Abstract
Importance
Although research on quality of life and dermatologic conditions is well represented in the literature, information on teledermatology’s effect on quality of life is virtually absent.
Objective
To determine the effect of store and forward teledermatology on quality of life.
Design
Two-site, parallel-group, superiority randomized controlled trial.
Setting
Dermatology clinics and affiliated sites of primary care at 2 US Department of Veterans Affairs medical facilities.
Participants
Patients being referred to a dermatology clinic were randomly assigned, stratified by site, to teledermatology or the conventional consultation process. Among the 392 patients who met the inclusion criteria and were randomized, 326 completed the allocated intervention and were included in the analysis.
Interventions
Store and forward teledermatology (digital images and a standardized history) or conventional text-based consultation processes were used to manage the dermatology consultations. Patients were followed up for 9 months.
Main Outcome Measures
The primary end point was change in Skindex-16 scores, a skin-specific quality-of-life instrument, between baseline and 9 months. A secondary end point was change in Skindex-16 scores between baseline and 3 months.
Results
Patients in both randomization groups demonstrated a clinically significant improvement in Skindex-16 scores between baseline and 9 months with no significant difference by randomization group (P=.66, composite score). No significant difference in Skindex-16 scores by randomization group between baseline and 3 months was found (P=.39, composite score).
Conclusions
Compared with the conventional consultation process, store and forward teledermatology did not result in a statistically significant difference in skin-related quality of life at 3 or 9 months after referral.
Trial Registration
clinicaltrials.gov Identifier: NCT00488293
For many dermatologic conditions, the health outcome measure of principal importance is self-reported quality of life.1,2 The effect skin disease may have on patient well-being and quality of life can be significant.3-6 For example, psoriasis was found to be more detrimental to quality of life than angina or hypertension and was associated with a reduction in physical and mental functioning comparable to that seen with cancer, arthritis, heart disease, diabetes, and depression.3,7 It has been reported that patients with pruritus are willing to forfeit 13% of their life expectancy to live without that symptom.8 Furthermore, the clinician’s judgment of skin disease severity is a poor proxy for the patient’s perspective.9-14
Although research on quality of life and dermatologic conditions is well represented in the literature, information on teledermatology’s effect on quality of life is virtually absent. To address this issue, we evaluated the effect of store and forward teledermatology on patients’ quality of life. We used the Skindex-16 as our quality-of-life measure. The Skindex-16 is a self-administered questionnaire developed using strict psychometric principles and is considered a superior measure for dermatology-specific quality-of-life research.15 Prior Skindex versions have been extensively tested and incrementally refined, resulting in a 16-item instrument that has internal consistency, reliability, reproducibility, evidence of content and construct validity, and responsiveness to clinical change.9,16-18 Skindex-16 measures 3 domains: symptoms, emotions, and functioning. Using the Skindex-16 and the Medical Outcomes Study 12-Item Short Form Health Survey, version 2 (SF-12 v2), we compared skin-specific quality of life and health status between patients undergoing a store and forward teledermatology consultation process with patients seen via a conventional clinic-based consultation process.
METHODS
DESIGN
The study was a 2-site, parallel-group, superiority randomized controlled trial. The study was designed to assess quality of life, clinical course, and economic outcomes. The primary outcome, quality of life, is presented in this article, and our hypothesis was that teledermatology would result in a significantly improved quality of life as measured by the Skindex-16. The study was powered using a 2-tailed test to assess for the alternative of conventional care superiority. Approval for this study was granted by the respective institutional review boards, and all participants provided informed consent.
SETTING
The setting for this study was the dermatology services of 2 Department of Veterans Affairs (VA) facilities: the Harry S Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, and the Minneapolis Veterans Affairs Health Care System (MVAHCS) in Minneapolis, Minnesota. These 2 medical centers serve as the site of dermatology care for the remote facilities from which patients were recruited. Patients in the catchment area for the Truman VA were recruited from 2 community-based outpatient clinics (primary care sites) affiliated with the Truman VA located 27 miles and 25 miles (to convert miles to kilometers, multiple by 1.6) from Columbia. Patients in the catchment area of the MVAHCS were recruited from the St Cloud VA Medical Center. The St Cloud VA Medical Center is located 66 miles from Minneapolis.
ELIGIBILITY CRITERIA
Eligible participants were adult patients being referred to one of the dermatology services from the remote sites of primary care. Patients were excluded if they had more than one skin condition for which they were being referred, did not have a visible or photographable skin condition, requested a fullbody examination, were unable to read or speak English, failed a single-question literacy assessment,19,20 had an emergent skin condition, had a pending dermatology clinic appointment within the next 9 months, had previously enrolled in this study, or had an impending move from the area in the next 9 months.
