This study examined the capability of human umbilical cord Wharton's jelly stem cells (HWJSCs) to differentiate in vitro and in vivo to oral mucosa and skin epithelial cells using a bioactive three-dimensional model that mimics the native epithelial-mesenchymal interaction. The HWJSCs were unable to fully differentiate to epithelial cells in vitro but were able to stratify and to express typical markers of epithelial differentiation, showing specific surface patterns. These results suggest that HWJSCs have the potential to differentiate to oral mucosa and skin epithelial cells in vivo and could be an appropriate novel cell source for the development of human oral mucosa and skin in tissue engineering protocols.
Keywords: Adult stem cells, Cell interactions, Cell transplantation, Tissue regeneration
Abstract
Perinatal stem cells such as human umbilical cord Wharton's jelly stem cells (HWJSCs) are excellent candidates for tissue engineering because of their proliferation and differentiation capabilities. However, their differentiation potential into epithelial cells at in vitro and in vivo levels has not yet been reported. In this work we have studied the capability of HWJSCs to differentiate in vitro and in vivo to oral mucosa and skin epithelial cells using a bioactive three-dimensional model that mimics the native epithelial-mesenchymal interaction. To achieve this, primary cell cultures of HWJSCs, oral mucosa, and skin fibroblasts were obtained in order to generate a three-dimensional heterotypical model of artificial oral mucosa and skin based on fibrin-agarose biomaterials. Our results showed that the cells were unable to fully differentiate to epithelial cells in vitro. Nevertheless, in vivo grafting of the bioactive three-dimensional models demonstrated that HWJSCs were able to stratify and to express typical markers of epithelial differentiation, such as cytokeratins 1, 4, 8, and 13, plakoglobin, filaggrin, and involucrin, showing specific surface patterns. Electron microscopy analysis confirmed the presence of epithelial cell-like layers and well-formed cell-cell junctions. These results suggest that HWJSCs have the potential to differentiate to oral mucosa and skin epithelial cells in vivo and could be an appropriate novel cell source for the development of human oral mucosa and skin in tissue engineering protocols.
Introduction
Human oral mucosa and skin play crucial roles as defensive barriers and are responsible for the maintenance of physiological homeostasis [1]. Both structures consist of two tissue layers: the epithelium and the underlying connective tissue. The most important cell populations of the epithelial layer are the keratinocytes, which are tightly attached to each other by cell-cell junctions and arranged in a number of distinct layers. Although these cells can be cultured in vitro, their doubling time is very long, and cell cultures tend to grow slowly. The roles of the connective tissue are related to the sustenance of the differentiation status of the overlying epithelium. In this context, the normal architecture of the oral mucosa and skin can be affected by numerous diseases associated with trauma, cancer, burns, infections, etc. In these cases, early coverage of the lesions is highly necessary, and several models of bioengineered oral mucosa and skin have been described for clinical use [2–4]. However, a new cell source allowing the generation of these artificial tissues without the need to obtain autologous biopsies is required, especially in patients with large areas affected by the disease.
Perinatal stem cells possess properties of both embryonic and adult stem cells [5], without the drawbacks that are often associated with the clinical use of embryonic stem cells (ethical issues, immune rejection, teratoma formation, etc.) and adult stem cells (limited proliferation potential and differentiation capability) [6]. Furthermore, procurement of adult stem cells from patients is an invasive technique and represents significant risk and discomfort. These limitations could be overcome with the use of additional cell sources such as perinatal cells.
Perinatal stem cells can be obtained from tissues that are normally discarded after delivery. These can be collected with low risk to the mother and the newborn and with few ethical concerns. Human Wharton's jelly stem cells (HWJSCs) are considered immunoprivileged perinatal cells that have high differentiation capability [7] and are easily available for therapeutic applications. As mesenchymal stem cells, HWJSCs retain the ability to differentiate to mesodermic tissues (cartilage, tendon, bone, and ligament). However, there is not clear evidence that HWJSCs are able to differentiate in epithelial cells.
In this work, we have developed a three-dimensional model of human oral mucosa and skin using stromal cells in combination with biomaterials and HWJSCs to determine the capabilities of HWJSCs to differentiate into oral mucosa and skin keratinocytes at in vitro and in vivo levels by using tissue engineering strategies.
Materials and Methods
Tissue Samples and Cellular Isolation
HWJSCs were isolated from umbilical cord samples. Human umbilical cords were obtained immediately after full-term births, rinsed in 1× phosphate-buffered saline (PBS), and then cut into small pieces (approximately 1.5 cm in length), which were sectioned longitudinally to expose the Wharton jelly below the amniotic membrane. Isolation of HWJSCs cells was performed by using type I collagenase (Gibco-BRL/Life Technologies, Karlsruhe, Germany, http://www.invitrogen.com) and a solution of 0.5 g/l trypsin and 0.2 g/l EDTA (Gibco-BRL) following previously described methods [8].
In order to obtain primary cell cultures of human oral mucosa and skin fibroblasts, biopsies of normal oral mucosa and skin were obtained at the Department of Plastic and Maxillofacial surgery of the University Hospital “Virgen de las Nieves” of Granada, Spain. Human oral mucosa and skin biopsies were washed in 1× PBS and enzymatically digested using 2 mg/ml Clostridium histolyticum collagenase I (Gibco-BRL) at 37°C for 6 hours [4]. Isolated fibroblasts were collected by centrifugation and expanded in culture flasks containing basal culture medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg /ml amphotericin B, all from Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and using standard cell culture conditions. This work was approved by the local ethical and research review committees. All patients gave their consent to participate in the study.
