Abstract
Investigators worldwide have for over forty years conducted case-control studies aimed at determining the causes of childhood cancer. The central challenge to conducting such research is the rarity of childhood cancer, thus many studies aggregate cases through clinical trials organizations such as COG. Rarity also precludes the use of prospective study designs, which are less prone to recall and selection biases. Despite these challenges a substantial literature on childhood cancer etiology has emerged but few strong environmental risk factors have been identified. Genetic studies are thus now coming to the fore with some success. The ultimate aim of epidemiologic studies is to reduce the population burden of childhood cancer by suggesting preventive measures or possibly by enabling early detection.
Keywords: Epidemiology, etiology, prevention
INTRODUCTION
Investigators worldwide have for over forty years conducted case-control studies aimed at determining the causes of childhood cancer, initially focusing on environmental risk factors but more recently examining genetics. The central challenge to conducting such research is the rarity of childhood cancer, thus many studies aggregate cases through clinical trials organizations such as COG. Rarity also precludes the use of prospective study designs, which are less prone to recall and selection biases. Despite these challenges a substantial literature on childhood cancer etiology has emerged and few strong environmental risk factors have been identified. Genetic studies are thus now coming to the fore with some success. The ultimate aim of epidemiologic studies is to reduce the population burden of childhood cancer by suggesting preventive measures or possibly by enabling early detection.
STATE OF THE DISCIPLINE
Our knowledge about the etiology of childhood cancer is still quite limited. One reason is the continued reliance on the case-control study design. Although the design is efficient for the study of rare diseases it is potentially more susceptible to recall and selection biases than the cohort design [1, 2]. The number of studies of each type of childhood cancer is roughly proportional to incidence, with dozens having been conducted in acute lymphoblastic leukemia [3] and only a handful having been conducted in Ewing sarcoma [4, 5], for instance. Most epidemiologic studies of childhood cancer have been conducted in Europe and North America, with COG and its predecessor groups contributing the bulk of research from the latter [6]..
The first generation of epidemiology studies gathered mainly by parental interview and medical record abstraction [6]. However, more recent studies have adopted the case-parent triad design for both practical and scientific reasons discussed below. This design allows estimation of genetic association, maternal genetic effects, and gene-by-environment interaction while avoiding the need to obtain a control group, which has become increasingly difficult in the last decade in many locales. Accordingly, many investigators now concentrate mainly on molecular epidemiology.
MAJOR RECENT ADVANCES
Epidemiologic research is by nature incremental, as careful replication of results is a hallmark of causality [7]. Consequently many recent advances in the field have been methodologic, such as aggregating cases through clinical trials networks [8], trying new methods of control selection [9–11], adopting new study designs, and improving exposure measurement [12–14]
Aggregating cases
In many jurisdictions, including European countries [15] and individual states in the U.S. [16], childhood cancers are centrally registered. In many instances cases may be contacted for research through registries, however few single jurisdictions are large enough to recruit adequate numbers of cases to studies. Hence, it is desirable to aggregate cases through national or international clinical trials organizations. The Childhood Cancer Research Network of COG provides a paradigmatic example.
Previously the conduct of epidemiologic studies within COG required local institutional IRB approval and physician permission before contacting patients, which resulted in a heavy administrative burden and a substantial loss of potential participants. In recognition of these difficulties, a pilot protocol was developed and activated at a 10% random sample of COG institutions in North America [8] involving two upfront consents for parents (and children, if age eligible). The first consent allowed name and contact information to be released to the COG registration system. In addition to this first consent, the second consent involved agreement to be potentially contacted in the future to consider taking part in a non-therapeutic study. These future studies would then be separately consented by the investigators conducting the study. By the end of the pilot study, over 2,200 individuals had been approached; 96% agreed to both levels of consent and only 1% refused both consent levels. Given the success of this pilot the CCRN opened groupwide within COG in late 2007 and as of mid-2012 there were over 27,000 registrations on the CCRN in the United States and Canada (Table I). Among CCRN registrants, ~93% gave permission for contact for future non-therapeutic research. For many tumors, the CCRN is the only entity worldwide that aggregates enough cases to enable a study.
Table I.