INTERVENTIONS
Potential study participants were identified when their primary care clinician generated a consultation with the dermatology service. Patients randomizing to usual care underwent the conventional consultation process used by each site. This process consisted of the referring clinician generating a consultation that was forwarded to the dermatology service via the VA’s electronic medical record. The electronic consultation includes text-based information only. In the case of Minneapolis, a template is used by the dermatology service with the option to include free text, whereas in Columbia the entire consultation is a free-text description. The dermatology services receive these consultations, review the text-based information, and typically schedule patients for a clinic-based visit. Patients are then asked to travel to the dermatology clinic at their respective medical center for a clinic-based evaluation.
Patients randomizing to teledermatology also used the electronic consultation request feature of the medical record because this is the required means of communicating referral requests and consultation results. In addition to the standard text field, the teledermatology consultation included a standardized history and a digital image set. The standardized history included a characterization of the condition as a rash vs growth, anatomical location, duration of presence, change in size (if any), symptoms or signs (pruritus, pain/burning, bleeding, change in color, or weeping/oozing), personal history of skin cancer, family history of skin cancer, history of childhood eczema, history of asthma or hay fever, previous treatments, and any other self-reported dermatologic history. All images were obtained with an 8-megapixel digital camera with an integrated flash (Canon Powershot S5 IS, Canon Inc). When necessary, a digital ring flash was used for short focal length or macroimages (SLR Digital Ring Light, Sakar International). The imaging protocol specified that the affected anatomical area be identifiable. Generally, this required at least one distance or midrange shot to show anatomical location and one close-up view of the affected area. However, a single image was allowable if the anatomical area was adequately represented by the close-up view. When necessary, additional images were obtained to show the entire distribution of the condition (eg, widespread rashes) or for close-up views that highlighted different areas of involvement.
Teledermatology as implemented in this study has been described as a teletriage system because the teledermatology consultation was not meant to replace the need or function as a surrogate for in-person visits for all referrals.21 A dermatologist reviewed the teledermatology consultation and either scheduled the patient for a clinic-based visit in the VA dermatology clinic or replied to the consultation with a diagnosis and/or management plan without scheduling the patient for a visit. In the latter case, the referring clinician was responsible for implementing the recommendations and relaying this information to the patient. Consistent with this teletriage concept, clinic-based procedure recommendations (eg, biopsies) were expected to result in an in-person dermatology clinic visit. However, medication recommendations (eg, trial of a topical steroid) would generally be implemented by the referring clinician. Patients could be re-referred by the primary care clinicians if they did not respond to recommendations or if they otherwise believed the patient needed to be seen in person by a dermatologist.
MEASUREMENTS AND OUTCOMES
Quality of life was assessed using Skindex-16 with the standard 1-week recall. Health status was assessed using the standard (4-week recall) SF-12 v2. All patients underwent baseline data collection that included the collection of demographic information, Skindex-16, SF-12 v2, and a comorbidity assessment. These last 3 instruments were self-administered. At 3 months after enrollment, the participants received the Skindex-16 and the SF-12 v2 via mail. Finally, the Skindex-16, SF-12 v2, and the comorbidity assessment were readministered (self-administration) at an in-person close-out dermatology clinic visit, scheduled 9 months after enrollment. If patients could not present for a close-out visit then the 9-month study instruments were mailed. A body diagram designating the location of the referred condition was completed at the baseline visit. To assist the patients in recalling their referred skin condition when filling out the Skindex-16, copies of this body diagram were provided to the patients at both the 3- and 9-month data collection points.
The instructions patients received for completion of the Skindex-16 instrument directed them to answer questions based on the skin condition that resulted in the dermatology referral (identified on the accompanying body diagram). Skindex-16 scoring and interpretation were performed in the standard method as described by Chren et al.18 Briefly, responses are recorded on a 0- (never bothered) to 100-point (always bothered) scale. Skindex-16 generates 3 domain scores (symptoms, emotions, and functioning) and a composite score (mean of 3 subscale scores).22 A Skindex-16 change score of 10 points is considered clinically significant—a value developed using an anchor-based approach to scale interpretation.23 In previous work, Skindex-16 scores were compared among patients before and after treatment for nonmelanoma skin cancer to a global “anchor” that assessed overall bother from the skin cancer.24 The anchor instrument had 7 response choices, and the minimum meaningful clinical difference was assumed to correspond to a difference in one response choice, based on previous interpretations and use of health transition global ratings.25-28 The mean change in Skindex-16 subscale scores of 10 points, for improvement or deterioration, correlated with the minimum change in the global rating response24 consistent with those observed for similar quality-of-life instruments.27
The SF-12 v2 scoring was performed according to the standard method described by Ware et al.29,30 The SF-12 v2 was used to determine the global health status of each study group at baseline, 3 months, and 9 months. A comorbidities checklist assessed whether the patients had any of 24 chronic medical conditions, whether they had any allergies, and if they took any over-the-counter or prescription medications. The checklist was a self-reported survey obtained on entry into the study and repeated at 9 months. A single question assessing global satisfaction with the care received for their skin condition was obtained from study participants at 9 months.
