clinical product. Additionally, the current procedure of requiring prior rather than concurrent ethics approval lengthens the process; the Clinical Trials Directive in Europe [5], faced with a similar problem, modified the prior approval process to a concurrent approval process to expedite the regulatory review. Imposing a similar, defined time limit of 30–60 days (Fig. 4) will expedite the review process considerably.
Figure 4.
Development path for cell therapies in India. Abbreviations: CBBTDEC, Cell Biology Based Therapeutic Drug Evaluation Committee; CDSCO, Central Drugs Standards Control Organization; d, days; DCGI, Drug Controller General of India; ICMR, Indian Council of Medical Research; IND, investigational new drug; Ph, phase; POC, proof of concept; R&D, research and development; w., with.
Standardizing Ethics Review Committees
Another source of confusion is the multiple and sequential ethics review processes, which can vary from region to region depending on the experience, expertise, and composition of the ethics committees. Although the Guidelines for Stem Cell Research and Therapy (2007) provide some guidance on the scope and membership of the NAC-SCRT and the Institutional Committee for Stem Cell Research and Therapy (IC-SCRT), and the Indian Council of Medical Research has issued guidelines (2006) [8] on the composition of the IECs, there is still considerable variability.
It would be best therefore to clearly define the role, responsibility, composition, and mandate of local and central institutional review boards (IRBs) as in other countries, for example, through the Code of Federal Regulations [9] in the United States. Additionally, central ethics committees, which have more experience, can step in when institutions performing FDA-regulated research are too small to establish an IRB. Importantly, all IRBs that review FDA-controlled trials are required to be registered with the Office of Human Research Protection (OHRP), which provides guidance and clarification, develops education programs, and affords quality oversight on biomedical and behavioral research.
A similarly well-defined and consistent review process should be applied across the country. This can be achieved by providing rules that govern the process for applying for ethics approval. These rules can be in the form of formal legal regulations, similar to the CFR in the United States, or can be provided by the NAC-SCRT, which can take on the role of an oversight committee. If this role is designated to NAC-SCRT, it can also take on the additional mandate of providing training and education to local institutes, similar to what OHRP does in the United States.
Uniform Regulatory Process for Market Authorization for National and International Sponsors
There is a lack of clarity on guidelines, agreement templates, and approval processes for the transfer of human materials between researchers, especially in the context of international multicenter trials. Current guidelines [2] call for the use of appropriate material transfer agreements (MTAs) from other nations to ensure that cell lines have been established in accordance with the existing guidelines of that country. A standardized MTA template would ensure that consistent quality-controlled, ethically procured cells are used in clinical trials in India that are in compliance with harmonized international guidelines [10].
Importation of materials for a clinical trial involves ethics approval from the IC-SCRT and the Health Ministry Screening Committee, and if a multinational company is involved, DCGI approval is also required. There is less clarity, however, on the importation of therapeutic products already approved in other jurisdictions. A reciprocal agreement with regulatory authorities in other jurisdictions could streamline or even eliminate this process.
In the United States, for example, the Food, Drug, and Cosmetics Act [11] prohibits the importation of therapeutic products that lack FDA approval. An unapproved new drug is any that is not manufactured in accordance with new drug application standards, including manufacturing standards [12], and therefore might require repeat preclinical or clinical testing in the United States.
A clear schematic representing a uniform regulatory and ethics approval process for national and international academic and industry sponsors is given in Figure 4. In this schematic, a presubmission process is recommended such as those prevalent in other jurisdictions (pre-IND application or pre-CTA) to expedite the review process [13]. For products designated under the therapeutic classification (Fig. 3), concurrent ethics and regulatory timed approval processes are prescribed. Phase I–III clinical trials can be initiated upon DCGI (via the CBBTDEC) approval. Market authorization requires complete review of the clinical data and the manufacturing facility, regardless of whether the premises are nationally or internationally based. A systematic review procedure to inspect and license manufacturing, fill-finish, and distribution facilities, which is currently lacking, is also highly recommended.
Under this schematic, additionally, there is an opportunity for the DCGI through the CBBTDEC to evaluate the use of investigational drugs, biologics, and devices for treatment of serious or life-threatening conditions when no satisfactory alternative treatment exists. Other jurisdictions, including the FDA, have several such mechanisms in place, including treatment INDs, compassionate use Investigational Device Exemptions (IDEs) and humanitarian use devices.