Enrollments on the Children’s Cancer Research Network (ACCRN07) protocol between July 1, 2008 and June 30, 2012
| Cancer | Number | Percent |
|---|---|---|
| Leukemia | 10109 | 36.81 |
| Hodgkin lymphoma | 1704 | 6.2 |
| Non-Hodgkin lymphoma | 1790 | 6.52 |
| CNS tumors | 4071 | 14.82 |
| Neuroblastoma | 1776 | 6.47 |
| Retinoblastoma | 375 | 1.37 |
| Wilms tumor | 1383 | 5.04 |
| Hepatoblastoma | 338 | 1.23 |
| Osteosarcoma | 884 | 3.22 |
| Ewing sarcoma | 547 | 1.99 |
| Rhabdomyosarcoma | 960 | 3.5 |
| Other soft tissue sarcoma | 918 | 3.34 |
| Germ cell tumors | 1045 | 3.81 |
| Thyroid carcinoma | 76 | 0.28 |
| Melanoma | 97 | 0.35 |
| Other tumors | 1390 | 5.06 |
Control selection
Control selection has become increasingly difficult on a nationwide basis in the United States [11] and elsewhere [17]. This has been documented by the Children’s Cancer Group (CCG)/COG, which conducted a number of case-control studies with control selection by random digit dialing over a twenty-five year period [11]. They found a significant decline in participant response, which mirrors many investigators’ recent experience [18–23]. Alternatives to random digit dialing include recruited through birth registries, friend controls, and genetic epidemiologic study designs [24]. Birth certificate controls were used in two recent COG studies [9, 10, 25] and were found to be comparable to random digit dialing controls there is a substantial administrative burden to dealing with more than thirty state birth registries. Hence, there is practical appeal to adopting the triad design discussed below.
Case-parent triad design
Due to the difficulty of recruiting controls as well as increasing interest in genetics, many have adopted the case-parent triad design [26, 27]. Scientifically the triad design is appealing because of its validity and flexibility; there are several statistical models by which one may analyze triad data, but all examine whether the inheritance of alleles by affected children deviates from Mendelian expectation [28–36]. The design allows estimation of risk ratios associated with alleles or haplotypes while being robust to confounding by ancestry (i.e., population stratification). It also allows the estimation of maternal genetic effects, parent-of-origin effects (i.e., imprinting), and gene-by-environment interaction. By adopting this design one precludes examination of environment only, however it is likely that the effect of most environmental factors is mediated through genetics.
Exposure assessment with dried blood spots
Dried blood spots (DBS) are stored for up to twenty years by a number of jurisdictions [37, 38] and have the potential of providing an unbiased assessment of exposures in late pregnancy. For instance, DBS have been used to examine prenatal exposure to tobacco[39] and folic acid [40], among many other possible analytes [41]. However, the ability to obtain DBS for childhood cancer research across multiple jurisdictions has not been tested. A pilot study in the United States was able to obtain written consent for release of DBS from 32% of participants, with the remainder being passive refusals or losses-to-follow-up. Five states sent DBS upon receipt of signed consent forms and the remainder did not provide them, either because they have been destroyed or because DBS release is not allowed [42]. It is thus possible to incorporate DBS into epidemiology studies for a small but useful fraction of patients in jurisdictions that retain them and allow release.
KEY INITATIVES
Exogenous risk factors
Many epidemiologic studies have sought to establish whether exogenous or environmental factors influence childhood cancer risk. Exploratory investigations in fact comprise the bulk of the literature for many types of childhood cancer [3, 43–45] and most candidate exposures have shown no strong associations. A few specific exposure-disease relationships based on strong hypotheses are however being investigated.
De novo infant leukemia frequently manifests MLL gene rearrangements, similar to secondary acute myeloid leukemia following etoposide therapy, leading to the hypothesis that maternal exposure to DNA topoismerase II inhibitors during pregnancy increases the risk of infant leukemia harboring MLL gene rearrangements [46], This hypothesis has been explored preliminarily in the United Kingdom [47] and Brazil [48], each of which found some associations of infant leukemia with maternal medication use during pregnancy. However, the most common source of exposure to DNA topoisomerase II inhibitors is diet. A single United States study has examined maternal intake of foods containing DNA topoismerase II inhibitors, which found a suggestive, but not significant, trend of increasing risk of MLL-rearrangement positive acute myeloid leukemia with increasing consumption [49].
Recent evidence indicates increased risk of hepatoblastoma in low (1,500–2,500 grams) and especially very low (<1,500 grams) birth weight infants [50]; among the smallest infants rates are as much as 20-fold higher than background. The association of hepatoblastoma with low birth weight has been reported in the United Kingdom, the United States, the Nordic countries, China, and Japan [45], suggesting a widespread exogenous risk factor. The most obvious possibility is that hepatoblastoma is initiated or promoted by iatrogenic exposures in neonatal intensive care units [51]. This hypothesis has been explored in two Japanese studies, which enrolled too few cases to credit the results [52, 53]. A much larger and more comprehensive case-control study of hepatoblastoma has been conducted by COG[54], the results of which will be available in the near future.