Analysis of the Mesenchymal Nature of HWJSCs
To confirm the mesenchymal stem cell profile of HWJSCs by flow cytometry, 1 × 106 HWJSCs were incubated with allophycocyanin-conjugated CD90 (clone Thy-1A1; mouse IgG2A) and phycoerythrin-conjugated CD45 (clone 2D1; mouse IgG1) antibodies (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) after being washed in staining buffer for 5 minutes. Then, Fc receptors were blocked and samples were transferred into a 5-ml flow cytometry tube and incubated with each antibody or each corresponding isotype control antibody at a concentration of 1:100. Following the incubation, any excess of antibody was removed by washing the cells with 2 ml of staining buffer, and they were analyzed on a FACSCalibur flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com) with the required compensation to remove the spillover fluorescence. For immunofluorescence, 0.5 × 104 HWJSCs were placed on cell culture chamber slides, fixed in 70% alcohol, and hybridized to specific monoclonal anti-CD90 (Thy1; Novus Biologicals, Littleton, CO, http://www.novusbio.com) and anti-CD105 (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) primary antibodies. After being washed, cells were incubated in fluorescein isothiocyanate and Cy3-labeled secondary antibodies and examined in a fluorescence microscope.
To confirm the differentiation capability of the cells, 0.5 × 104 HWJSCs were placed on cell culture chamber slides for 4 weeks using osteogenic, adipogenic, and chondrogenic induction media, as we previously described [9]. The composition of these media is shown in supplemental online Table 1.
To demonstrate the acquisition of the osteogenic phenotype, alizarin red S staining was used. Briefly, cells were fixed in 4% paraformaldehyde and stained with a 2% solution of alizarin red. Stained cells were rinsed with water three times to remove excess stain and then evaluated under a light microscope. To evaluate the adipogenic differentiation of HWJSCs, cells were stained with Oil Red O (0.7 mg in 100 ml of propylene glycol). Finally, the chondrogenic potential was analyzed by using Alcian blue solution (1% Alcian blue 8GX and 3% glacial acetic acid, pH adjusted to 2.5).
Development of Three-Dimensional Bioactive Systems to Induce Epithelial Differentiation of HWJSCs
To induce the epithelial differentiation of HWJSCs using three-dimensional bioactive systems, tissue models of heterotypical human oral mucosa (H-hOM) and heterotypical human skin (H-hS) were developed on the basis of previously described bioengineered tissues [3, 10]. Briefly, a stroma substitute was first generated by using a mixture of human fibrin obtained from frozen human plasma and 0.1% agarose. An average of 250,000 cultured oral mucosa and skin fibroblasts were added to 5 ml of the mixture immediately before inducing the polymerization of the artificial stroma on Transwell (Corning Enterprises, Corning, NY, http://www.corning.com) porous inserts. Once the stromas jellified, HWJSCs were seeded on top of the oral mucosa and skin artificial stromas and cultured for 7 days (1-week samples) submerged in preconditioning epithelial culture medium (supplemental online Table 1) for 4 weeks at 37°C in 5% carbon dioxide. Finally, samples were subjected to air-liquid culture technique for 1 additional week (2-week samples) to induce the final differentiation of the HWJSCs into a multilayered oral mucosa and skin epithelium.
In Vivo Evaluation of the Epithelial Differentiation Potential of HWJSCs
To analyze the differentiative potential of HWJSCs, both H-hOM and H-hS were grafted on immunodeficient athymic mice. Briefly, 6-week-old Fox 1nu/nu nude mice (Harlan, Indianapolis, IN, http://www.harlan.com) were anesthetized with acepromazine (Calmo-Neosan; Pfizer Inc., New York, NY, http://www.pfizer.com; 0.001 mg per gram of weight of the animal) and ketamine (Imalgene 1000; Merial, Duluth, GA, http://us.merial.com; 0.15 mg per gram of weight of the animal), and a segment of skin 2.5 × 2.5 cm was excised from the backs of the animals. Then, H-hOM and H-hS were engrafted on the surgical wounds just above the muscular fascia using absorbable suture material. Analgesia was used in the drinking water. Mice were euthanatized by lethal administration of anesthetics at 10, 20, 30, and 40 days after implantation of the three-dimensional heterotypical models of oral mucosa and skin (H-hOM and H-hS), and all grafted tissues were harvested for histological analysis.
Histological Analysis of In Vitro and In Vivo Samples
For light microscopy, in vitro and in vivo engrafted H-hOM and H-hS samples were fixed with 4% formaldehyde, dehydrated, and embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin and eosin. For immunofluorescence labeling of typical epithelial markers, sections were incubated with cytokeratin 1 (CK1) (1:500; Sigma-Aldrich), CK4 (1:1,000; Sigma-Aldrich), CK8 (prediluted; Master Diagnostica, Granada, Spain, http://www.masterdiagnostica.com), CK13 (1:400; Sigma-Aldrich), involucrin (1:500; Sigma-Aldrich), filaggrin (1:50; Abcam, Cambridge, MA, http://www.abcam.com), and plakoglobin (1:20, Abcam) primary antibodies. Subsequently, sections were stained with goat anti-rabbit or anti-mouse IgG secondary antibodies. CK1 and involucrin were specific anti-human antibodies, and they did not react with host mouse tissues. The control samples used in this work corresponded to native human oral mucosa (N-hOM) and native human skin (N-hS). For scanning electron microscopy, in vitro and in vivo H-hOM and H-hS samples were fixed in cacodylate-buffered 2.5% glutaraldehyde, dehydrated in increasing concentrations of acetone, and critical point dried, mounted on aluminum stubs, sputter-coated with gold according to pre-established protocols [14], and examined in a scanning electron microscope (Quanta 200; FEI, Eindhoven, The Netherlands, http://www.fei.com). For transmission electron microscopy, samples were fixed, postfixed in 1% osmium tetroxide for 90 minutes, and then embedded in Spurr's resin and cut into ultrathin sections. For analysis, the sections were stained with aqueous uranyl acetate and lead citrate and observed with a transmission electron microscope (EM902; Carl Zeiss Meditec, Inc., Oberkochen, Germany, http://www.zeiss.com).