Cell Manufacturing and Quality Control Issues
Current guidelines minimally call for safety and toxicity evaluation of stem cell products in appropriate animal models in a good laboratory practice-certified laboratory [2]. The International Council on Harmonization of Pharmaceuticals for Technical Use has published guidance documents on preclinical safety studies for biotechnology products, but cell therapies do not fit perfectly into the format for conventional biologics [14, 15] The EU has issued guidelines for preclinical studies in relevant animal models of disease or injury for cell-based medicinal products [16]. In the United States, the FDA's Office of Cellular, Tissue and Gene Therapies typically provides informal and formal guidance to sponsors via pre-pre-IND and pre-IND meetings and prefers to use a case-by-case approach. A similar combination of guidelines and preapproval meetings should be followed by the DCGI/CBBTDEC to facilitate translational research in India.
Current guidelines are similarly sparing in terms of chemistry, manufacturing, and control (CMC) [2] and specify only that the cells used in clinical trials must be processed under good manufacturing practice/GTP standards and must be free from animal products and microbial conditions. Other jurisdictions, including the FDA, Health Canada, and the EMA, have a Drug Master File (DMF) procedure that allows manufacturers of active pharmaceutical ingredients (APIs) to file proprietary information with regulatory authorities on a confidential basis, thus enabling other sponsors using the APIs to provide relevant quality information without being privy to confidential information. A DMF procedure, along with more explicit CMC guidelines, should be made available for cell therapy products.
Currently, there are no validated assays available in India for assessing standard safety parameters such as mycoplasma or endotoxin. Most investigators import FDA or Conformité Européenne (CE)-Marked diagnostic kits. There is thus a need for licensed and regulated laboratories to develop and validate both standard and product-specific assays. This would ensure consistent and standardized quality control of stem cell therapeutic products, similar to the Central Drug Laboratories, which provide statutory quality control for all drugs and cosmetics manufactured in, and imported to India.
The national guidelines for stem cell research are currently undergoing revision, and a new version will address clinical investigation efforts using pluripotent stem cells. Developing common formats for consent, banking, expansion, and characterization of iPSCs will require some effort, but it will ensure that Indian regulations are harmonized with those of other jurisdictions and facilitate ready participation in international clinical trials.
Stem Cell Clinics
Although approximately 22 public and 7 private research institutes are authorized to conduct stem cell research in India, many private clinics and hospitals offer unproven therapies. Reports indicate that more than 600 patients with conditions including Alzheimer's, multiple sclerosis, renal failure, cerebral palsy, cardiac disorders, strokes and traumatic neurological deficits, and diabetes have been treated using hESCs at a cost of $20,000–$30,000 at a single clinic alone [17].
The NAC-SCRT has declared that cell-based therapies (outside of proven therapies) can be conducted only as regulated and approved clinical trials in India. Use of unproven medicine outside a duly approved clinical trial should therefore be considered malpractice. Malpractice is punishable both under the current Consumer Forum Act [18] and the Indian Medical Council Act [19]. Additionally, under the Drugs and Magic Remedies (Objectionable Advertisement) Act [20], false claims may also be prosecuted.
Legitimate stem cell research and development activities by the private and public sectors in India are compromised by the presence and operation of clinics that use unproven cell-based therapies, and vulnerable patients are exploited. If unregulated practices in stem cell clinics continue and result in adverse events (tumor formation or death) that garner publicity, this may hinder the entire momentum of legitimate stem cell research in India and prevent India from being a site for global research. Recognizing this as a problem, the regulators are working closely with industry sponsors and academic investigators to revise current guidelines in an attempt to ease translation of peer-reviewed research into the clinics. Regulators should also consider legal action, including prosecution. This would send a strong message nationally and globally and emphasize the legitimacy of approved stem cell clinical trials in India. Streamlining current regulatory practice and offering treatment INDs would (a) enable local investigators and commercial industry to offer such therapeutics, and (b) enable Indians to access regulated experimental medicinal products in a timely and regulated manner.
