Abstract
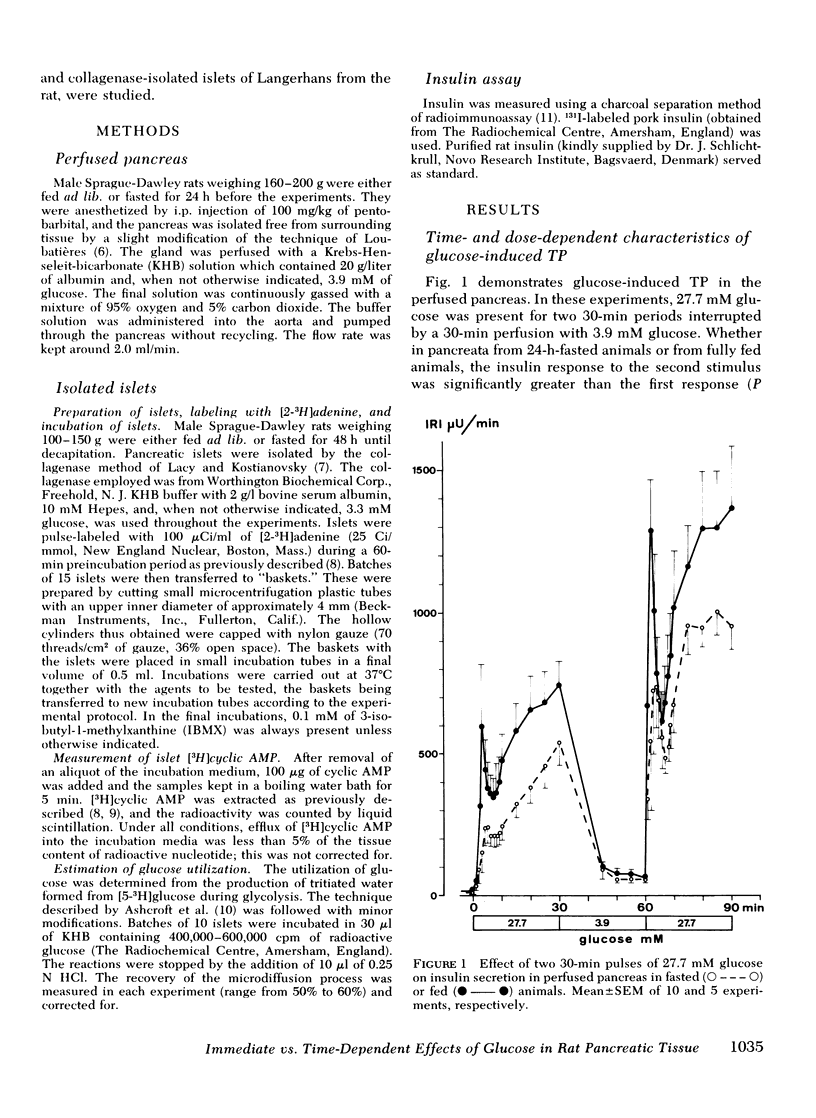
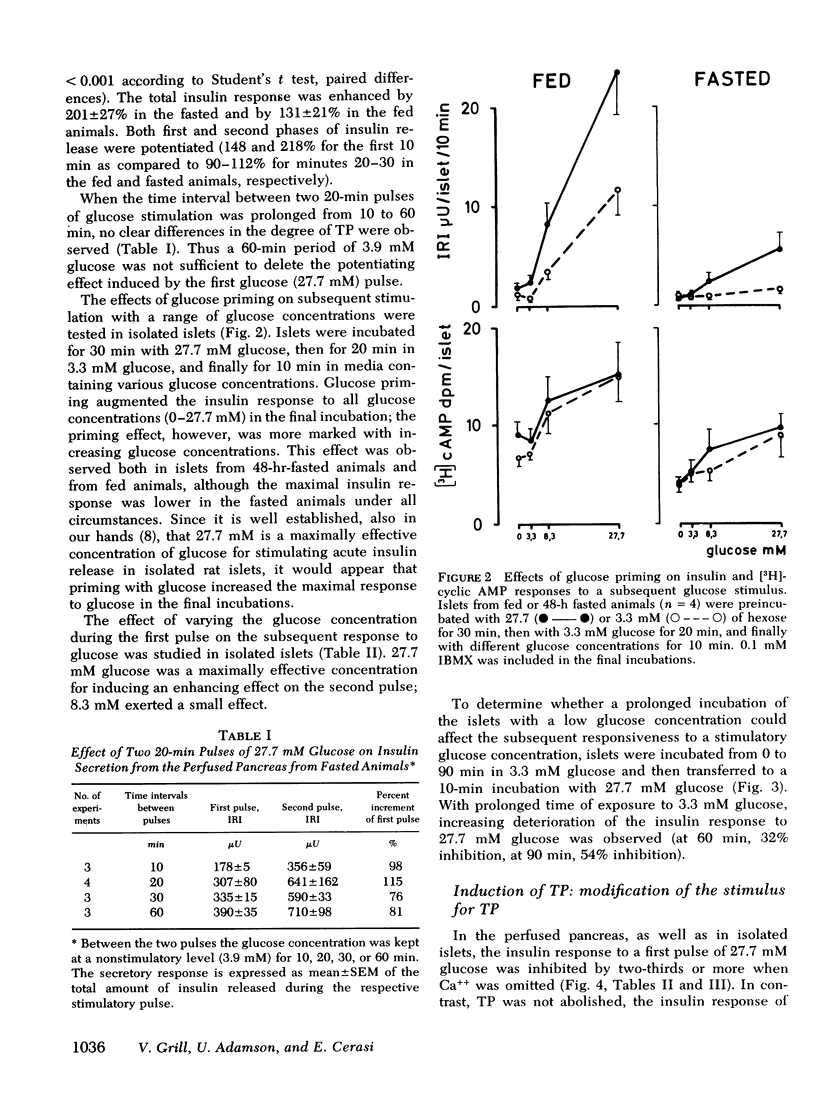
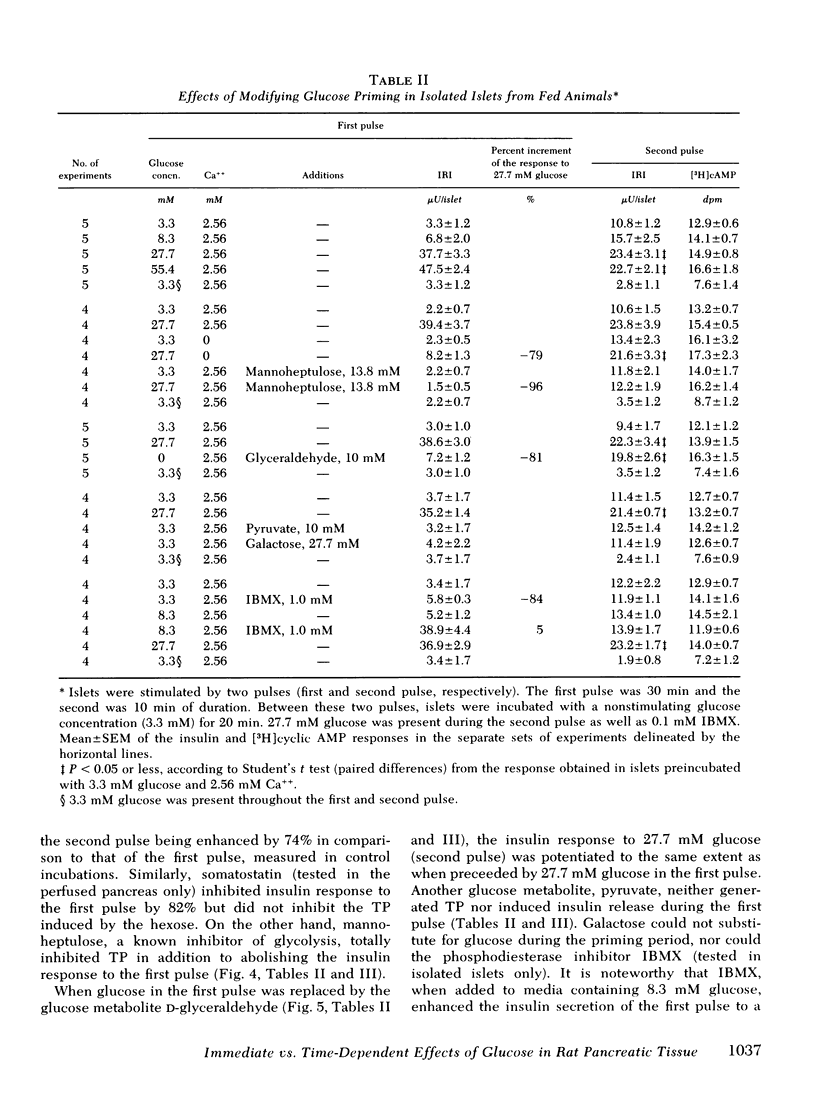
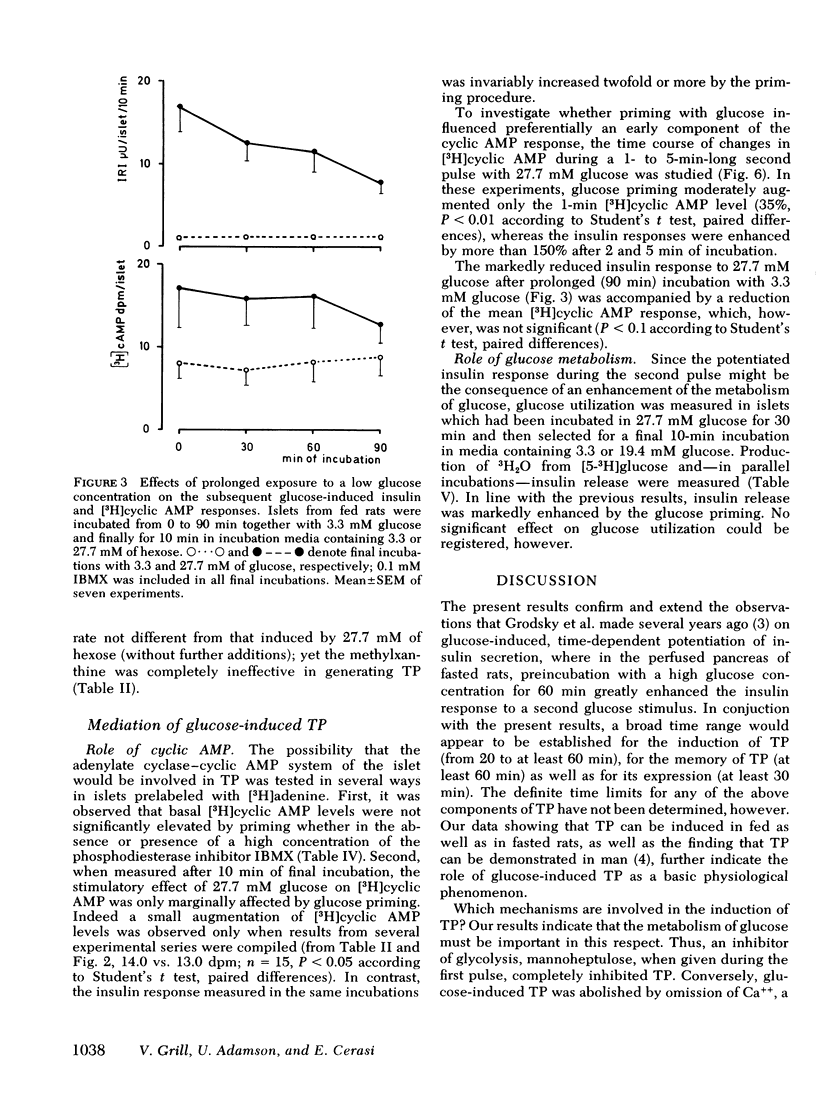
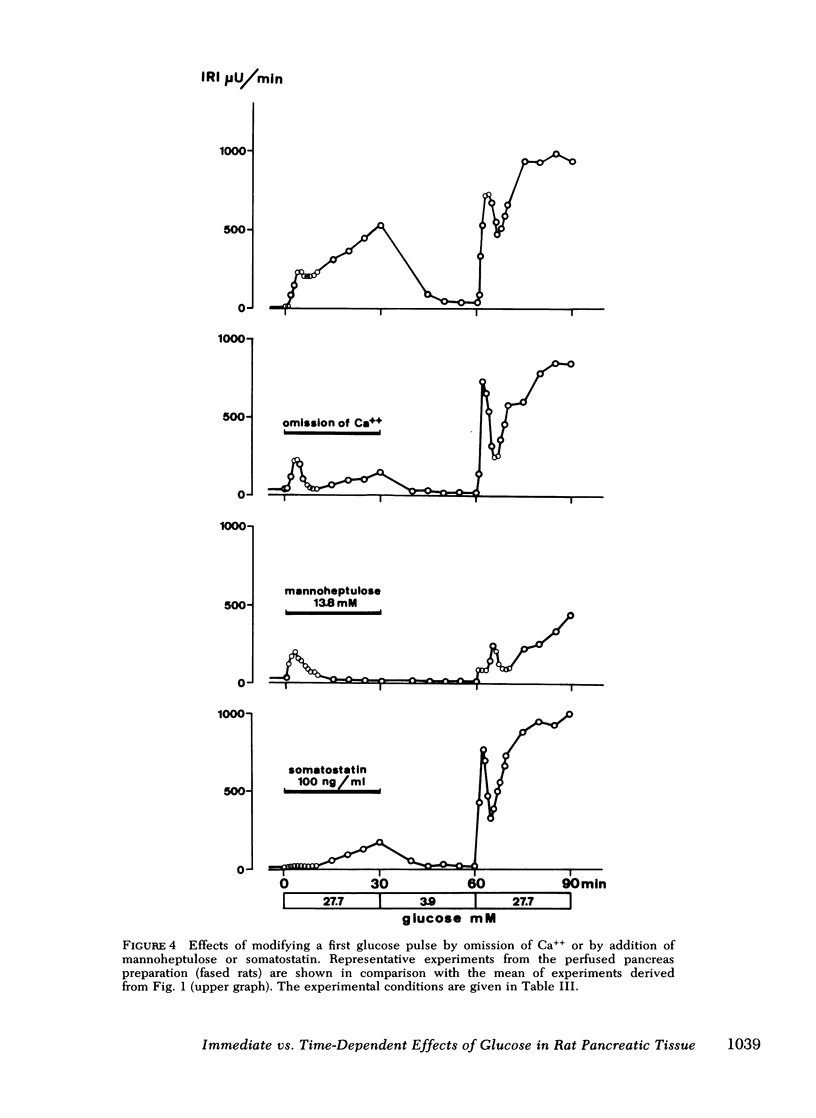
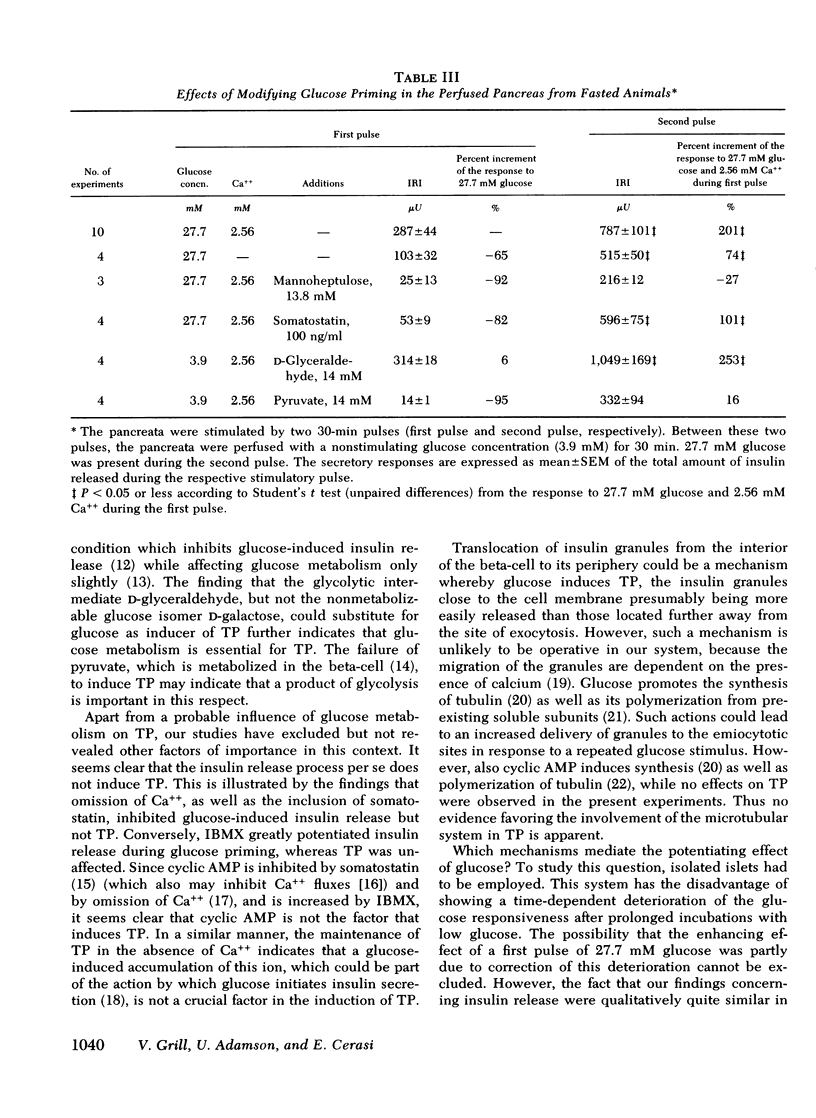
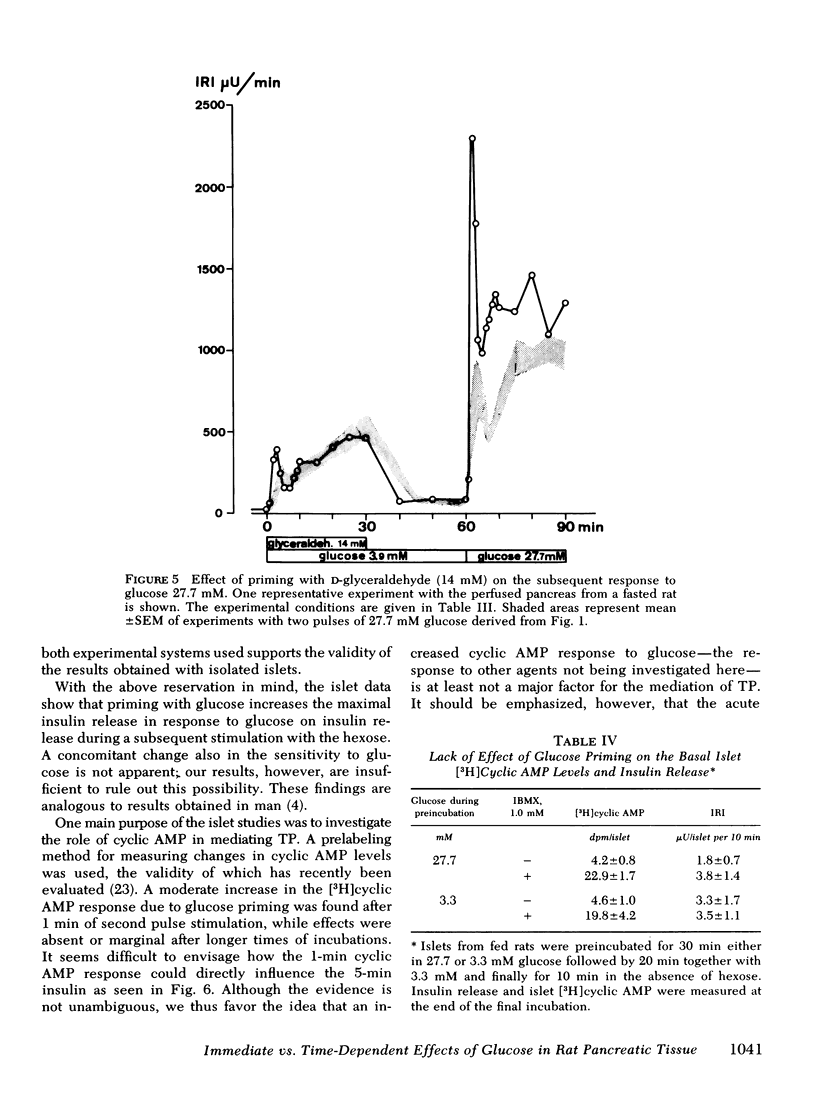
Glucose-induced insulin secretion is enhanced by a preceeding glucose stimulus. The characteristics of this action of glucose were investigated in perfused pancreas and collagenase-isolated islets of Langerhans. A 20- to 30-min pulse of 27.7 mM glucose enhanced both the first and second phase of insulin release in response to a second glucose stimulus by 76-201%. This enhancement was apparent as an augmented maximal insulin release response to glucose. The effect of priming with glucose was seen irrespective of whether the pancreatic tissue was obtained from fed or fasted rats. Separating the two pulses of hexose by a 60-min time interval of exposure to 3.3 mM glucose did not abolish the potentiation of the second pulse. Omission of Ca++ as well as the inclusion of somatostatin or mannoheptulose during the first pulse abolished insulin secretion during this time period; however, only the inclusion of mannoheptulose deleted the potentiation of the second pulse. d-Glyceraldehyde, but not pyruvate, d-galactose, or 3-isobutyl-1-methylxanthine, could substitute for glucose in inducing potentiation.