Results
Analysis of the Mesenchymal Nature of the HWJSCs
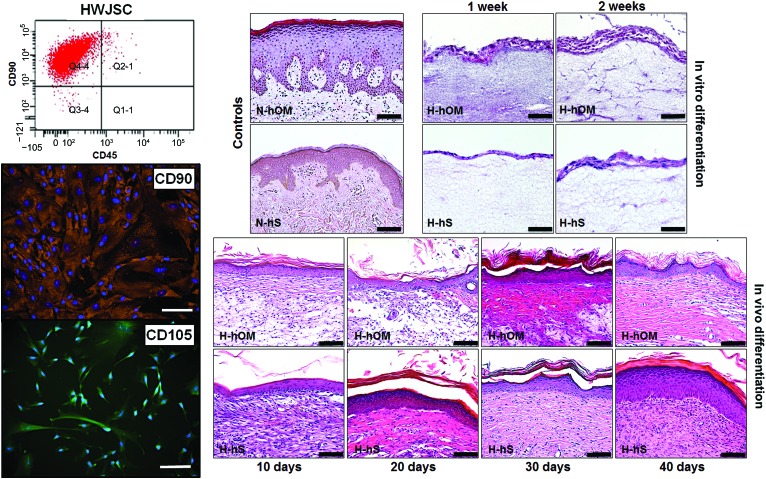
The analysis of HWJSCs used in this work revealed that these cells were adherent, with an elongated and spindle-shape morphology. HWJSCs were able to properly differentiate to osteogenic, chondrogenic, and adipogenic cell lineages, as determined by alizarin red S, Alcian blue, and Oil Red O staining (supplemental online Fig. 1). In addition, the flow cytometry or immunofluorescence analysis of specific mesenchymal stem cell markers showed high expression of CD90 (99.4% positive cells) and CD105 (81.5% positive cells for the immunofluorescence analysis) and negative expression of CD45 (1.3% positive cells), with controls being negative (Fig. 1). All this demonstrates that the cells used in this work were undifferentiated mesenchymal stem cells that were not committed toward specific differentiation pathways.
Figure 1.
Determination of the mesenchymal profile of HWJSCs by flow cytometry analysis for CD90 and CD45 and immunofluorescence for CD90 and CD105 (left) and light microscopy histological analysis of controls and three-dimensional bioactive models of H-hOM and H-hS stained with hematoxylin and eosin (right). In vitro differentiation samples corresponding to tissues kept in culture for 1 and 2 weeks showed a stratified epithelium with two to eight cell layers. In vivo samples implanted on nude mice for 10, 20, 30, and 40 days showed a large number of epithelial layers that were organized in basal, spinosum, granulosum and, corneum strata. N-hOM and N-hS were used as controls. Scale bars = 50 μm (left) and 100 μm (right). Abbreviations: H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; HWJSC, human umbilical cord Wharton's jelly stem cell; N-hOM, native human oral mucosa; N-hS, native human skin.
Epithelial Differentiative Capability of HWJSCs as Determined by Histological Analysis
Our results showed that the use of three-dimensional systems based on bioengineered human tissues was able to efficiently induce the epithelial differentiation of the HWJSCs. Initially bioengineered tissues cultured ex vivo showed a stratified epithelium with a number of cell layers oscillating between two for the H-hS kept in vitro for 1 week and seven or eight layers for the H-hOM cultured ex vivo for 2 weeks (Fig. 1). These layers were not differentiated in specific basal, spinosum, granulosum, and corneum strata, and the typical rete ridges and stromal papillae were not detected. This was considerably different from the structure of the N-hOM and the N-hS, which showed a greater number of layers and higher levels of differentiation. On the other hand, the histological analysis showed that in vivo grafted N-hOM and N-hS had more differentiation levels than ex vivo samples, showing a large number of epithelial layers that were organized in basal, spinosum, granulosum, and corneum strata, especially after 20 days in vivo (Fig. 1).
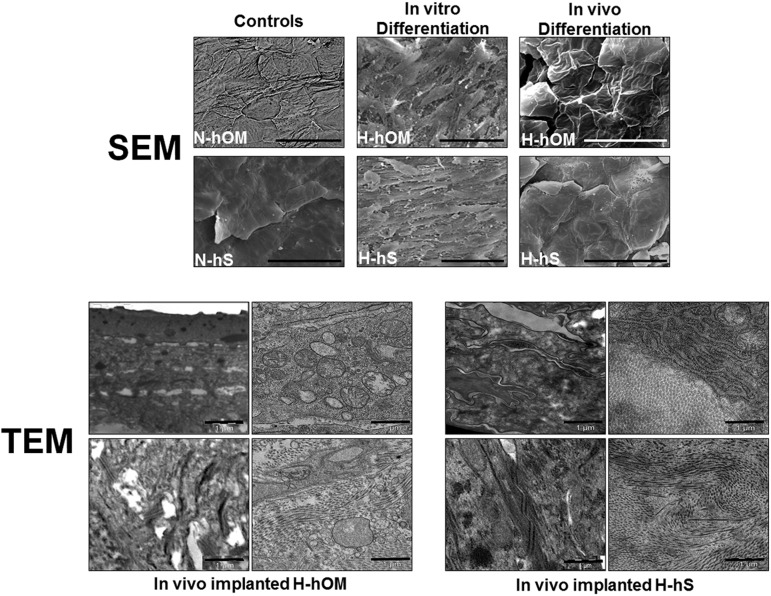
This was in agreement with the scanning electron microscopy (SEM) analysis of the ex vivo samples, which revealed that the patterns of surface differentiation of the cells on both tissues were very immature as compared with the control native tissues (Fig. 2). In fact both control N-hOM and N-hS showed a tight superficial layer of flat polygonal cells with desquamation signs in which cells were covering the entire surface. In the case of the N-hOM, typical patterns of terminal differentiation were identified as parallel and curved microplicae and pits. In contrast, H-hOM and H-hS cultured in vitro had a superficial layer of elongated, spindle-shaped cells, with spaces among them, and no surface patterns were identifiable. Finally, when these bioengineered tissues were grafted in vivo, cells tended to resemble the structure of the native control tissues, with flattened cells and evident signs of desquamation. Cells on the surface of the H-hOM displayed some parallel and curved microplicae, but pits were completely absent. The transmission electron microscopy ultrastructural analysis of the in vivo grafted samples (Fig. 2) confirmed the presence of an epithelial-like cell layer on top of the in vivo implanted tissues, with cells organized in different layers with some intercellular junctions. In addition, the presence of abundant rough endoplasmic reticulum and mitochondria suggests that the cells were metabolically active. On the other hand, the bioengineered stroma was rich in collagen fibers that were very similar to those of the control native samples.