Genetic risk factors
A largely endogenous etiology is suggested for several types of childhood cancer. For instance, the close correlation of osteosarcoma incidence with the childhood growth curve, the earlier peak in incidence in females, and the frequent occurrence of tumors in the long bones of the leg suggest that the etiology of pediatric osteosarcoma is linked to bone growth [55, 56]. The implication of this descriptive epidemiology is that children with a more rapid or sustained growth spurt have a higher risk of osteosarcoma. As the timing and extent of adolescent bone growth are under substantial genetic control a number of investigations have concentrated on germline genetics in osteosarcoma [57, 58]. The largest such study was recently conducted by COG and initial analyses focused on 798 single nucleotide polymorphisms in 42 genes involved in estrogen metabolism and the insulin-like growth factor/growth hormone axis [26]; three variants were significantly associated with osteosarcoma after correction for multiple comparisons.
Risk of germ cell tumors also appears to be influenced largely by inherited propensity of germ cells to survive and resist apoptosis during prenatal migration. Compelling pilot data suggests that genes associated with adult testicular germ cell tumors, KITLG, SPRY4, BAK1 and DMRT1, are also associated with pediatric germ cell tumors in both sexes [59], and COG is currently investigating this hypothesis in a much larger ongoing study.
Ewing sarcoma occurs with an incidence of greater than 9:1 in children with European ancestry compared to those with primarily African or Asian ancestry [60]. While a recent genome-wide association study (GWAS) in France has identified single nucleotide polymorphisms (SNPs) associated with Ewing sarcoma in Europeans [61], it is unlikely that these explain such a wide gap in incidence between ancestries. Admixture mapping [62] of cases with African-American or Hispanic ancestry is likely to uncover additional loci that Ewing sarcoma cases inherited in common from European ancestors. In addition, recent data suggests that the length of microsatellites which are binding sites of the of EWS-FLI1 fusion protein influences downstream expression of Ewing sarcoma signaling pathways [63], suggesting that cases will have inherited a large number of consecutive GGAA-repeats in specific EWS-FLI1 transcriptional target genes. Both hypotheses are the subject of current investigation within COG.
Gene-environment interaction
Identification of gene-environment interaction can strengthen the evidence that exogenous exposures are associated with childhood cancers. For example, several studies in Europe and the United States have suggested that maternal vitamin supplementation during pregnancy may lower the risk of neuroblastoma [44, 64]. Because these associations have been determined by maternal interview in case-control studies, they may be due to recall or selection biases. However, mothers’ responses to interview should be unrelated to their genotypes- hence the appeal of studying interaction between maternal vitamin supplementation and common genetic polymorphisms involved in folate, vitamin A, and related metabolic and transport pathways in an ongoing COG study.
STRATEGIC APPROACH: ADVANCING THE DISCIPLINE
In the coming years most epidemiologic investigations of childhood cancer will incorporate genetics and further will move from mainly investigations of candidate genes to GWAS, as several have recently succeeded despite sample sizes that fall far short of recommendation [65] and despite a distinct lack of familial aggregation or twin concordance apart from known high-penetrance syndromes [66–71] (note that the high concordance for leukemia in monozygotic twins is thought to be due to in utero transmission rather than genetics[72]).