The study was designed to include the spectrum of skin disease expected in the target population and did not systematically include or exclude any specific conditions or attempt to analyze diagnostic-specific outcomes. However, we collected diagnostic information at close-out that assigned the patient’s diagnosis to 1 of 16 categories (or “other” diagnosis) that was used to provide descriptive diagnostic information of the skin conditions encountered among the study participants.
SAMPLE SIZE AND RANDOMIZATION
The study was powered based on detecting a mean absolute difference of 10 points in the change score for Skindex-16 between baseline and 9 months using a 2-sided t test. The estimate of SD (30 points) was obtained from Chren et al.24 The study was powered at 90% with an α of .05 (2-tailed), which required 190 patients per enrollment group. Consenting patients were randomized using a simple randomization scheme stratified by site to either a store and forward teledermatology consultation or the conventional consultation process (usual care). Randomization assignments were made by placing a telephone call to the statistical coordinating center after a prerandomization checklist was completed by the enrolling sites and verified by the coordinating center. Masking to study assignment was impossible with the study interventions.
STATISTICAL ANALYSIS
Demographic characteristics were compared between groups using χ2 tests for categorical variables and the t test for continuous variables. Quality of life as measured by the Skindex-16 was analyzed by the t test for the domains and composite score between groups. The SF-12 v2 was analyzed by the t test. Comorbidity assessments at baseline and 9 months were compared between study groups by χ2 tests. When baseline differences were found, an adjusted analysis was performed. Longitudinal analyses for each domain and composite Skindex-16 score using a mixed-effects model with random intercept and trend model to account for correlation among observations for each patient were performed. Finally, a global satisfaction assessment was obtained from the patients at 9 months. Satisfaction with the care they received for their skin condition was assessed using a 5-point Likert scale and was analyzed using the χ2 test. All analyses were conducted using SAS statistical software for Windows, version 9.2 (SAS Institute Inc).
RESULTS
RECRUITMENT
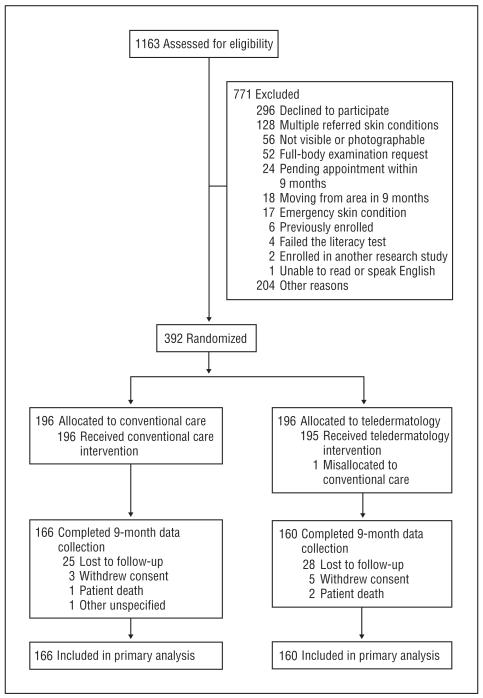
Recruitment, occurring between 2008 and 2011, was terminated when the scheduled enrollment deadline was reached. A total of 1163 consultations for patients being referred to the dermatology services was available for inclusion, and 392 patients enrolled in the study for an enrollment rate of 34%. Reasons for nonenrollment appear in the Figure. A total of 196 patients were randomized to the teledermatology intervention (160 patients at the Minneapolis site and 36 patients at the Columbia site), and 196 patients were randomized to the conventional consultation process (159 patients at the Minneapolis site and 37 patients at the Columbia site). A total of 166 patients in the usual care group and 160 patients in the teledermatology group completed the 9-month close-out procedures. Two patients in usual care did not complete a Skindex-16 survey at close-out, and 1 patient in teledermatology did not complete the symptoms domain questions at baseline.