Figure 2.
Electron microscopy analysis of controls and three-dimensional bioactive models of H-hOM and H-hS. SEM images (top) corresponding to N-hOM and N-hS controls showed a tight superficial layer of flat polygonal cells with desquamation signs in which cells were covering the entire surface, whereas samples kept in vitro for 2 weeks showed immature differentiation patterns, and samples implanted in vivo for 40 days tended to resemble the structure of the native control tissues, with flattened cells and evident signs of desquamation. Scale bars = 50 μm. TEM samples (bottom) were analyzed after 40 days of in vivo implantation and demonstrated that in vivo-implanted tissues were mature and well-differentiated, with numerous intercellular junctions, abundant cell organelles, and a collagen-rich stroma. Scale bars = 1 μm. Abbreviations: H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Epithelial Differentiative Capability of the HWJSCs as Determined by Cytokeratin Expression Analysis
As shown in supplemental online Table 2, the analysis of cytokeratin expression showed that the typical markers of simple epithelia CK7 and CK8, which were negative in control N-hOM and N-hS, were slightly positive in ex vivo cultured tissues, especially in H-hOM cultured for 2 weeks and in H-hS samples corresponding to 1 week of culture. In addition, CK8 expression was positive in H-hOM after 10 and 20 days of in vivo implantation. However, the CK7 and CK8 expression was negative in all H-hS implanted samples, as it was the case of the control samples (illustrative examples of the analysis of CK8 are shown in Fig. 3).
Figure 3.
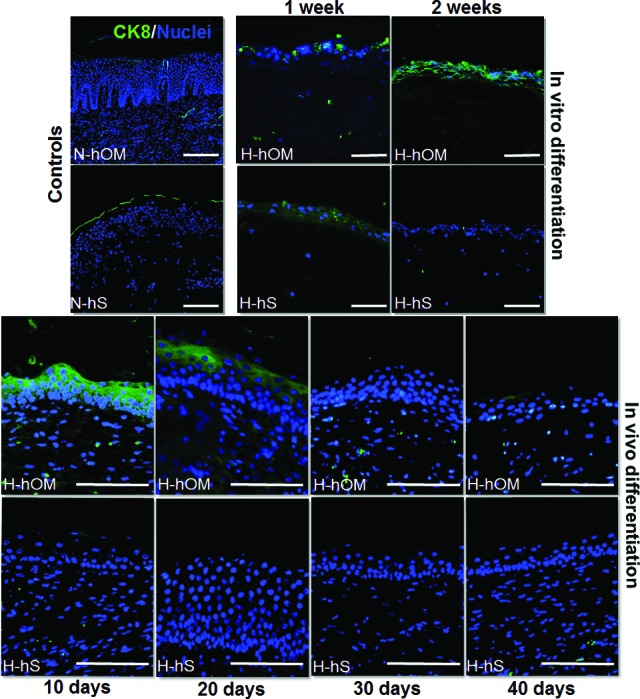
CK8 expression analysis as determined by immunofluorescence in three-dimensional bioactive models of H-hOM and H-hS. In vitro differentiation samples corresponding to tissues kept in culture for 1 and 2 weeks showed slightly positive expression of this cytokeratin. In vivo samples implanted on nude mice for 10, 20, 30, and 40 days were positive in H-hOM only after 10 and 20 days. N-hOM and N-hS were used as controls and showed negative expression of CK8. CK8 protein was labeled in green, and the cell nuclei were counterstained in blue. Scale bars = 100 μm. Abbreviations: CK, cytokeratin; H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin.
The analysis of the markers of stratified epithelia CK13 and CK4 demonstrated them to be positive in N-hOM and N-hS. The specific analysis of CK13 showed that this protein was expressed in all layers of H-hOM samples after 1 and 2 weeks of ex vivo development and at 20, 30, and 40 days of in vivo implantation, whereas H-hS samples showed positive CK13 expression only at the first week of ex vivo development. The expression of CK4 (supplemental online Fig. 2) was very similar, although it was negative in ex vivo H-hOM and positive in samples grafted in vivo for 10 days. Images of the analysis of CK13 are shown in Figure 4.
Figure 4.
