Abstract
Importance
Nonmelanoma skin cancer (NMSC) is the most common cancer and predominantly affects older patients. Because NMSCs do not typically affect survival or short-term quality of life, the decision about whether and how to treat patients with limited life expectancy (LLE) is challenging, especially for asymptomatic tumors.
Objective
To compare treatment patterns and clinical outcomes of patients with NMSC with and without LLE.
Design, Setting, and Participants
A prospective cohort study of 1536 consecutive patients diagnosed with NMSC at 2 dermatology clinics: a university-based private practice and a Veterans Affairs Medical Center in San Francisco, California. Patients were recruited in 1999 through 2000 and followed up for a median of 9 years. A total of 1360 patients with 1739 tumors (90%) were included in the final analysis. Limited life expectancy was defined as patients either 85 years or older at the time of diagnosis or patients with multiple comorbidities (Charlson Comorbidity Index of ≥3). Treatment options included no treatment, destruction, or 2 types of surgery— elliptical excision or Mohs surgery.
Main Outcomes and Measures
Treatment type.
Results
Most NMSCs (69%) were treated surgically, regardless of patient life expectancy. The choice of surgery was not influenced by patient prognosis in univariate or multivariable models adjusted for tumor and patient characteristics. Many patients with LLE (43%) died within 5 years, none of NMSC. Tumor recurrence was rare (3.7% at 5 years [95% CI, 2.6%-4.7%]) in all patients. Although serious complications were unusual, approximately 20% of patients with LLE reported complications of therapy, compared with 15% of other patients.
Conclusions and Relevance
Most NMSCs are treated surgically, regardless of the patient’s life expectancy. Given the very low tumor recurrence rates and high mortality from causes unrelated to NMSC in patients with LLE, clinicians should consider whether these patients would prefer less invasive treatment strategies.
Determining appropriate care of nonfatal conditions for patients with limited life expectancy (LLE) is challenging.1 For example, rates of prostate cancer screening in patients with LLE are probably excessive, given the known risks of screening and limited benefits of treatment.2,3 Similarly, a significant proportion of patients with metastatic cancer receive routine screening tests that are unlikely to provide any benefit.4 A study of Medicare beneficiaries showed that more than 30% of patients underwent surgery in the last year of life, and almost 20% underwent a procedure in the last month of life.5 Together, these studies argue for prudent use of procedures toward the end of life. Although nonmelanoma skin cancer (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is the most common malignant neoplasm and is typically nonfatal, few studies have examined its treatment in patients who may not survive long enough to benefit. The objective of this study was to compare treatment patterns and clinical outcomes of patients with NMSC with and without LLE.
Almost 1 in 5 Americans6 will be diagnosed as having NMSC; most of these patients are elderly. Nonmalignant skin cancers grow slowly, rarely metastasize or affect survival, and typically do not result in significant morbidity or diminished quality of life.7 In fact, a significant proportion of these tumors are asymptomatic, and patients are often unaware of them. The primary goals of treatment are to prevent expansion and local recurrence of the tumor. Despite its low im-pact on most patients, the high prevalence of NMSC and the fact that it is routinely treated, make it the fifth most costly cancer for Medicare.8,9
The current standard of care in the United States is to treat NMSCs, and no guidelines exist about whether physicians should consider patient age or functional status in choosing treatments.10 Treatment decisions for patients with NMSC with LLE require consideration that the benefits of treatment may not occur within the patient’s remaining life span, but any risks are immediate.