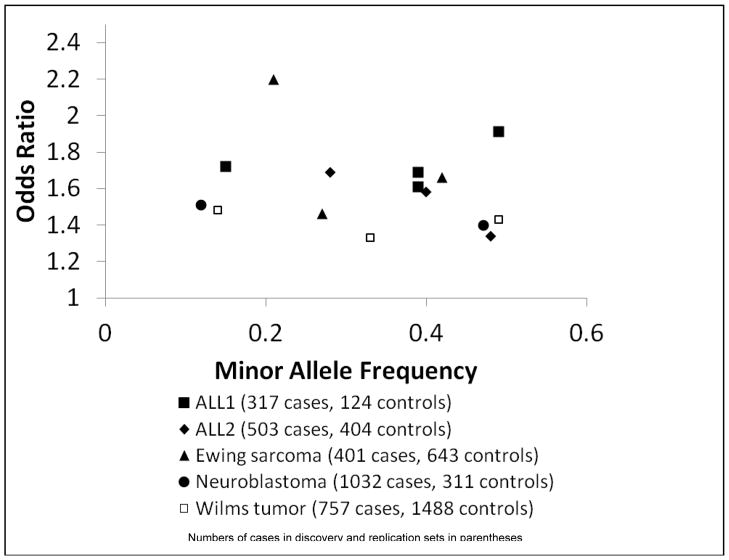
The apparent reason for the success of GWAS in pediatric cancers is that the magnitude of association, described by the odds ratio (OR) per allele, is greater in childhood compared to adult cancers. The median odds ratio (OR) per allele in GWAS of adult cancer was ~1.25 in a recent review [73], with 75% having an OR <1.5. In contrast, SNPs associated with four childhood cancers in GWAS have ORs per allele of between 1.4 and 2.2 [61, 74–77]. These ORs are plotted versus minor allele frequency in Figure 1; the legend includes the number of cases included in discovery and replication phases. Based on this empirical track record of childhood cancer GWAS, it is reasonable to expect a higher effect size for variants associated with other childhood cancers. Thus, although the achievable samples sizes will remain small compared to adult cancers, it is likely that the GWAS approach will to continue to succeed in pediatric tumors.
Figure 1.
Odds ratios per risk allele and minor allele frequencies for SNPs with genome-wide significance in the first five GWAS of childhood cancers.
Footnotes
THE AUTHORS HAVE NO CONFLICTS OF INTEREST TO DECLARE
References
- 1.Rudant J, Clavel J, Infante-Rivard C. Selection bias in case-control studies on household exposure to pesticides and childhood acute leukemia. J Expo Sci Environ Epidemiol. 2010;20:299–309. doi: 10.1038/jes.2009.61. [DOI] [PubMed] [Google Scholar]
- 2.Schuz J, Spector LG, Ross JA. Bias in studies of parental self-reported occupational exposure and childhood cancer. Am J Epidemiol. 2003;158:710–716. doi: 10.1093/aje/kwg192. [DOI] [PubMed] [Google Scholar]
- 3.Spector LG, Charbonneau B, Robison LL. Epidemiology and etiology. In: Pui CH, editor. Childhood Leukemias. 3. Cambridge: Cambridge University Press; 2012. pp. 49–71. [Google Scholar]
- 4.Valery PC, Holly EA, Sleigh AC, Williams G, Kreiger N, Bain C. Hernias and Ewing’s sarcoma family of tumours: a pooled analysis and meta-analysis. Lancet Oncol. 2005;6:485–490. doi: 10.1016/S1470-2045(05)70242-4. [DOI] [PubMed] [Google Scholar]
- 5.Valery PC, Williams G, Sleigh AC, Holly EA, Kreiger N, Bain C. Parental occupation and Ewing’s sarcoma: pooled and meta-analysis. Int J Cancer. 2005;115:799–806. doi: 10.1002/ijc.20933. [DOI] [PubMed] [Google Scholar]
- 6.Ross JA, Olshan AF. Pediatric cancer in the United States: the Children’s Oncology Group Epidemiology Research Program. Cancer Epidemiol Biomarkers Prev. 2004;13:1552–1554. [PubMed] [Google Scholar]
- 7.Linet MS, Wacholder S, Zahm SH. Interpreting epidemiologic research: lessons from studies of childhood cancer. Pediatrics. 2003;112:218–232. [PubMed] [Google Scholar]
- 8.Steele JR, Wellemeyer AS, Hansen MJ, Reaman GH, Ross JA. Childhood cancer research network: a North American Pediatric Cancer Registry. Cancer Epidemiol Biomarkers Prev. 2006;15:1241–1242. doi: 10.1158/1055-9965.EPI-06-0447. [DOI] [PubMed] [Google Scholar]
- 9.Puumala SE, Spector LG, Robison LL, Bunin GR, Olshan AF, Linabery AM, Roesler MA, Blair CK, Ross JA. Comparability and representativeness of control groups in a case-control study of infant leukemia: a report from the Children’s Oncology Group. Am J Epidemiol. 2009;170:379–387. doi: 10.1093/aje/kwp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spector LG, Ross JA, Puumala SE, Roesler M, Olshan AF, Bunin GR. Feasibility of nationwide birth registry control selection in the United States. Am J Epidemiol. 2007;166:852–856. doi: 10.1093/aje/kwm143. [DOI] [PubMed] [Google Scholar]
- 11.Bunin GR, Spector LG, Olshan AF, Robison LL, Roesler M, Grufferman S, Shu XO, Ross JA. Secular trends in response rates for controls selected by random digit dialing in childhood cancer studies: a report from the Children’s Oncology Group. Am J Epidemiol. 2007;166:109–116. doi: 10.1093/aje/kwm050. [DOI] [PubMed] [Google Scholar]