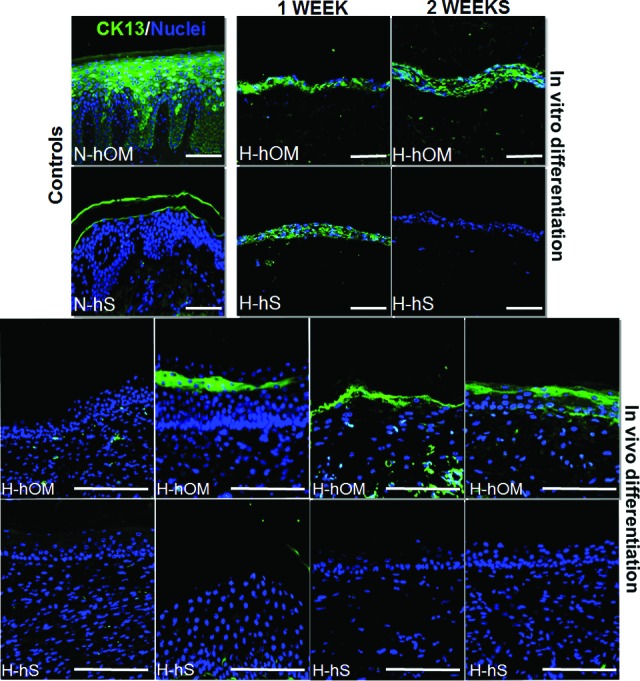
CK13 expression analysis as determined by immunofluorescence in three-dimensional bioactive models of H-hOM and H-hS. H-hOM samples were positive after 1 and 2 weeks of ex vivo development and at 20, 30, and 40 days of in vivo implantation on nude mice, whereas H-hS samples showed positive CK13 expression only at the first week of ex vivo development. N-hOM and N-hS were used as controls. CK13 protein was labeled in green, and the cell nuclei were counterstained in blue. Scale bars = 100 μm. Abbreviations: CK, cytokeratin; H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin.
Finally, the analysis of CK1 marker of keratinization in the suprabasal cells which were positive in both control tissues revealed that H-hOM and H-hS cultured ex vivo were negative for this cytokeratin. The in vivo analysis showed strong positive expression for most of the H-hOM and H-hS samples, especially after 40 days of in vivo grafting (Fig. 5). The anti-human nature of the CK1 antibody allowed us to determine that grafted tissues had human origin.
Figure 5.
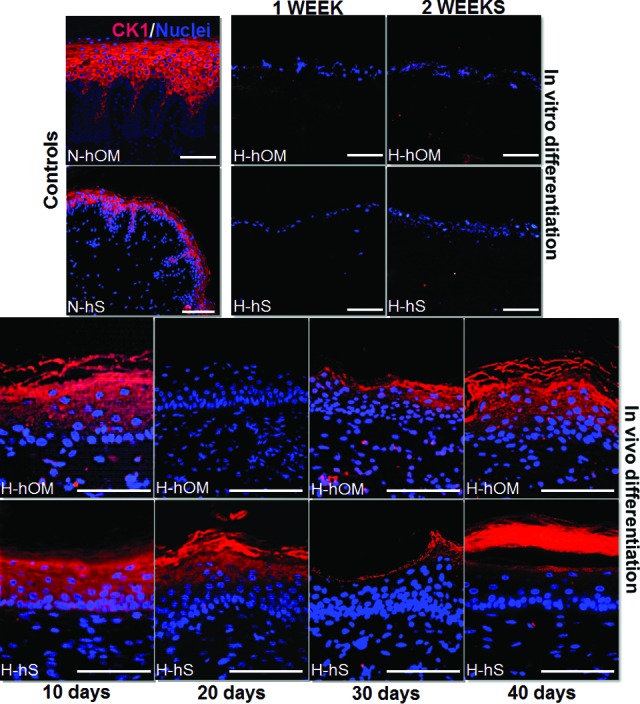
CK1 expression analysis as determined by immunofluorescence in three-dimensional bioactive models of H-hOM and H-hS. In vitro differentiation samples corresponding to tissues kept in culture for 1 and 2 weeks were negative for CK1 expression, whereas most in vivo samples implanted on nude mice for 10, 20, 30, and 40 days expressed this protein. N-hOM and N-hS used as controls were positive for CK1. CK1 protein was labeled in red, and the cell nuclei were counterstained in blue. Scale bars = 500 μm (N-hS panel) and 100 μm (other panels). Abbreviations: CK, cytokeratin; H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin.
Epithelial Differentiative Capability of the HWJSCs as Determined by Filaggrin and Involucrin Expression Analysis
Our results revealed that the expression of filaggrin (Fig. 6) and involucrin (supplemental online Fig. 3), markers of well-differentiated epithelia that were positive in H-hOM and H-hS, was initially negative in both H-hOM and H-hS cultured ex vivo for 1 week. However, the expression of these proteins increased after 2 weeks ex vivo only for H-hOM tissues. Once grafted in vivo the expression of both markers tended to become positive, especially after 20 days for the skin and 40 days for the oral mucosa. In the case of the oral mucosa, the expression of filaggrin was slightly positive from day 10 of in vivo grafting and from day 20 for the H-Hs (Fig. 6; supplemental online Table 2). The anti-human origin of involucrin antibody allowed us to confirm that grafted tissues were of human origin.
Figure 6.