METHODS
DESIGN, SETTING, AND PATIENTS
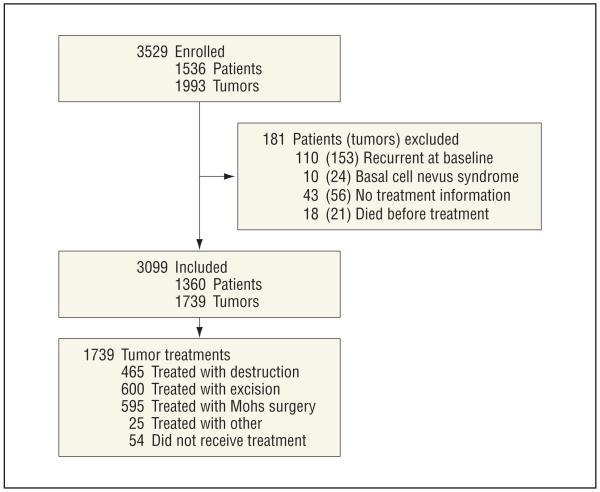
We conducted a prospective cohort study of consecutive patients with NMSC diagnosed and treated in January 1999 through December 2000, and followed up for 10 years after treatment in a university-based dermatology practice (private) or the dermatology clinic of the nearby university-affiliated Veterans Affairs Medical Center (VAMC). Details of the study have been described elsewhere.11,12 Overall, 1536 patients with 1993 NMSCs were eligible for our study (Figure 1). Our cohort was assembled based on histopathology records, including tumors treated by dermatology or other specialties. Eligible NMSCs were those biopsied with final histopathological diagnoses of BCC or SCC. After exclusions, the final cohort consisted of 1360 patients with 1739 tumors (Figure 1). The VAMC and the University of California, San Francisco, institutional review board approved this study.
Figure 1.
Study flowchart
LIMITED LIFE EXPECTANCY
We defined LLE based on age and medical comorbidities: patients of LLE were either 85 years or older or had a Charlson Comorbidity Index of 3 or higher.13 The rationale for these criteria is that they indicate LLE: a person 85 years old in the United States has a median life expectancy of approximately 5 years,14 and a Charlson Comorbidity Index of 3 or higher indicates poorest survival and has been used to define poor prognosis.15,16
TREATMENT TYPES
Treatment types were categorized as the following: (1) Mohs micrographic surgery (Mohs); (2) simple elliptical excision (excision); (3) destruction, including electrodessication and curettage, cryotherapy, laser, or radiation; (4) other, including topical fluorouracil cream or other topical treatment; and (5) no treatment, which included patients who refused treatment or for whom no treatment was deemed necessary.
DATA COLLECTION AND MEASURES
Using structured data forms, trained research staff extracted data from clinician notes and pathology records. Patient data included age, sex, history of NMSC, organ transplantation, human immunodeficiency virus infection, and number of NMSCs on enrollment in the cohort. Tumor information included clinical descriptions, size, location, and histopathology. Tumors were classified according to the presence or absence of histological risk factors for recurrence,10 and facial tumors were further categorized by location in the H-zone of the face, an area believed to be at higher risk for recurrence.17 Information was also collected about the clinic location (VAMC or private), and training level of the clinician who performed the biopsy (attending physician, resident physician, or nurse practitioner).
Before treatment, patients were also requested to complete a detailed survey that included items measuring income, education, and photosensitivity (Fitzpatrick skin type)18 and patient-reported Charlson Comorbidity Index.19 We measured how much a tumor bothered the patient with the question, “Overall, during the past week how often have you been bothered by your skin cancer?” Seven response choices were anchored by “never” and “always.” Responses to the global item were classified into 3 groups according to tertiles of bother: rarely (1 and 2), moderately (3 through 5), and frequently (6 and 7). Patients with multiple tumors were asked about the tumor that bothered them the most.
A total of 671 patients (43.7%) responded to the questionnaire. Compared with nonrespondents to the questionnaire, responders were slightly older (median age, 70 vs 68 years), more likely to be men (74.7% vs 69.2%), more likely to have had a previous NMSC (53.7% vs 46.4%), and had more frequent dermatology visits (1.7 vs 1.2). Respondents were more likely to have a tumor present on the central face (36.7% vs 32.4%). Follow-up information was available for 93.7% patients. Patients lost to follow-up were similar to those with follow-up in most features but were more likely to be female (38% vs 26%), to have worse mental health status (median Short Form-12 Mental Component Score, 41.2 vs 51.5), and to have BCC rather than SCC (89% vs 75%).