Figure.
Participant enrollment diagram. Eligibility assessments at Minneapolis included a review of all incoming electronic consults for eligibility, whereas the Columbia site could not use that same mechanism. Eligibility assessments at Columbia required interaction with potential participants. Other reasons for exclusion included inability to follow up with the potential participant after the consultation was placed to determine interest or eligibility. More than one reason for not meeting inclusion criteria was possible among the 271 patients not meeting the inclusion criteria. The patient mislocated to conventional care was excluded from all analyses.
BASELINE CHARACTERISTICS
Demographic characteristics of the 2 study groups are listed in Table 1. No differences were found in demographic characteristics or baseline Skindex-16 scores between the 2 randomization groups, although “type of skin cancer–personal” approached statistical significance (P=.053). There was likewise no difference in demographic characteristics within each site of enrollment. The baseline Skindex-16 by site of enrollment found a significantly different mean (SD) functioning domain score (12.1 [21.5] vs 19.2 [26.4]; P=.02), with the lower score being recorded by the Columbia site. In an adjusted analysis by site of enrollment that used a longitudinal mixed-effects model, no differences in site of enrollment were found. The model included visit (baseline, 3 months, and 9 months), randomization group, site of enrollment, and their interaction terms. Only the visit variable showed significance with a negative slope (improved Skindex-16 score) for each domain.
Table 1. Characteristics of Patients at Baseline by Randomization Group.
| No. (%) of Patientsa |
|||
|---|---|---|---|
| Characteristic | Conventional Care (n = 196) |
Teledermatology (n = 195) |
P Value |
| Male sex | 192 (98.0) | 190 (97.4) | .73 |
| Ethnicity | |||
| Non-Hispanic | 191 (97.4) | 193 (99.0) | .33 |
| Hispanic | 2 (1.0) | 0 | |
| Unknown | 3 (1.5) | 2 (1.0) | |
| Race | |||
| Non-Hispanic white | 185 (94.4) | 188 (96.4) | .47 |
| Hispanic white | 2 (1.0) | 0 | |
| Black | 4 (2.0) | 4 (2.1) | |
| Other | 5 (2.6) | 3 (1.5) | |
| Age, mean (SD), y | 62.9 (13.9) | 61.7 (14.9) | .42 |
| Skindex-16 score at baseline, mean (SD) |
|||
| Symptoms | 31.4 (28.5) | 34.0 (29.5) | .38 |
| Emotions | 41.1 (30.9) | 43.9 (32.1) | .38 |
| Functioning | 17.4 (25.7) | 18.4 (25.8) | .70 |
| Composite | 30.0 (24.9) | 32.1 (25.6) | .41 |
| Skin condition Type |
|||
| Rash | 106 (54.1) | 101 (51.8) | |
| Growth | 90 (45.9) | 94 (47.1) | .65 |
| Duration | |||
| Acute | 16 (8.2) | 16 (8.3) | .99 |
| Subacute | 75 (35.8) | 75 (39.1) | |
| Chronic | 104 (53.3) | 101 (52.6) | |
| Change in size | |||
| Increase | 70 (35.7) | 79 (40.7) | .47 |
| Decrease | 10 (5.1) | 10 (5.2) | |
| Varies | 25 (12.8) | 16 (8.2) | |
| None | 91 (46.4) | 89 (45.9) | |
| Pruritus | 107 (54.6) | 104 (53.3) | .80 |
| Pain or burning | 66 (33.7) | 74 (37.9) | .38 |
| Bleeding | 45 (23.0) | 53 (27.2) | .34 |
| Color change | 66 (33.7) | 69 (35.4) | .72 |
| Weeping/oozing | 29 (14.8) | 30 (15.4) | .87 |
| Other symptom | 14 (7.1) | 6 (3.1) | .07 |
| Skin cancer history– personal |
25 (12.8) | 25 (12.8) | .98 |
| Type of skin cancer– personal |
|||
| Melanoma | 0 | 3 (12.0) | .053 |
| Nonmelanoma | 18 (72.0) | 20 (80.0) | |
| Unknown | 7 (28.0) | 2 (8.0) | |
| Skin cancer history– family |
31 (15.8) | 43 (22.4) | .10 |
| Type of skin cancer– family |
|||
| Melanoma | 3 (9.7) | 5 (11.6) | .94 |
| Nonmelanoma | 11 (35.5) | 16 (37.2) | |
| Unknown | 17 (54.8) | 22 (51.2) | |
| Skin disease– personal |
62 (31.6) | 53 (27.2) | .33 |
Data are presented as number (percentage) of patients unless otherwise indicated.