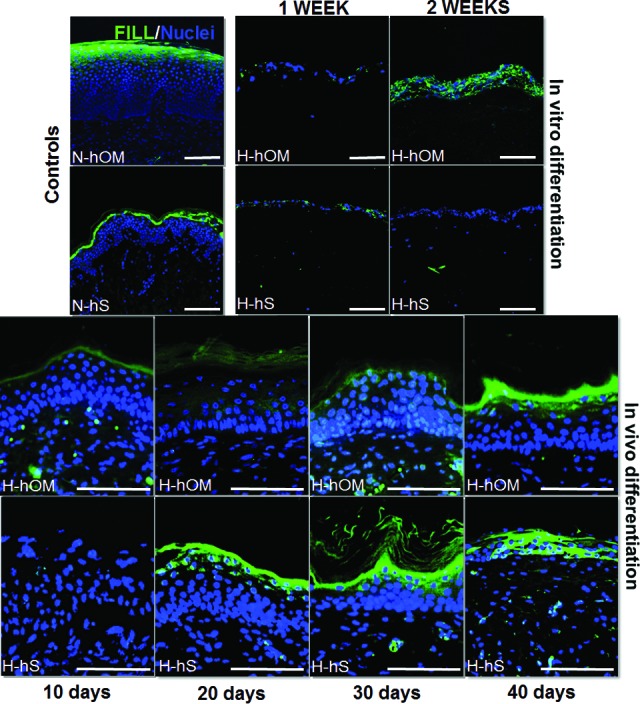
Filaggrin expression analysis as determined by immunofluorescence in three-dimensional bioactive models of H-hOM and H-hS. In vitro differentiation samples corresponding to tissues kept in culture for 1 and 2 weeks showed negative or slight expression of filaggrin, whereas in vivo samples implanted on nude mice for 10, 20, 30, and 40 days tended to express this protein with time. N-hOM and N-hS were used as controls and showed suprabasal expression of this marker. Filaggrin protein was labeled in green, and the cell nuclei were counterstained in blue. Scale bars = 100 μm. Abbreviations: FILL, filaggrin; H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin.
Determination of the Epithelial Differentiative Capability of the HWJSCs as Determined by Plakoglobin Expression Analysis
Our data showed that the expression of plakoglobin (PKG), a valuable marker of epithelial cell function by the determination of cell-cell adhesion, was positive in N-hOM and N-hS, especially at the suprabasal layers. It was negative for all ex vivo developed samples (H-hOM and H-hS). The in vivo results demonstrated that H-hOM and H-hS implanted onto athymic mice were gradually positive for PKG. In this regard, after 10 days of implantation the PKG signal was weak, increasing at 20 days with a suprabasal localization restricted to the granulosum strata. After 20 days H-hOM and H-hS samples showed a wide expression of PKG at the basal, spinosum, and granular strata, being stronger on H-hS samples (Fig. 7).
Figure 7.
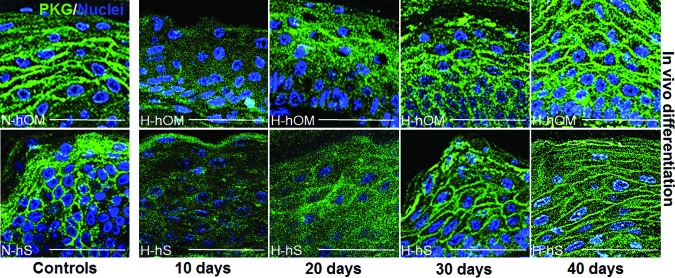
Plakoglobin expression analysis as determined by immunofluorescence in three-dimensional bioactive models of H-hOM and H-hS. In vivo samples implanted on nude mice showed positive expression of this protein, especially after long times of development in vivo, and became similar to the N-hOM and N-hS that were used as controls. Plakoglobin protein was labeled in green, and the cell nuclei were counterstained in blue. Scale bars = 50 μm. Abbreviations: H-hOM, heterotypical human oral mucosa; H-hS, heterotypical human skin; N-hOM, native human oral mucosa; N-hS, native human skin; PKG, plakoglobin.
Discussion
Over the past 5 years, scientific evidence has indicated that the intrinsic properties of both the stem cells and the cell niche have to be considered as key parameters for allogenic and autologous transplantation. HWJSCs can be easily harvested at low cost and efficiently expanded and cryopreserved. Many studies have previously demonstrated that HWJSCs are a promising cell source in regenerative medicine [6, 11]. In addition to this, numerous reports have indicated that HWJSCs are more efficient in terms of stem cell potency as compared with other mesenchymal cell types such as bone marrow stem cells. Also, these cells show shorter doubling time and more rapid propagation and expansion in culture [12]. The increasing interest in HWJSCs as alternative cell sources derives from their perinatal origin, representing a bridge between embryonic and adult stem cells. Although the differentiation potential of HWJSCs to mesodermic cell lineages, including the myogenic cell lineage, has been elucidated previously [13], further studies should verify whether the cells can also differentiate into endodermic and ectodermic cell types [14]. In this work we have studied the potential of HWJSCs to differentiate into ectodermic cell lineages by using tridimensional heterotypical models of H-hOM and H-hS at in vitro and in vivo levels.
First, our in vitro results suggest that HWJSCs have certain capabilities to differentiate to the epithelial lineage as revealed by the fact that cells subcultured on top of the fibrin-agarose stromal substitute tended to stay on the surface and to form a well-defined epithelial-like structure, especially in the case of the (H-hOM) substitutes. In addition, this structure expressed some simple epithelial markers such as CK8 and a marker of stratification such as CK13 in (H-hOM) samples. However, the differentiation level of HWJSCs under in vitro conditions was very limited, as demonstrated by the lack of typical features of differentiated skin and oral mucosa (well-defined strata, rete ridges, chorial papillae, and surface patterns), the absence of proper cell-cell contacts and intercellular junctions, and the negative expression of well-differentiated epithelial markers. In fact, both the hematoxylin and eosin staining and the SEM results confirmed the presence of spaces among the cells, whereas the immunofluorescence studies revealed the negative expression of PKG in all in vitro samples. This could be explained by the fact that HWJSCs in the umbilical cord are normally committed to synthesize high amounts of extracellular matrix (ECM), components, mainly collagen and several sulfated proteoglycans [15]. Probably, HWJSCs cultured in vitro for 2 weeks still retain some of their features of mesenchymal cells and synthesize small amounts of ECM components that could be responsible for the absence of direct cell-cell contacts in the newly formed and immature epithelial layer. In addition, the lack of rete ridges and stromal papillae and the absence of epithelial strata have been previously reported by our group at in vitro levels in models of orthotypical human oral mucosa and skin [3, 10].