CLINICAL OUTCOMES
Outcomes of interest were treatment patterns, tumor recurrence, mortality, and complications. Data about tumor recurrence were determined from the medical record and patient examination. At a median of 9.0 years after treatment (interquartile range [IQR], 8.5-9.7) trained dermatologic nurse practitioners who were masked to treatment type reviewed the records. Additionally, consenting patients were examined a median of 8.6 years (IQR, 8.3-9.0) after therapy by a dermatologist masked to treatment type. If any suspicion of recurrence was noted, the patient was referred to a dermatologist for further assessment. For all tumors with evidence of recurrence by medical record review or examination, the entire record was reviewed again by an additional clinician masked to details from the original review. A tumor was defined as recurrent if the tumor type (BCC or SCC) and body location were identical to those of the pri-mary tumor, and the lesion was described by the clinician as recurrent or previously treated.
Data about vital status were obtained from the medical record as well as the Center for Disease Control and Prevention’s National Death Index20 through 2009. Information about treatment complications was obtained both from review of the medical record and from patient questionnaires. At 3, 18, and 24 months after therapy, patients were asked to respond to the following item: “In your opinion, were there any complications of your treatment during or after the treatment itself?” and “If yes, how serious was the complication?” Choices included minimal, mild, moderate, very, or extremely serious. Patients were also asked to describe the complication.
STATISTICAL ANALYSIS
Characteristics of patients and tumors were compared between LLE and non-LLE subgroups using a χ2 test for categorical characteristics and the Wilcoxon rank sum test for continuous characteristics. Unadjusted 5-year tumor recurrence and mortality rates (at 5 and 10 years) in the overall sample and by LLE status were calculated using the Kaplan-Meier method. Data were censored in the following way for each outcome: For recurrence, data were rightcensored at the last date on which a patient received any care; a patient was considered lost to follow-up if there was no record of care after treatment, and excluded from analyses related to the tumor recurrence outcome. Formortality, if a death date was not found in the National Death Index as of December 2009, the observation was right-censored to January 1, 2010. We also examined treatment patterns in patients who ultimately died within 2 years of diagnosis.
A multivariable regression model21 was also fit to determine if LLE status influences treatment. Multivariable models adjusted for income of $30 000/year or higher, college education, whether the tumor bothered the patient, history of NMSC and 2 or more NMSCs at enrollment, care setting (VAMC vs private), practitioner performing biopsy (attending vs resident vs nurse practitioner), tumor location, tumor diameter, histologic subtype, and high-risk histologic characteristics. A model that excluded LLE status and included age 85 years or older and Charlson Comorbidity Index of 3 or higher as 2 independent variables was also fit with the variables listed earlier. Relative risks and CIs were calculated using a modified Poisson approach with robust error variances.21 We performed sensitivity analysis after multiple imputation under the assumption that the data were missing at random. Results were not different using imputation; therefore, we did not report these findings. We used a generalized estimating equation approach (Huber-White method) with independent working correlation structure to correct for multiple tumors from the same patient. Analyses were performed in R (version 2.13) and STATA (version 11.2; StataCorp Inc).
RESULTS
COHORT CHARACTERISTICS
Table 1 lists the overall characteristics of cohort participants, which included 1360 patients with 1739 NMSCs. The median patient age was 69.0 years (IQR, 55.0-78.0years), and 72.7% were male. Overall, most patients had a Charlson Comorbidity Index of 1.0 (IQR, 0.0-3.0) and 22% of patients were frequently bothered by their tumor. A total of 332 patients with 428 tumors were classified as having LLE at baseline, based on advanced age and multiple comorbidities. As expected, patients with LLE were generally older and had more comorbidities than patients without LLE. Also patients with LLE had lower incomes and educational status and their tumors were larger and more likely to be SCCs and located on the central face. Also patients with LLE were more likely to report being bothered by their tumor than patients without LLE, although most patients were not bothered by their tumors.