One hundred twenty patients (61.5%) who randomized to teledermatology were seen at least once for an in-person dermatology clinic visit. Teledermatology patients had a mean of 1.10 (range, 0-5) in-person dermatology clinic visits compared with a mean of 1.55 (range, 0-6) for usual care patients.
QUALITY-OF-LIFE OUTCOMES
Between baseline and 3 months, the point estimate Skindex-16 scores improved for both study groups among all domains and by composite score (Table 2). The improved mean scores are indicated by a negative mean difference. For the teledermatology group, a mean (SD) clinically significant improvement in the emotions domain was found (−11.6 [27.2]). No evidence suggested a difference in the change in quality of life as measured by the Skindex-16 between treatment groups. Likewise, between baseline and 9 months, the Skindex-16 scores improved for both study groups among all domains and by composite score (Table 2). Clinically significant improvements were reported for symptoms, emotions, and composite score for both randomization groups. In addition, no evidence suggested a difference in quality of life as measured by the Skindex-16 between treatment groups.
Table 2. Change in Skindex-16 Scores Among Treatment Groups.
| Skindex-16 Domain and Composite Scoresa |
||||
|---|---|---|---|---|
| Randomization Group | Symptoms | Emotions | Functioning | Composite |
| Baseline to 3 months | ||||
| Conventional care | ||||
| Mean (SD) | −8.0 (22.9) | −8.9 (25.3) | −0.5 (20.9) | −5.8 (19.1) |
| No. | 171 | 171 | 171 | 171 |
| Teledermatology | ||||
| Mean (SD) | −8.7 (29.8) | −11.6 (27.2) | −3.2 (20.2) | −7.8 (21.9) |
| No. | 160 | 160 | 160 | 161 |
| P value | .81 | .36 | .22 | .39 |
| Baseline to 9 months | ||||
| Conventional care | ||||
| Mean (SD) | −14.4 (28.2) | −18.1 (25.1) | −6.9 (22.3) | −13.2 (21.6) |
| No. | 164 | 164 | 164 | 164 |
| Teledermatology | ||||
| Mean (SD) | −10.3 (30.6) | −19.7 (30.7) | −6.0 (24.5) | −12.0 (24.5) |
| No. | 159 | 160 | 160 | 160 |
| P value | .22 | .61 | .73 | .66 |
Negative score indicates an improved Skindex-16 score.
HEALTH STATUS OUTCOMES
The SF-12 v2 found no evidence to suggest a difference in health status for any scale between study groups at any time point with one exception (data not shown). For the bodily pain assessed at 3 months scale, a significantly lower score (worse health state) was reported for the teledermatology group. The SF-12 v2 scores remained essentially unchanged during the study for both randomization groups. Likewise, the comorbidity assessment found no differences in self-reported morbidity with one exception (data not shown). Significantly more patients reported a history of transient ischemic attack among the conventional care group compared with teledermatology at baseline (13 vs 3; P = .01). However, no difference was noted for this disease entity at 9 months because 3 fewer conventional care patients reported this history and 2 additional teledermatology patients reported transient ischemic attacks.
GLOBAL SATISFACTION ASSESSMENT AND DIAGNOSTIC CATEGORIZATION
The global satisfaction assessment found no evidence for a difference in the level of satisfaction reported between patients managed by the 2 consultation modalities (Table 3). Few patients were unsatisfied with their care in either randomization group, although 3 patients in the teledermatology group expressed strong disagreement to the satisfaction query compared with no patients expressing that sentiment in usual care. Two of these 3 patients were not seen in a dermatology clinic for an in-person visit during the 9-month study period.
Table 3. Global Satisfaction With Dermatologic Care at 9 Monthsa.
| Overall, I Am Satisfied With the Care I Received for My Skin Problem |
No. (%) of Patients |
|
|---|---|---|
| Conventional Care (n = 166) |
Teledermatology (n = 159) |
|
| Strongly agree | 118 (71.5) | 106 (66.7) |
| Agree | 34 (20.6) | 32 (20.1) |
| Neutral | 11 (6.7) | 14 (8.8) |
| Disagree | 2 (1.2) | 4 (2.5) |
| Strongly disagree | 0 | 3 (1.9) |
P = .33.
The diagnostic categorization data are given in Table 4. As descriptive data, Table 4 indicates a similar spectrum of skin disease among the 2 groups.