Strikingly, once the H-hOM and H-hS tissues were grafted in vivo in animal models, HWJSCs was demonstrated to be able to efficiently differentiate to both oral mucosa and skin epithelia with higher maturation levels. In fact, the analysis of the samples revealed the formation of well-defined basal, spinosum, granulosum, and corneum cell layers, especially after 20 days in vivo. These findings, along with the presence of some specific differentiation patterns on the cell surface [16, 17], evidence the high differentiation capability of HWJSCs under in vivo conditions. However, the absence of typical rete ridges, the absence of chorial papillae, and the lack of type V cell surface patterns (pits) suggest that the differentiation process could require longer in vivo grafting times. In addition, both the H-hOM and the H-hS showed numerous cell-cell contacts, which became similar to those of the control tissues after 30 days in vivo. This implies that cells tend to join and form a tight epithelial barrier, which is important for the integrity and function of the epithelium [18]. The differentiated status of the heterotypical tissues grafted in vivo was confirmed by the expression of key markers of epithelial differentiation, such as CK1, filaggrin, and involucrin for both H-hOM and H-hS samples and CK4/13 in the case of the H-hOM, with the absence of CK8 expression. This suggests that the protein expression profile of these tissues was analogous to that of the native epithelia and may point out the functionality of these heterotypical epithelia generated from HWJSCs.
The analysis of the expression of CK8 demonstrated that its expression was evident at in vitro levels in both H-hOM and H-hS samples along with H-hOM in vivo samples. CK8 is considered a primary cytokeratin because it is the first to be produced in simple epithelia of embryos [19, 20]. Previous studies developed in our group revealed that the expression of CK8 in artificial human oral mucosa was associated with early stages of differentiation of epithelial cells and turned negative after in vivo implantation. Our results suggest that heterotypical tissues may have a low differentiation status at in vitro levels, which would change once implanted in vivo, especially in the case of H-hOM. CK4 and its partner CK13 are produced in the differentiating suprabasal cells of stratified epithelia, especially in oral mucosa. In this experiment, CK13 was expressed at in vitro and in vivo levels with high affinity for H-hOM samples. This notable affinity demonstrates that different bioengineered stromas could exert different and specific inductive roles that could determine the fate of the overlying differentiating epithelium. Besides, CK1 expression has been a gold standard marker of skin epithelial cells that is produced in the suprabasal cells and it is important for postmitotic differentiation in stratified cornified epithelia. In connection with cytokeratins, keratin filament-associated proteins (KFAPs), such as filaggrin, involucrin, and PKG cell-adhesive complex, provide a network that maintains cell integrity and cell function [21].
KFAPs are nonfilamentous structural proteins of the stratum granulosum of stratified cornified epithelia that also showed suprabasal expression after in vivo grafting. The conjugated expression of cytokeratins and KFAPs from the filament-matrix complex could stabilize the cytoskeleton of cornifying keratinocytes, thus ratifying the functional status of HWJSCs as epithelial cells. Apart from this, cytokeratins bind to PKG, maintaining the integrity of the cytoskeleton and acting as a signaling molecule during stratification. The presence of PKG could therefore favor the stratification of the epithelia, and this may explain the high number of cell layers observed at in vivo H-hOM and H-hS samples [22]. These results support the hypothesis that heterotypical tissues reached high maturation levels in response to the in vivo environment, with negativization of simple epithelium markers and positivization of typical markers of well-differentiated epithelia.
All these results suggest that HWJSCs might be able to differentiate into cells of ectodermal layers, thus allowing replacement of ectodermal tissues. To do so, in this study we have taken advantage of the intrinsic potential of HWJSCs in combination with inductive strategies. In fact, the high potentiality of these cells allowed different researchers to differentiate them to nonmesodermic cell lineages, such as the vascular endothelium [23] and neural tissues [24]. Interestingly, HWJSCs were shown to constitutively express some cytokeratins, and they are able to form cell layers on tridimensional collagen gels [25]. In addition, microarray studies reveal that HWJSCs express markers of all three primordial cell layers. For these reasons, several researchers suggested that HWJSCs could be in an intermediate differentiation state between embryonic stem cells and adult mesenchymal stem cells [6]. This would support the pluripotential capability of these cells under certain conditions [5].
To induce the nonmesenchymal differentiation of these cells, we used a double strategy using three-dimensional coculture systems based on human bioengineered tissues able to mimic the native epithelial-mesenchymal interaction in vitro and in vivo. The in vitro developed three-dimensional systems were grafted in vivo to determine the role of paracrine signaling from the host animal. As expected, HWJSCs were not able to efficiently differentiate to skin and oral mucosa epithelia in vitro, as previously demonstrated by Schneider et al. [25]. However, the use of in vivo strategies was able to demonstrate that these cells were fully capable of differentiating to epithelial keratinocytes, thus confirming the crucial role of paracrine signaling. The direct cell-cell contact between cells derived from different germ layers and the secretion of different tissue-specific factors by host mature adult keratinocytes may represent important differentiation stimuli for the in vivo epithelial differentiation of the mesenchymal cells.
