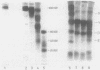
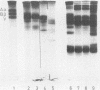
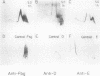
Abstract
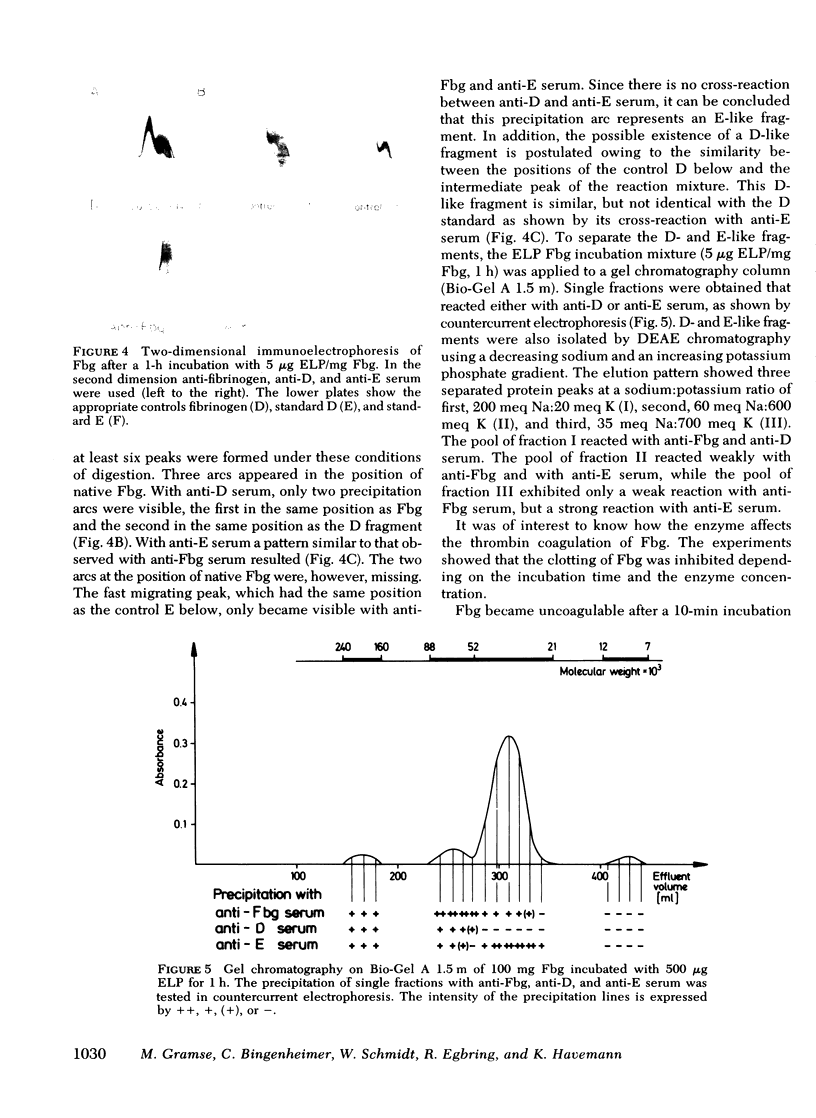
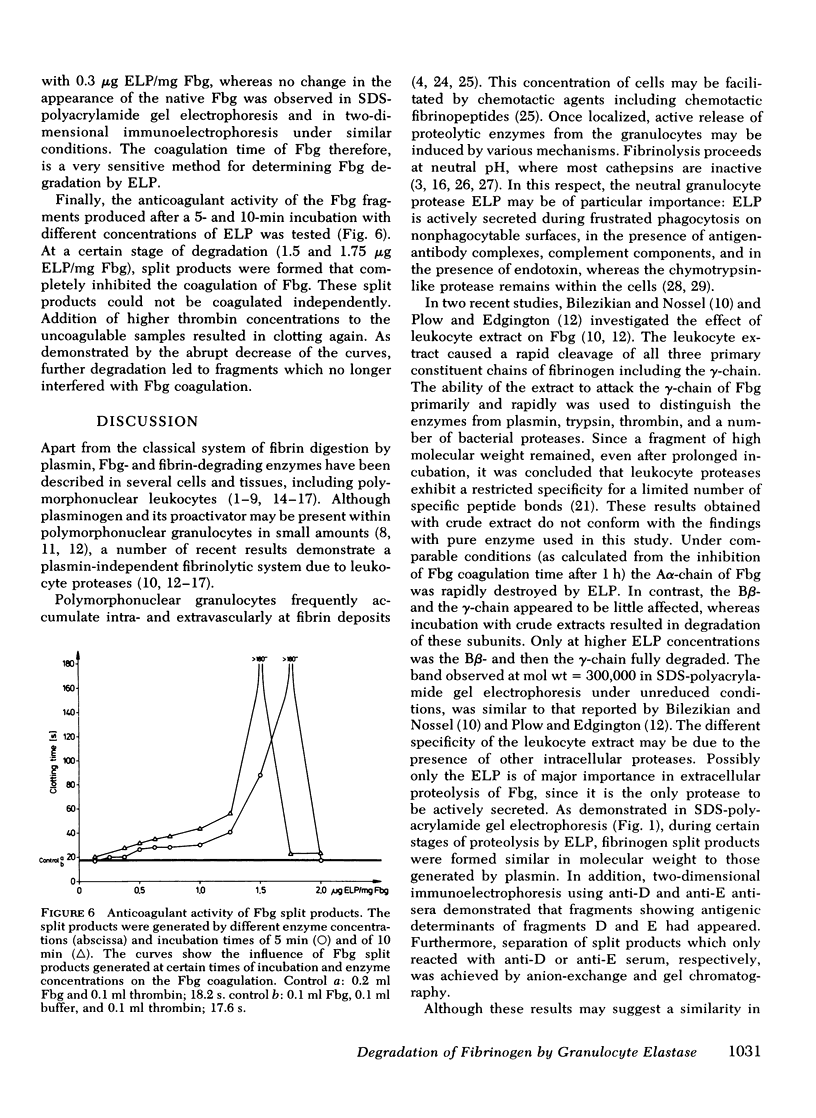
We investigated the effect of elastase-like neutral protease isolated from human granolocytes on human fibrinogen. Dependent on enzyme concentration and time of incubation, the elastase-like protease induced a progressive degradation of fibrinogen. Analysis of the remaining polypeptide chains showed a high susceptibility of the Aalpha- and low susceptibility of the gamma-chain of fibrinogen towards the proteolytic action of the enzyme. The split products were characterized by polyacrylamide gel electrophoresis and two-dimensional immunoelectrophoresis. They showed antigenic determinants of fibrinogen and of plasmin-induced proteolysis products D and E. The cleavage fragments isolated by gel chromatography had distinct molecular weights. Coagulability of fibrinogen by thrombin was inhibited according to the concentration of the protease and the time of incubation. Split products of fibrinogen with higher molecular weight prolonged the coagulation time of native fibrinogen, whereas low molecular weight fragments were ineffective.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup T., Henrichsen J., Kwaan H. C. Protease content and fibrinolytic activity of human leukocytes. Blood. 1967 Jan;29(1):134–138. [PubMed] [Google Scholar]
- Barnhart M. I. Importance of neutrophilic leukocytes in the resolution of fibrin. Fed Proc. 1965 Jul-Aug;24(4):846–853. [PubMed] [Google Scholar]
- Bilezikian S. B., Nossel H. L. Unique pattern of fibrinogen cleavage by human leukocyte proteases. Blood. 1977 Jul;50(1):21–28. [PubMed] [Google Scholar]
- CAVIEZEL O., VOLLERY M., VANNOTTI A. FIBRINOLYSE LEUCOCYTAIRE. Schweiz Med Wochenschr. 1964 Jul 18;94:1016–1020. [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Egbring R., Schmidt W., Fuchs G., Havemann K. Demonstration of granulocytic proteases in plasma of patients with acute leukemia and septicemia with coagulation defects. Blood. 1977 Feb;49(2):219–231. [PubMed] [Google Scholar]
- GANS H. FIBRINOLYTIC PROPERTIES OF PROTEASES DERIVED FROM HUMAN, DOG AND RABBIT LEUKOCYTES. Thromb Diath Haemorrh. 1964 Jan 1;10:379–389. [PubMed] [Google Scholar]
- HENRY R. L. LEUKOCYTES AND THROMBOSIS. Thromb Diath Haemorrh. 1965 Mar 15;13:35–46. [PubMed] [Google Scholar]