- 12.Linabery AM, Slater ME, Spector LG, Olshan AF, Stork SK, Roesler MA, Reaman GH, Ross JA. Feasibility of neonatal dried blood spot retrieval amid evolving state policies (2009–2010): a Children’s Oncology Group study. Paediatr Perinat Epidemiol. 2011;25:549–558. doi: 10.1111/j.1365-3016.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurek AM, Greenland S, Spector LG, Roesler MA, Robison LL, Ross JA. Self-report versus medical record - perinatal factors in a study of infant leukaemia: a study from the Children’s Oncology Group. Paediatr Perinat Epidemiol. 2011;25:540–548. doi: 10.1111/j.1365-3016.2011.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosco JL, Tseng M, Spector LG, Olshan AF, Bunin GR. Reproducibility of reported nutrient intake and supplement use during a past pregnancy: a report from the Children’s Oncology Group. Paediatr Perinat Epidemiol. 2010;24:93–101. doi: 10.1111/j.1365-3016.2009.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiller CA, Desandes E, Danon SE, Izarzugaza I, Ratiu A, Vassileva-Valerianova Z, Steliarova-Foucher E. Cancer incidence and survival in European adolescents (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2006–2018. doi: 10.1016/j.ejca.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Thompson TD, Miller JW, Pollack LA, Stewart SL. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121:e1470–1477. doi: 10.1542/peds.2007-2964. [DOI] [PubMed] [Google Scholar]
- 17.Law GR, Smith AG, Roman E. The importance of full participation: lessons from a national case-control study. Br J Cancer. 2002;86:350–355. doi: 10.1038/sj.bjc.6600092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle T, Landrigan J, Bulsara C, Fritschi L, Heyworth J. Increasing study participation. Epidemiology. 2011;22:279. doi: 10.1097/EDE.0b013e3182087666. [DOI] [PubMed] [Google Scholar]
- 19.Castano-Vinyals G, Nieuwenhuijsen MJ, Moreno V, Carrasco E, Guino E, Kogevinas M, Villanueva CM. Participation rates in the selection of population controls in a case-control study of colorectal cancer using two recruitment methods. Gac Sanit. 2011;25:353–356. doi: 10.1016/j.gaceta.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Cogswell ME, Bitsko RH, Anderka M, Caton AR, Feldkamp ML, Hockett Sherlock SM, Meyer RE, Ramadhani T, Robbins JM, Shaw GM, Mathews TJ, Royle M, Reefhuis J. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009;170:975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- 21.Mazloum M, Bailey HD, Heiden T, Armstrong BK, de Klerk N, Milne E. Participation in population-based case-control studies: does the observed decline vary by socio-economic status? Paediatr Perinat Epidemiol. 2012;26:276–279. doi: 10.1111/j.1365-3016.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- 22.Tam CC, Higgins CD, Rodrigues LC. Effect of reminders on mitigating participation bias in a case-control study. BMC Med Res Methodol. 2011;11:33. doi: 10.1186/1471-2288-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson DM, Marrie RA, Ashley-Koch A, Schiffer R, Trottier J, Wagner L. Design, methodological issues and participation in a multiple sclerosis case-control study. Acta Neurol Scand. 2011 doi: 10.1111/j.1600-0404.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross JA, Spector LG, Olshan AF, Bunin GR. Invited commentary: Birth certificates--a best control scenario? Am J Epidemiol. 2004;159:922–924. doi: 10.1093/aje/kwh137. discussion 925. [DOI] [PubMed] [Google Scholar]
- 25.Puumala SE, Ross JA, Wall MM, Spector LG. Pediatric germ cell tumors and parental infertility and infertility treatment: a Children’s Oncology Group report. Cancer Epidemiol. 2011;35:e25–31. doi: 10.1016/j.canep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musselman JR, Bergemann TL, Ross JA, Sklar C, Silverstien KAT, Langer EK, Savage SA, Nagarajan R, Krailo M, Malkin D, Spector LG. Case-parent analysis of variation in pubertal hormone genes and pediatric osteosarcoma: A Children’s Oncology Group (COG) study. Int J Mol Epidemiol Genet. 2012:3. [PMC free article] [PubMed] [Google Scholar]