Conclusion
In this work we have demonstrated that HWJSCs have the potential to differentiate to skin and oral mucosa epithelial cells in vivo. The host diffusible factors must be considered major regulatory factors, as the most elevated levels of differentiation of HWJSCs into keratinocytes were found at in vivo levels. All these findings support the idea that HWJSCs could be useful for the development of human skin and oral mucosa tissues for clinical use in patients with large skin and oral mucosa injuries.
See www.StemCellsTM.com for supporting information available online.
Supplementary Material
Acknowledgments
This study was supported by the Spanish Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III), Grants FIS PI11/1582 and FIS PI11/2668. We are grateful to Sam Hamzi for assistance with the English version.
Author Contributions
I.G.: collection and assembly of data, data analysis and interpretation, manuscript writing; J.M.: collection and assembly of data; M.G.-A.: provision of material of study, collection of data; R.C. and C.C.: collection of data; M.d.C.S.-Q.: conception and design, financial support; A.C.: data analysis and interpretation, final approval of the manuscript; M.A.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Ramos-e-Silva M, Jacques C. Epidermal barrier function and systemic diseases. Clin Dermatol. 2012;30:277–279. doi: 10.1016/j.clindermatol.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Bian Z, Kuijpers-Jagtman AM, et al. Skin and oral mucosa equivalents: Construction and performance. Orthod Craniofac Res. 2010;13:11–20. doi: 10.1111/j.1601-6343.2009.01475.x. [DOI] [PubMed] [Google Scholar]
- 3.Carriel V, Garzon I, Jimenez JM, et al. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. Cells Tissues Organs. 2012;196:1–12. doi: 10.1159/000330682. [DOI] [PubMed] [Google Scholar]
- 4.Garzon I, Serrato D, Roda O, et al. In vitro cytokeratin expression profiling of human oral mucosa substitutes developed by tissue engineering. Int J Artif Organs. 2009;32:711–719. doi: 10.1177/039139880903201002. [DOI] [PubMed] [Google Scholar]
- 5.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 6.Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton's Jelly stem cells: Future clinical applications. Placenta. 2011;32(suppl 4):S311–S315. doi: 10.1016/j.placenta.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Witkowska-Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton's jelly, amnion and chorion. Cell Mol Biol Lett. 2011;16:493–514. doi: 10.2478/s11658-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garzón I, Perez-Kohler B, Garrido-Gomez J, et al. Evaluation of the cell viability of human Wharton's jelly stem cells for use in cell therapy. Tissue Eng Part C Methods. 2012;18:408–419. doi: 10.1089/ten.tec.2011.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieto-Aguilar R, Serrato D, Garzon I, et al. Pluripotential differentiation capability of human adipose-derived stem cells in a novel fibrin-agarose scaffold. J Biomater Appl. 2011;25:743–768. doi: 10.1177/0885328209360425. [DOI] [PubMed] [Google Scholar]
- 10.Garzón I, Sanchez-Quevedo MC, Moreu G, et al. In vitro and in vivo cytokeratin patterns of expression in bioengineered human periodontal mucosa. J Periodontal Res. 2009;44:588–597. doi: 10.1111/j.1600-0765.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzuka T, Rachakatla RS, Doi C, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70:28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 13.Lupu M, Khalil M, Andrei E, et al. Integration properties of Wharton's jelly-derived novel mesenchymal stem cells into ventricular slices of murine hearts. Cell Physiol Biochem. 2011;28:63–76. doi: 10.1159/000331714. [DOI] [PubMed] [Google Scholar]
- 14.Conconi MT, Burra P, Di Liddo R, et al. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089–1096. [PubMed] [Google Scholar]
- 15.Malkowski A, Sobolewski K, Jaworski S, et al. FGF binding by extracellular matrix components of Wharton's jelly. Acta Biochim Pol. 2007;54:357–363. [PubMed] [Google Scholar]
- 16.Moreu G, Sanchez-Quevedo MC, Lopez-Escamez JA, et al. Cell surface patterns in normal human oral gingival epithelium: A quantitative scanning electron microscopy approach. Histol Histopathol. 1993;8:47–50. [PubMed] [Google Scholar]
- 17.Alaminos M, Garzon I, Sanchez-Quevedo MC, et al. Time-course study of histological and genetic patterns of differentiation in human engineered oral mucosa. J Tissue Eng Regen Med. 2007;1:350–359. doi: 10.1002/term.38. [DOI] [PubMed] [Google Scholar]
- 18.Golinski PA, Groger S, Herrmann JM, et al. Oral mucosa model based on a collagen-elastin matrix. J Periodontal Res. 2011;46:704–711. doi: 10.1111/j.1600-0765.2011.01393.x. [DOI] [PubMed] [Google Scholar]
- 19.Marceau N, Schutte B, Gilbert S, et al. Dual roles of intermediate filaments in apoptosis. Exp Cell Res. 2007;313:2265–2281. doi: 10.1016/j.yexcr.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 20.Kopan R, Fuchs E. A new look into an old problem: Keratins as tools to investigate determination, morphogenesis, and differentiation in skin. Genes Dev. 1989;3:1–15. doi: 10.1101/gad.3.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnemann J, Sullivan KH, Magee AI, et al. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J Cell Sci. 1993;104:741–750. doi: 10.1242/jcs.104.3.741. [DOI] [PubMed] [Google Scholar]
- 23.Alaminos M, Perez-Kohler B, Garzon I, et al. Transdifferentiation potentiality of human Wharton's jelly stem cells towards vascular endothelial cells. J Cell Physiol. 2010;223:640–647. doi: 10.1002/jcp.22062. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235–243. doi: 10.1016/j.brainresbull.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Schneider RK, Pullen A, Kramann R, et al. Long-term survival and characterisation of human umbilical cord-derived mesenchymal stem cells on dermal equivalents. Differentiation. 2010;79:182–193. doi: 10.1016/j.diff.2010.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.