Table 4. Diagnostic Categorization at 9 Months by Randomization Group.
| No. (%) of Patientsa |
||
|---|---|---|
| Diagnostic Categoryb | Conventional Care (n = 196) |
Teledermatology (n = 193)c |
| Benign keratosis | 16 (8.2) | 21 (10.9) |
| Benign nonmelanocytic neoplasm, other than | 15 (7.7) | 12 (6.2) |
| seborrheic keratosis | ||
| Actinic keratosis | 32 (16.3) | 21 (10.9) |
| Benign melanocytic neoplasm | 2 (1.0) | 11 (5.7) |
| Indeterminant melanocytic neoplasm | 2 (1.0) | 4 (2.1) |
| Melanoma | 0 | 1 (0.5) |
| Nonmelanoma skin cancer | 20 (10.2) | 16 (8.3) |
| Psoriasis | 12 (6.1) | 9 (4.7) |
| Papulosquamous dermatosis, other than psoriasis | 6 (3.1) | 2 (1.0) |
| Eczematous dermatitis | 50 (25.5) | 49 (25.4) |
| Collagen vascular disease | 0 | 1 (0.5) |
| Fungal infection | 4 (2.0) | 13 (6.7) |
| Bacterial infection | 2 (1.0) | 6 (3.1) |
| Viral infection | 1 (0.5) | 6 (3.1) |
| Other | 34 (17.3) | 21 (10.9) |
Percentages do not total 100% because of rounding.
No cases of “immunobullous disease” or “infestation” were recorded.
Two patients’ skin conditions could not be categorized.
COMMENT
Compared with the conventional (in-person) consultation process, store and forward teledermatology did not result in a statistically significant difference in skin-related quality of life at 3 or 9 months after referral. Participants in both randomization groups demonstrated improved point estimate skin-related quality-of-life scores at the 3- and 9-month periods. The randomization groups were comparable at baseline, health status and comorbidities were similar, and completion of the 3- and 9-month study procedures was high. Thus, our findings indicate that skin-related quality of life is not affected by consultation modality, and clinically significant improvements in quality of life are achieved with conventional care and store and forward teledermatology.
Only one prior study31 has assessed quality of life among recipients of teledermatology services. With no conventional care group for comparison, patients using teledermatology services were found to have mean Dermatology Quality of Life scores comparable to dermatology outpatients with a wide range of skin conditions (mean [SD] Dermatology Quality of Life score, 6.34 [6.68]). Overall, participants were very satisfied with the teledermatology intervention, with some evidence to suggest a relationship between reported quality of life and satisfaction.
Our findings are consistent with previous evidence for Skindex responsiveness in a heterogeneous group of skin conditions.9 We found that patients in both study groups, representing a wide range of ambulatory skin disease, had improved Skindex-16 scores at the 2 postreferral time points. Further, clinically significant improvements in 2 domains and the composite score were noted 9 months after referral for both study groups.
Contrary to our Skindex-16 findings, overall health status as measured by the SF-12 v2 remained relatively stable in both study groups throughout the study. It is, perhaps, not surprising that a skin-specific measure of quality of life would be more responsive to change over time to a dermatology intervention than would a generic measure, although our cohort had a fairly high level of competing comorbidities that could influence a generic measure, such as the SF-12 v2. These findings support the assertion that quality-of-life assessments in ambulatory skin disease populations should include a skin-specific instrument as an evaluation tool because other more generic instruments may lack discriminatory ability.16,32-34
The lack of evidence for a difference in the satisfaction level expressed by patients in both study groups is also an important finding. This finding suggests that patients’ judgment of the experience with the modality itself, which in the case of teledermatology was likely a novel experience, probably did not influence the outcomes, or perceived outcomes, of the care they received.
Our findings have some limitations. First, our demographic was primarily an elderly, male, and white population, which may influence the case mix of skin disease and the generalizability of our findings. Second, most of our data were based on self-report and required the ability to recall the referred skin condition. However, we do not believe this feature had an important influence on our data because we used valid and reliable instruments, we administered them in the standard fashion, and at each administration a body diagram marked with the location of the referred lesion was provided with the skin-specific study instrument (Skindex-16). Third, the missing data percentage for our primary outcome (Skindex-16) was 17%. Although this is a relatively low missing data rate, it is, nonetheless, possible that the nonresponders may have altered our findings. Nonresponse rates were similar between randomization groups. Fourth, our findings only apply to a teledermatology application that provides the option of in-person dermatology clinic follow-up after the teledermatology consultation is reviewed. The fact that most teledermatology patients also received in-person dermatology care may have contributed to the homogeneity of our findings. Our results may not be reproduced in teledermatology systems that are meant to replace all in-person contact with a dermatologist.35
An important strength of this study was that we assessed true site-to-site implementation of store and forward teledermatology with actual health care provision occurring via teledermatology. Thus, as an effectiveness study, it evaluated store and forward teledermatology in the manner that it would actually be provided in this setting. We believe this feature of the study design and the lack of evidence to suggest a difference in quality of life and global satisfaction has important operational implications for teledermatology. Before implementation, it is important to determine how a store and forward teledermatology consultation process performs compared with conventional care. Assessing an outcome of principal importance in ambulatory dermatology populations, we found no evidence to suggest a difference in quality of life between consultation modalities.