- 27.Healy J, Bourgey M, Richer C, Sinnett D, Roy-Gagnon MH. Detection of fetomaternal genotype associations in early-onset disorders: evaluation of different methods and their application to childhood leukemia. J Biomed Biotechnol. 2010;2010:369534. doi: 10.1155/2010/369534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H. The interpretation of the parameters in the transmission/disequilibrium test. Am J Hum Genet. 1999;64:326–328. doi: 10.1086/302208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun F, Flanders WD, Yang Q, Khoury MJ. Transmission disequilibrium test (TDT) when only one parent is available: the 1-TDT. Am J Epidemiol. 1999;150:97–104. doi: 10.1093/oxfordjournals.aje.a009923. [DOI] [PubMed] [Google Scholar]
- 30.Laird NM, Blacker D, Wilcox M. The sib transmission/disequilibrium test is a Mantel-Haenszel test. Am J Hum Genet. 1998;63:1915–1916. doi: 10.1086/302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 32.Shi M, London SJ, Chiu GY, Hancock DB, Zaykin D, Weinberg CR. Using imputed genotypes for relative risk estimation in case-parent studies. Am J Epidemiol. 2011;173:553–559. doi: 10.1093/aje/kwq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi M, Umbach DM, Weinberg CR. Testing haplotype-environment interactions using case-parent triads. Hum Hered. 2010;70:23–33. doi: 10.1159/000298326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg CR. Allowing for missing parents in genetic studies of case-parent triads. Am J Hum Genet. 1999;64:1186–1193. doi: 10.1086/302337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg CR. Methods for detection of parent-of-origin effects in genetic studies of case-parents triads. Am J Hum Genet. 1999;65:229–235. doi: 10.1086/302466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg CR, Wilcox AJ, Lie RT. A log-linear approach to case-parent-triad data: assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parental imprinting. Am J Hum Genet. 1998;62:969–978. doi: 10.1086/301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olney RS, Moore CA, Ojodu JA, Lindegren ML, Hannon WH. Storage and use of residual dried blood spots from state newborn screening programs. J Pediatr. 2006;148:618–622. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 38.Agergaard P, Olesen C, Ostergaard JR, Christiansen M, Sorensen KM. Chromosome 22q11. 2 duplication is rare in a population-based cohort of Danish children with cardiovascular malformations. Am J Med Genet A. 2012;158A:509–513. doi: 10.1002/ajmg.a.34441. [DOI] [PubMed] [Google Scholar]
- 39.Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16:1902–1905. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 40.O’Broin S, Gunter E. Dried-serum spot assay for folate. Clin Chem. 2002;48:1128–1130. [PubMed] [Google Scholar]
- 41.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 42.Linabery AM, Slater ME, Spector LG, Olshan AF, Stork SK, Roesler MA, Reaman GH, Ross JA. Feasibility of neonatal blood spot retrieval amid evolving state policies (2009–2010): A Children’s Oncology Group study. Paediatr Perinat Epidemiol. 2011 doi: 10.1111/j.1365-3016.2011.01228.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu A, Heck JE, Ribeiro KB, Brennan P, Boffetta P, Buffler P, Hung RJ. Wilms’ tumour: a systematic review of risk factors and meta-analysis. Paediatr Perinat Epidemiol. 2010;24:449–469. doi: 10.1111/j.1365-3016.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 44.Heck JE, Ritz B, Hung RJ, Hashibe M, Boffetta P. The epidemiology of neuroblastoma: a review. Paediatr Perinat Epidemiol. 2009;23:125–143. doi: 10.1111/j.1365-3016.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 45.Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59:776–779. doi: 10.1002/pbc.24215. [DOI] [PubMed] [Google Scholar]
- 46.Ross JA, Potter JD, Robison LL. Infant leukemia, topoisomerase II inhibitors, and the MLL gene. J Natl Cancer Inst. 1994;86:1678–1680. doi: 10.1093/jnci/86.22.1678. [DOI] [PubMed] [Google Scholar]