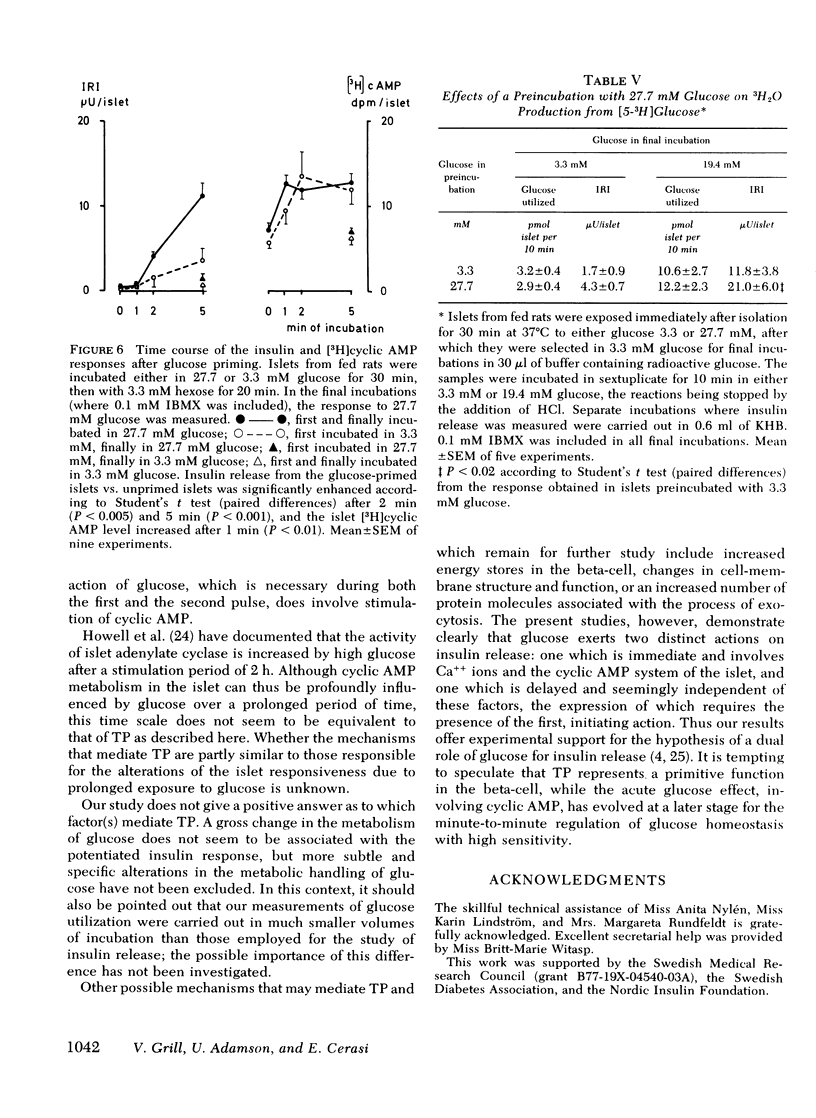
In islets labeled with [2-3H]adenine, the [3H]cyclic AMP response to glucose was increased by 35% when measured after 1 min, but was increased only marginally after 2-10 min of stimulation with a second pulse of glucose. The production of 3H2O from glucose was not affected by glucose priming.