Acknowledgments
Funding/Support: Dr Chren is supported by grant K24 AR052667 from the National Institute of Arthritis and Musculoskeletal and Skin Disease, National Institutes of Health. This study was supported by grant HSR&D IIR 05-278 from the US Department of Veterans Affairs Health Services Research and Development Service.
Additional Information: Skindex-16 was used with the permission of Mary-Margaret Chren, MD.
Footnotes
Dr Whited is currently with the Durham VA Medical Center, Durham, North Carolina.
Author Contributions: Drs Whited, Warshaw, and Kapur had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Whited, Warshaw, Reda, Moritz, and Datta. Acquisition of data: Raju, Cook, Engasser, Pullen, Parks, Warshaw, Edison, and Marty. Analysis and interpretation of data: Whited, Warshaw, Kapur, Thottapurathu, Moritz, Reda, Motyka, Sindowski, Brown, and Datta. Drafting of the manuscript: Whited. Critical revision of the manuscript for important intellectual content: Warshaw, Kapur, Moritz, Reda, Datta, Edison, Chren, and Marty. Statistical analysis: Kapur, Thottapurathu, Motyka, Moritz, and Reda. Administrative, technical, and material support: Moritz, Sindowski, Motyka, Brown, and Chren. Study supervision: Whited, Warshaw, Reda, and Moritz. Obtained funding: Whited and Warshaw.
Conflict of Interest Disclosures: Drs Whited and Edison are coeditors of the book Teledermatology: A User’s Guide published by Cambridge University Press and receive royalties based on sales. Dr Chren is a consultant to Genetech Inc (on patient-reported outcomes).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government.
Contributor Information
Dr John D. Whited, Harry S Truman Memorial Veterans’ Hospital, Columbia.
Dr Erin M. Warshaw, Minneapolis Veterans Affairs Health Care System, Minneapolis; Department of Dermatology, University of Minnesota, Minneapolis.
Dr Karen E. Edison, Department of Dermatology, University of Missouri, Columbia.
Dr Kush Kapur, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Ms Lizy Thottapurathu, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Dr Srihari Raju, Minneapolis Veterans Affairs Health Care System, Minneapolis.
Dr Bethany Cook, Minneapolis Veterans Affairs Health Care System, Minneapolis.
Dr Holly Engasser, Minneapolis Veterans Affairs Health Care System, Minneapolis.
Dr Samantha Pullen, Minneapolis Veterans Affairs Health Care System, Minneapolis.
Ms Patricia Parks, Minneapolis Veterans Affairs Health Care System, Minneapolis.
Messr Tom Sindowski, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Ms Danuta Motyka, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Messr Rodney Brown, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Messr Thomas E. Moritz, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
Dr Santanu K. Datta, Durham Veterans Affairs Medical Center, Division of General Internal Medicine, Duke University Medical Center, Durham, North Carolina.
Dr Mary-Margaret Chren, Department of Dermatology, University of California, San Francisco, San Francisco Veterans Affairs Medical Center, San Francisco.
Dr Lucinda Marty, St Cloud Veterans Affairs Health Care System, St Cloud, Minnesota.
Dr Domenic J. Reda, Cooperative Studies Program Coordinating Center, Hines Veterans Affairs Hospital, Hines, Illinois.