Conclusion
We have made several recommendations to accelerate stem cell research and development in India, including the urgent need to streamline and unify current regulatory and ethics approval processes to ensure consistency for national and international industrial and academic applicants. A risk stratification strategy based largely on the extent of cell manipulation to identify the required level of regulation, the creation of reciprocal agreements with other regulatory agencies, and standardization of the various ethics committees will enable a more rapid and regulated translation of stem cell products. Implementing these and other changes will position India globally as a leader in stem cell-based therapies. Participants (leading stem cell researchers from around India, industry champions, and regulators) at the 2012 Policies for Regulations of Cell Therapy Trials in India workshop in Vellore were extremely supportive of streamlining the regulatory process.
Acknowledgments
The workshop on Policies for Regulations of Cell Therapy Trials in India at Vellore, India, was funded partly by the Indian Council of Medical Research and the Department of Biotechnology, Ministry of Science and Technology, Government of India, as well as the Centre for Stem Cell Research, a unit of inStem (Bangalore, India) at the Christian Medical College (Vellore, India).
Author Contributions
S.V.: conception and design, collection and/or assembly of data, manuscript writing; M.R.: conception and design, collection and/or assembly of data; A.K.: collection and/or assembly of data, final approval of manuscript; A.S.: conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.V. is a compensated consultant for a nonprofit organization and is uncompensated for research funds.
References
- 1.Sangwan VS, Vemuganti GK, Singh S, et al. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci Rep. 2003;23:169–174. doi: 10.1023/b:bire.0000007690.43273.73. [DOI] [PubMed] [Google Scholar]
- 2.Director General, Indian Council of Medical Research. Guidelines for Stem Cell Research and Therapy. New Delhi, India: Indian Council of Medical Research; 2007. [Google Scholar]
- 3.Central Drugs Standards Control Organization. Guidance for industry on submission of clinical trial application for evaluating safety and efficacy. [Accessed November 2012]. Document no. CT/71108, version 1.1, 2008. Available at http://www.cdsco.nic.in.
- 4.Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. European Parliament. 2001 [Google Scholar]
- 5.Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. J Eur Commun. 2001;L121:34–44. [PubMed] [Google Scholar]
- 6.Health Mo., editor. Canadian Gazette Part II, 2007(2007-06-22) Gatineau, QC: Public Services and Government Services Canada; Safety of Human Cells, Tissues and Organs for Transplantation Regulations. [Google Scholar]
- 7. Code of Federal Regulations, Title 21. 21 Part 1271 Human Cells, Tissues, and Cellular and Tissue-Based Products.
- 8.Indian Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Participants. New Delhi, India: 2006. [Google Scholar]
- 9. Code of Federal Regulations, Title 21. 21 Part 56 Institutional Review Board. Vol. Part 56 Institutional Review Board.
- 10.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Good Clinical Practice E6(R1) Efficacy Guidelines. 1996 doi: 10.1111/j.1365-2125.1994.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Federal Food, Drug, and Cosmetics Act (21USC351), 1938. United States Code, 2006 Edition, Supplement 3. Available at http://www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/
- 12. Code of Federal Regulations, Title 21. Vol. 4. 21 Part 210 Current Good Manufacturing Practice in Manufacturing, Processing, Packing or Holding of Drugs: General, April 1, 2012. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=210&showFR=1.
- 13.U.S. Food and Drug Administration. Guidance for Industry Formal Meetings Between the FDA and the Sponsors or Applicants. 2009.
- 14.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals (S6). Safety Guidelines 1997. Federal Register. 1997;62(222):61515. [Google Scholar]
- 15.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Addendum to ICH S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals (S6R1). Safety Guidelines 2009. Federal Register. 2012;77(97):29665–29666. [PubMed] [Google Scholar]
- 16.European Medicines Agency (EMEA) Reflection paper on stem cell-based medicinal products. London, United Kingdom: Committee for Advanced Therapies; 2010. [Google Scholar]
- 17.Khullar M. Unfettered by regulations, India pulls ahead on stem cell treatments [Google Scholar]
- 18.Government of India. New Delhi, India: Government of India Press; 1986. Consumer Protection Act India. Gazette of India. [Google Scholar]
- 19.Government of India. New Delhi, India: Government of India Press; 1956. Indian Medical Council Act. Gazette of India. [Google Scholar]
- 20.Government of India. New Delhi, India: Government of India Press; 1954. Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954. Gazette of India. [Google Scholar]