- Hermann G., Miescher P. A. Differentiation of leukocytic fibrinolytic enzymes from plasmin by the use of plasmatic proteolytic inhibitors. Int Arch Allergy Appl Immunol. 1965;27(6):346–354. doi: 10.1159/000229618. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Pepper D. S., Ewart M. R. Generation of chemotactic activity for leukocytes by the action of thrombin on human fibrinogen. Nat New Biol. 1973 May 9;243(123):56–57. [PubMed] [Google Scholar]
- MOUNTER L. A., ATIYEH W. Proteases of human leukocytes. Blood. 1960 Jan;15:52–59. [PubMed] [Google Scholar]
- Niemetz J. Coagulant activity of leukocytes. Tissue factor activity. J Clin Invest. 1972 Feb;51(2):307–313. doi: 10.1172/JCI106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K. Neutral leucocyte proteases and elastase inhibited by plasma alpha 1 -antitrypsin. Scand J Clin Lab Invest. 1971 Nov;28(3):251–253. doi: 10.3109/00365517109095696. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. An alternative pathway for fibrinolysis. I. The cleavage of fibrinogen by leukocyte proteases at physiologic pH. J Clin Invest. 1975 Jul;56(1):30–38. doi: 10.1172/JCI108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopowicz J. Distribution of fibrinolytic and proteolytic enzymes in subcellular fractions of human granulocytes. Thromb Diath Haemorrh. 1968 Mar 31;19(1):84–93. [PubMed] [Google Scholar]
- Prokopowicz J. Purification of plasminogen from human granulocytes using DEAE-Sephadex column chromatography. Biochim Biophys Acta. 1968 Jan 22;154(1):91–95. doi: 10.1016/0005-2795(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Egbring R., Havemann K. Effect of elastase-like and chymotrypsin-like neutral proteases from human granulocytes on isolated clotting factors. Thromb Res. 1975 Apr;6(4):315–329. doi: 10.1016/0049-3848(75)90081-x. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Chymotrypsin-like neutral protease from lysosomes of human polymorphonuclear leukocytes. Hoppe Seylers Z Physiol Chem. 1977 May;358(5):555–564. doi: 10.1515/bchm2.1977.358.1.555. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Wasi S., Murray R. K., Macmorine D. R., Movat H. Z. The role of PMN-leucocyte lysosomes in tissue injury, inflammation and hypersensitivity. II. Studies on the proteolytic activity of PMN-leucocyte lysosomes of the rabbit. Br J Exp Pathol. 1966 Aug;47(4):411–423. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]