REFERENCES
- 1.Chren MM. Giving “scale” new meaning in dermatology: measurement matters. Arch Dermatol. 2000;136(6):788–790. doi: 10.1001/archderm.136.6.788. [DOI] [PubMed] [Google Scholar]
- 2.Chren MM. Understanding research about quality of life and other health outcomes. J Cutan Med Surg. 1999;3(6):312–316. doi: 10.1177/120347549900300608. [DOI] [PubMed] [Google Scholar]
- 3.Finlay AY, Khan GK, Luscombe DK, Salek MS. Validation of Sickness Impact Profile and Psoriasis Disability Index in psoriasis. Br J Dermatol. 1990;123(6):751–756. doi: 10.1111/j.1365-2133.1990.tb04192.x. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Quality of life, health-state utilities and willingness to pay in patients with psoriasis and atopic eczema. Br J Dermatol. 1999;141(6):1067–1075. doi: 10.1046/j.1365-2133.1999.03207.x. [DOI] [PubMed] [Google Scholar]
- 5.Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140(4):672–676. doi: 10.1046/j.1365-2133.1999.02768.x. [DOI] [PubMed] [Google Scholar]
- 6.Kent G, Al’Abadie M. Psychologic effects of vitiligo: a critical incident analysis. J Am Acad Dermatol. 1996;35(6):895–898. doi: 10.1016/s0190-9622(96)90112-7. [DOI] [PubMed] [Google Scholar]
- 7.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 8.Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153–1156. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- 9.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107(5):707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 10.Finlay AY, Kelly SE. Psoriasis—an index of disability. Clin Exp Dermatol. 1987;12(1):8–11. doi: 10.1111/j.1365-2230.1987.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 11.Medansky RS, Handler RM, Medansky DL. Self-evaluation of acne and emotion: a pilot study. Psychosomatics. 1981;22(5):379–383. doi: 10.1016/S0033-3182(81)73507-2. [DOI] [PubMed] [Google Scholar]
- 12.Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17(1):1–3. doi: 10.1111/j.1365-2230.1992.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 13.Salek MS, Finlay AY, Luscombe DK, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 1993;129(4):422–430. doi: 10.1111/j.1365-2133.1993.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 14.Wessely SC, Lewis GH. The classification of psychiatric morbidity in attenders at a dermatology clinic. Br J Psychiatry. 1989;155:686–691. doi: 10.1192/s0007125000018201. [DOI] [PubMed] [Google Scholar]
- 15.De Korte J, Mombers FM, Sprangers MA, Bos JD. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol. 2002;138(9):1221–1227. doi: 10.1001/archderm.138.9.1221. [DOI] [PubMed] [Google Scholar]
- 16.Chren MM, Lasek RJ, Quinn LM, Covinsky KE. Convergent and discriminant validity of a generic and a disease-specific instrument to measure quality of life in patients with skin disease. J Invest Dermatol. 1997;108(1):103–107. doi: 10.1111/1523-1747.ep12285650. [DOI] [PubMed] [Google Scholar]
- 17.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11):1433–1440. [PubMed] [Google Scholar]
- 18.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 19.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 20.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21–27. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Veterans Affairs Patient Care Services [Accessed June 1, 2012];Teledermatology Operations Manual. 2011 Nov; Available only on the VA’s Intranet website. http://vaww.telehealth.va.gov/clinic/tdrm/index.asp.
- 22.Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134(4):454–458. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 23.Chren MM. Interpretation of quality-of-life scores. J Invest Dermatol. 2010;130(5):1207–1209. doi: 10.1038/jid.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351–1357. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, Clinical Significance Consensus Meeting Group Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 27.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 28.Sprangers MA, Moinpour CM, Moynihan TJ, Patrick DL, Revicki DA, Clinical Significance Consensus Meeting Group Assessing meaningful change in quality of life over time: a users’ guide for clinicians. Mayo Clin Proc. 2002;77(6):561–571. doi: 10.4065/77.6.561. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Kosinski N, Turner-Bowker DM, Gandek B. User’s Manual for the SF12v2® Health Survey (With a Supplement Documenting SF-12® Health Survey) QualityMetric Inc; Lincoln, RI: 2002. [Google Scholar]
- 31.Williams TL, Esmail A, May CR, et al. Patient satisfaction with teledermatology is related to perceived quality of life. Br J Dermatol. 2001;145(6):911–917. doi: 10.1046/j.1365-2133.2001.04472.x. [DOI] [PubMed] [Google Scholar]
- 32.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127(12):2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- 33.Kini SP, DeLong LK. Overview of health status quality-of-life measures. Dermatol Clin. 2012;30(2):209–221. xiii. doi: 10.1016/j.det.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 34.van Cranenburgh OD, Prinsen CAC, Sprangers MAG, Spuls PI, de Korte J. Health-related quality-of-life assessment in dermatologic practice: relevance and application. Dermatol Clin. 2012;30(2):323–332. x. doi: 10.1016/j.det.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Warshaw EM, Hillman YJ, Greer NL, et al. Teledermatology for diagnosis and management of skin conditions: a systematic review. J Am Acad Dermatol. 2011;64(4):759–772. doi: 10.1016/j.jaad.2010.08.026. [DOI] [PubMed] [Google Scholar]