- 47.Alexander FE, Patheal SL, Biondi A, Brandalise S, Cabrera ME, Chan LC, Chen Z, Cimino G, Cordoba JC, Gu LJ, Hussein H, Ishii E, Kamel AM, Labra S, Magalhaes IQ, Mizutani S, Petridou E, de Oliveira MP, Yuen P, Wiemels JL, Greaves MF. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61:2542–2546. [PubMed] [Google Scholar]
- 48.Pombo-de-Oliveira MS, Koifman S. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev. 2006;15:2336–2341. doi: 10.1158/1055-9965.EPI-06-0031. [DOI] [PubMed] [Google Scholar]
- 49.Spector LG, Xie Y, Robison LL, Heerema NA, Hilden JM, Lange B, Felix CA, Davies SM, Slavin J, Potter JD, Blair CK, Reaman GH, Ross JA. Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children’s oncology group. Cancer Epidemiol Biomarkers Prev. 2005;14:651–655. doi: 10.1158/1055-9965.EPI-04-0602. [DOI] [PubMed] [Google Scholar]
- 50.Spector LG, Puumala SE, Carozza SE, Chow EJ, Fox EE, Horel S, Johnson KJ, McLaughlin CC, Reynolds P, Behren JV, Mueller BA. Cancer risk among children with very low birth weights. Pediatrics. 2009;124:96–104. doi: 10.1542/peds.2008-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai TT, Bearer CF. Iatrogenic environmental hazards in the neonatal intensive care unit. Clin Perinatol. 2008;35:163–181. ix. doi: 10.1016/j.clp.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oue T, Kubota A, Okuyama H, Kawahara H, Nara K, Kawa K, Kitajima H. Hepatoblastoma in children of extremely low birth weight: a report from a single perinatal center. J Pediatr Surg. 2003;38:134–137. doi: 10.1053/jpsu.2003.50027. discussion 134–137. [DOI] [PubMed] [Google Scholar]
- 53.Maruyama K, Ikeda H, Koizumi T, Tsuchida Y, Tanimura M, Nishida H, Takahashi N, Fujimura M, Tokunaga Y. Case-control study of perinatal factors and hepatoblastoma in children with an extremely low birthweight. Pediatr Int. 2000;42:492–498. doi: 10.1046/j.1442-200x.2000.01287.x. [DOI] [PubMed] [Google Scholar]
- 54.Puumala SE, Ross JA, Feusner JH, Tomlinson GE, Malogolowkin MH, Krailo MD, Spector LG. Parental infertility, infertility treatment and hepatoblastoma: a report from the Children’s Oncology Group. Hum Reprod. 2012;27:1649–1656. doi: 10.1093/humrep/des109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirabello L, Pfeiffer R, Murphy G, Daw NC, Patino-Garcia A, Troisi RJ, Hoover RN, Douglass C, Schuz J, Craft AW, Savage SA. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22:899–908. doi: 10.1007/s10552-011-9763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011;2011:548151. doi: 10.1155/2011/548151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirabello L, Yu K, Berndt SI, Burdett L, Wang Z, Chowdhury S, Teshome K, Uzoka A, Hutchinson A, Grotmol T, Douglass C, Hayes RB, Hoover RN, Savage SA. A comprehensive candidate gene approach identifies genetic variation associated with osteosarcoma. BMC Cancer. 2011;11:209. doi: 10.1186/1471-2407-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruza E, Sotillo E, Sierrasesumaga L, Azcona C, Patino-Garcia A. Analysis of polymorphisms of the vitamin D receptor, estrogen receptor, and collagen Ialpha1 genes and their relationship with height in children with bone cancer. J Pediatr Hematol Oncol. 2003;25:780–786. doi: 10.1097/00043426-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Poynter JN, Hooten AJ, Frazier AL, Ross JA. Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes Chromosomes Cancer. 2012;51:266–271. doi: 10.1002/gcc.20951. [DOI] [PubMed] [Google Scholar]
- 60.Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973–2005. Cancer. 2009;115:3526–3536. doi: 10.1002/cncr.24388. [DOI] [PubMed] [Google Scholar]
- 61.Postel-Vinay S, Veron AS, Tirode F, Pierron G, Reynaud S, Kovar H, Oberlin O, Lapouble E, Ballet S, Lucchesi C, Kontny U, Gonzalez-Neira A, Picci P, Alonso J, Patino-Garcia A, de Paillerets BB, Laud K, Dina C, Froguel P, Clavel-Chapelon F, Doz F, Michon J, Chanock SJ, Thomas G, Cox DG, Delattre O. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat Genet. 2012;44:323–327. doi: 10.1038/ng.1085. [DOI] [PubMed] [Google Scholar]
