Abstract
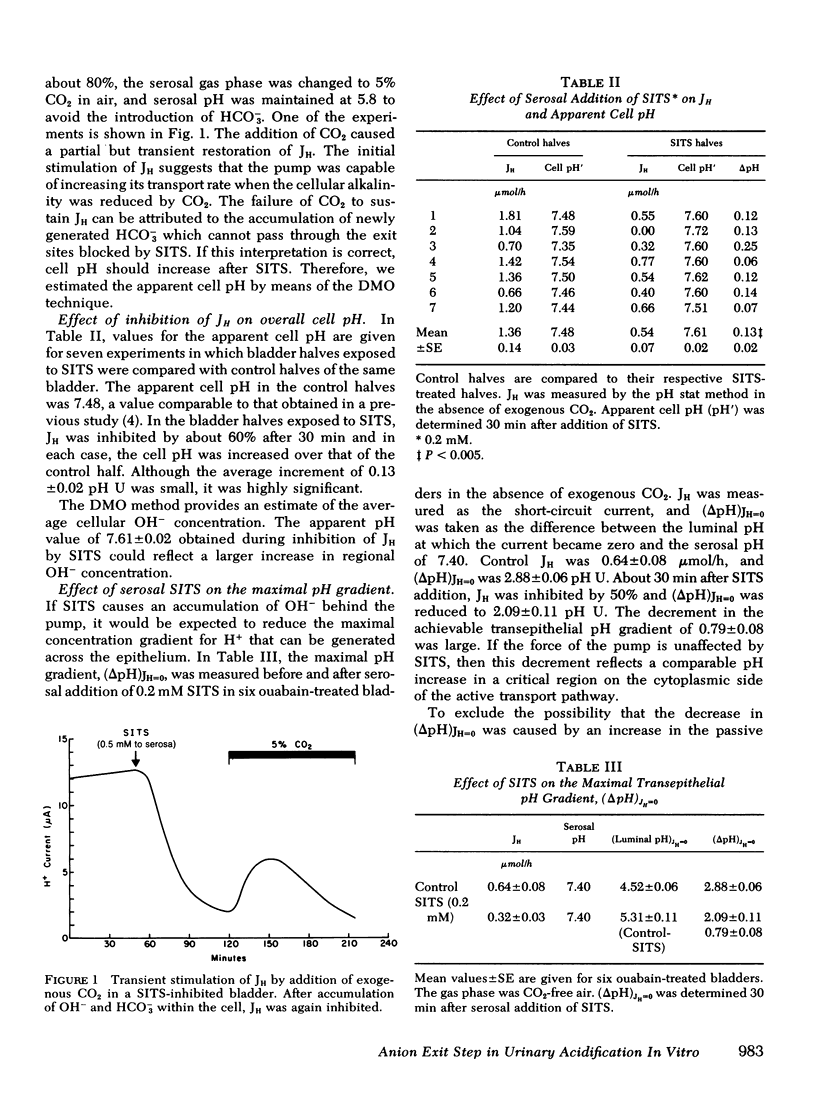
Acidification of the luminal solution by the isolated turtle bladder involves H+ secretion by a pump at the luminal membrane. The OH− dissociated in this process reacts with CO2 and forms HCO3− which moves passively out of the cell across the serosal cell membrane. In the present study, this exit step for HCO3− was inhibited by serosal addition of the disulfonic stilbene, SITS, an agent which is thought to bind to a transport protein at the serosal cell membrane. 90 min after serosal addition of 0.5 mM SITS, H+ secretion decreased by > 80%. In contrast, luminal addition of SITS had no effect. During inhibition of H+ secretion by serosal SITS, overall cell pH, measured by the 5, 5-dimethyl-2, 3-oxazolidinedione method, increased from 7.48±0.03 to 7.61±0.02. This increase of 0.13±0.02 pH U was associated with a much larger regional pH increase as judged from the decrement in the attainable pH gradient across the epithelium. After serosal SITS, this gradient was reduced from 2.88±0.06 to 2.09±0.11 pH U. In the absence of evidence for increased H+ permeability or a change in the force of the H+ pump, the gradient decrement of 0.79±0.08 U reflects a similar pH increment on the cytoplasmic side of the pump.
SITS inhibits the exit of bicarbonate across the serosal cell membrane and, thereby, creates a compartment of high alkalinity in series with the pump. The increased electrochemical gradient across the active transport pathway is the primary factor in the inhibition of urinary acidification.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauwens R., Al-Awqati Q. Active H+ transport in the turtle urinary bladder. Coupling of transport to glucose oxidation. J Gen Physiol. 1976 Oct;68(4):421–439. doi: 10.1085/jgp.68.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. II. Effects of proteolytic enzymes on disulfonic stilbene sites of surface proteins. J Membr Biol. 1974;15(3):227–248. doi: 10.1007/BF01870089. [DOI] [PubMed] [Google Scholar]
- Ehrenspeck G., Brodsky W. A. Effects of 4-acetamido-4'-isothiocyano-2,2-disulfonic stilbene on ion transport in turtle bladders. Biochim Biophys Acta. 1976 Feb 6;419(3):555–558. doi: 10.1016/0005-2736(76)90268-6. [DOI] [PubMed] [Google Scholar]
- Filho E. M., Malnic G. H in cortical peritubular capillaries of rat kidney. Pflugers Arch. 1976 Jun 22;363(3):211–217. doi: 10.1007/BF00594603. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie B. R., Schwartz J. H., Steinmetz P. R. Coupling between Cl- absorption and HCO3- secretion in turtle urinary bladder. Am J Physiol. 1973 Sep;225(3):610–617. doi: 10.1152/ajplegacy.1973.225.3.610. [DOI] [PubMed] [Google Scholar]
- Levinson C. The interaction of chloride with the sulfate transport system of Ehrlich ascites tumor cells. J Cell Physiol. 1975 Dec;87(2):235–244. doi: 10.1002/jcp.1040870212. [DOI] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Oliver J. A., Himmelstein S., Steinmetz P. R. Energy dependence of urinary bicarbonate secretion in turtle bladder. J Clin Invest. 1975 May;55(5):1003–1008. doi: 10.1172/JCI108000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein A., Cabantchik Z. I., Knauf P. Mechanism of anion transport in red blood cells: role of membrane proteins. Fed Proc. 1976 Jan;35(1):3–10. [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H. H+ current response to CO2 and carbonic anhydrase inhibition in turtle bladder. Am J Physiol. 1976 Aug;231(2):565–572. doi: 10.1152/ajplegacy.1976.231.2.565. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Rosen S., Steinmetz P. R. Carbonic anhydrase function and the epithelial organization of H+ secretion in turtle urinary bladder. J Clin Invest. 1972 Oct;51(10):2653–2662. doi: 10.1172/JCI107083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. Metabolic energy and PCO2 as determinants of H+ secretion by turtle urinary bladder. Am J Physiol. 1977 Aug;233(2):F145–F149. doi: 10.1152/ajprenal.1977.233.2.F145. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Acid-base relations in epithelium of turtle bladder: site of active step in acidification and role of metabolic CO2. J Clin Invest. 1969 Jul;48(7):1258–1265. doi: 10.1172/JCI106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Characteristics of hydrogen ion transport in urinary bladder of water turtle. J Clin Invest. 1967 Oct;46(10):1531–1540. doi: 10.1172/JCI105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Effect of luminal pH on ion permeability and flows of Na+and H+ in turtle bladder. Am J Physiol. 1971 Jun;220(6):1573–1580. doi: 10.1152/ajplegacy.1971.220.6.1573. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976 Jul 1;262(5563):54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