It is concluded that (a) the induction of the glucose-induced, time-dependent potentiation described here is dependent on glucose metabolism but not on stimulation of cyclic AMP, calcium fluxes, or insulin release per se; (b) the mechanisms that mediate the pancreatic “memory” for glucose are unknown but do not seem to involve to a major extent an increased activity of the adenylate cyclase-cyclic AMP system of the beta-cell; (c) the evidence presented supports the hypothesis of a dual role of glucose for insulin release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967 Jun;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- Cerasi E. Potentiation of insulin release by glucose in man. I. Quantitative analysis of the enhancement of glucose-induced insulin secretion by pretreatment with glucose in normal subjects. Acta Endocrinol (Copenh) 1975 Jul;79(3):483–501. [PubMed] [Google Scholar]
- Cerasi E. Potentiation of insulin release by glucose in man. II. Role of the insulin response, and enhancement of stimuli other than glucose. Acta Endocrinol (Copenh) 1975 Jul;79(3):502–510. [PubMed] [Google Scholar]
- Charles M. A., Lawecki J., Pictet R., Grodsky G. M. Insulin secretion. Interrelationships of glucose, cyclic adenosine 3:5-monophosphate, and calcium. J Biol Chem. 1975 Aug 10;250(15):6134–6140. [PubMed] [Google Scholar]
- Claro A., Grill V., Efendić S., Luft R. Studies on the mechanisms of somatostatin action on insulin release. IV. effect of somatostatin on cyclic AMP levels and phosphodiesterase activity in isolated rat pancreatic islets. Acta Endocrinol (Copenh) 1977 Jun;85(2):379–388. [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L. Reversal of somatostatin inhibition of insulin secretion by calcium. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1015–1019. doi: 10.1016/0006-291x(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Grill V., Borglund E., Cerasi E. Evidence for uniform labeling of precursor and product with [3H]adenine. Biochim Biophys Acta. 1977 Sep 29;499(2):251–258. doi: 10.1016/0304-4165(77)90007-1. [DOI] [PubMed] [Google Scholar]
- Grill V., Cerasi E. Stimulation by D-glucose of cyclic adenosine 3':5'-monophosphate accumulation and insulin release in isolated pancreatic islets of the rat. J Biol Chem. 1974 Jul 10;249(13):4196–4201. [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Curry D., Landahl H., Bennett L. [Further studies on the dynamic aspects of insulin release in vitro with evidence for a two-compartmental storage system]. Acta Diabetol Lat. 1969 Sep;6 (Suppl 1):554–578. [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Green I. C., Montague W. A possible role of adenylate cyclase in the long-tern dietary regulation of insulin secretion from rat islets of Langerhans. Biochem J. 1973 Oct;136(2):343–349. doi: 10.1042/bj1360343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Lacy P. E., Finke E. H., Codilla R. C. Cinemicrographic studies on beta granule movement in monolayer culture of islet cells. Lab Invest. 1975 Nov;33(5):570–576. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Loubatières A., Mariani M. M., Ribes G., de Malbosc H., Chapal J. Etude expérimentale d'un nouveau sulfamide hypoglycémiant particulièrement actif, le HB 419 ou glibenclamide. Diabetologia. 1969 Feb;5(1):1–10. doi: 10.1007/BF01212212. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Insulin secretion: multifactorial regulation for a single process of release. The Minkowski award lecture delivered on September 7, 1972 before the European Association for the study of Diabetes at Madrid, Spain. Diabetologia. 1973 Jun;9(3):167–173. doi: 10.1007/BF01219778. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E., Krzanowski J., Kotler-Brajtburg J., Landgraf R., Fertel R. The dual function of glucose in islets of Langerhans. J Biol Chem. 1971 Feb 25;246(4):1007–1011. [PubMed] [Google Scholar]
- Montague W., Howell S. L., Green I. C. Insulin release and the microtubular system of the islets of Langerhans: effects of insulin secretagogues on microtubule subunit pool size. Horm Metab Res. 1976 May;8(3):166–169. doi: 10.1055/s-0028-1093653. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Kipnis D. M. Microtubule assembly and the intracellular transport of secretory granules in pancreatic islets. Science. 1976 Jan 9;191(4222):88–90. doi: 10.1126/science.1108194. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Kipnis D. M. Regulation of tubulin synthesis in islets of Langerhans. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3188–3191. doi: 10.1073/pnas.73.9.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]