- 62.Winkler CA, Nelson GW, Smith MW. Admixture mapping comes of age. Annu Rev Genomics Hum Genet. 2010;11:65–89. doi: 10.1146/annurev-genom-082509-141523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gangwal K, Close D, Enriquez CA, Hill CP, Lessnick SL. Emergent Properties of EWS/FLI Regulation via GGAA Microsatellites in Ewing’s Sarcoma. Genes Cancer. 2010;1:177–187. doi: 10.1177/1947601910361495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olshan AF, Smith JC, Bondy ML, Neglia JP, Pollock BH. Maternal vitamin use and reduced risk of neuroblastoma. Epidemiology. 2002;13:575–580. doi: 10.1097/00001648-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Spencer CC, Su Z, Donnelly P, Marchini J. Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 2009;5:e1000477. doi: 10.1371/journal.pgen.1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Couto E, Chen B, Hemminki K. Association of childhood acute lymphoblastic leukaemia with cancers in family members. Br J Cancer. 2005;93:1307–1309. doi: 10.1038/sj.bjc.6602867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemminki K, Mutanen P. Parental cancer as a risk factor for nine common childhood malignancies. Br J Cancer. 2001;84:990–993. doi: 10.1054/bjoc.2000.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buckley JD, Buckley CM, Breslow NE, Draper GJ, Roberson PK, Mack TM. Concordance for childhood cancer in twins. Med Pediatr Oncol. 1996;26:223–229. doi: 10.1002/(SICI)1096-911X(199604)26:4<223::AID-MPO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 69.Hemminki K, Jiang Y. Risks among siblings and twins for childhood acute lymphoid leukaemia: results from the Swedish Family-Cancer Database. Leukemia. 2002;16:297–298. doi: 10.1038/sj.leu.2402351. [DOI] [PubMed] [Google Scholar]
- 70.Inskip PD, Harvey EB, Boice JD, Jr, Stone BJ, Matanoski G, Flannery JT, Fraumeni JF., Jr Incidence of childhood cancer in twins. Cancer Causes Control. 1991;2:315–324. doi: 10.1007/BF00051671. [DOI] [PubMed] [Google Scholar]
- 71.Rodvall Y, Hrubec Z, Pershagen G, Ahlbom A, Bjurman A, Boice JD., Jr Childhood cancer among Swedish twins. Cancer Causes Control. 1992;3:527–532. doi: 10.1007/BF00052749. [DOI] [PubMed] [Google Scholar]
- 72.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102:2321–2333. doi: 10.1182/blood-2002-12-3817. [DOI] [PubMed] [Google Scholar]
- 73.Hindorff LA, Gillanders EM, Manolio TA. Genetic architecture of cancer and other complex diseases: lessons learned and future directions. Carcinogenesis. 2011;32:945–954. doi: 10.1093/carcin/bgr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maris JM, Mosse YP, Bradfield JP, Hou C, Monni S, Scott RH, Asgharzadeh S, Attiyeh EF, Diskin SJ, Laudenslager M, Winter C, Cole KA, Glessner JT, Kim C, Frackelton EC, Casalunovo T, Eckert AW, Capasso M, Rappaport EF, McConville C, London WB, Seeger RC, Rahman N, Devoto M, Grant SF, Li H, Hakonarson H. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papaemmanuil E, Hosking FJ, Vijayakrishnan J, Price A, Olver B, Sheridan E, Kinsey SE, Lightfoot T, Roman E, Irving JA, Allan JM, Tomlinson IP, Taylor M, Greaves M, Houlston RS. Loci on 7p12.2, 10q21.2 and 14q11. 2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41:1006–1010. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trevino LR, Yang W, French D, Hunger SP, Carroll WL, Devidas M, Willman C, Neale G, Downing J, Raimondi SC, Pui CH, Evans WE, Relling MV. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet. 2009;41:1001–1005. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turnbull C, Perdeaux ER, Pernet D, Naranjo A, Renwick A, Seal S, Munoz-Xicola RM, Hanks S, Slade I, Zachariou A, Warren-Perry M, Ruark E, Gerrard M, Hale J, Hewitt M, Kohler J, Lane S, Levitt G, Madi M, Morland B, Neefjes V, Nicholdson J, Picton S, Pizer B, Ronghe M, Stevens M, Traunecker H, Stiller CA, Pritchard-Jones K, Dome J, Grundy P, Rahman N. A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat Genet. 2012;44:681–684. doi: 10.1038/ng.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]