Table 1. Characteristics of NMSC Cohort According to LLE Category.
| LLE Subgroups, %a |
||||
|---|---|---|---|---|
| Characteristic | Overall Cohort, % (N = 1360) |
Yes (n = 332) |
No (n = 536) |
P Value |
| Patient | ||||
| Age, median (IQR), y | 69.0 (55.0-78.0) | 79.0 (69.0-86.0) | 63.0 (52.0-74.0) | <.001 |
| Sex, male | 72.7 | 78.0 | 72.6 | .09 |
| Charlson Comorbidity Index,13 median (IQR) | 1.0 (0.0-3.0) | 6.0 (3.0-7.0) | 0.0 (0.0-1.0) | <.001 |
| Fitzpatrick skin type 1 or 218 | 39.0 | 39.8 | 38.8 | .85 |
| History of prior NMSC | 51.1 | 55.7 | 52.4 | .38 |
| No. of NMSC at baseline, median (IQR) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | .68 |
| History of HIV | 3.1 | 3.0 | 3.7 | .71 |
| History of organ transplantation | 2.7 | 4.5 | 1.1 | .003 |
| Annual income, <$30 000 | 50.7 | 63.1 | 45.1 | <.001 |
| Education, high school or less | 37.1 | 46.5 | 32.6 | <.001 |
| Tumor | n = 1739 | n = 428 | n = 678 | |
| Tumor diameter, mean (IQR), mm | 8.0 (5.0-13.0) | 9.0 (6.0-15.0) | 8.0 (5.0-12.0) | <.001 |
| Tumor on central face | 35.1 | 40.7 | 34.1 | .03 |
| Basal cell carcinoma | 74.6 | 63.1 | 81.3 | <.001 |
| Superficial pathology | 30.7 | 30.8 | 30.2 | .88 |
| High-risk histology,10 | 19.3 | 17.3 | 21.7 | .09 |
| Bothers patient frequently | 21.7 | 27.5 | 18.8 | .03 |
| Care setting | ||||
| Treated at VAMC | 39.5 | 51.2 | 41.0 | .004 |
| Treated at private hospital | 60.5 | 48.8 | 59.0 | |
| Attending physician performed biopsy | 58.4 | 44.6 | 61.3 | |
| Resident physician performed biopsy | 24.7 | 37.6 | 19.2 | <.001 |
| Nurse practitioner performed biopsy | 16.9 | 17.8 | 19.5 | |
| No. of annual patient visits to dermatology, median (IQR) | 1.6 (0.5-2.8) | 1.9 (0.7-3.1) | 1.7 (0.6-3.0) | .18 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; LLE, limited life expectancy; NMSC, nonmelanoma skin cancer; VAMC, Veterans Affairs Medical Center.
The sum of the number of patients in LLE plus non-LLE is smaller than the total cohort because we had 492 missing data points for patient-reported Charlson index, which is part of the definition of LLE.
RECURRENCE AND MORTALITY
Tumor recurrence follow-up was available for 1269 patients with 1618 tumors (93%). The 5-year recurrence rates were low in the entire cohort and in the LLE subgroup (3.7% [95% CI, 2.6%-4.7%] vs 3.7% [95% CI, 1.5%-5.9%]) (Table 2). Of 14 patients with LLE whose tumors recurred, 9 died soon after their recurrence of other causes (median survival after recurrence, 21 months [IQR, 15-27 months]).
Table 2. Clinical Outcomes After Treatment for NMSC in the Overall Cohort and Patients With LLE.
| Clinical Outcome | Overall Cohort (No. of Patients/No. of Tumors) (1360/1739) |
LLE (No. of Patients/No. of Tumors) |
|
|---|---|---|---|
| Yes (332/428) |
No (536/678) |
||
| 5-y Tumor recurrence rate | 3.7 (2.6-4.7) | 3.7 (1.5-5.9) | 3.8 (2.1-5.4) |
| Mortality | |||
| 5-y | 22.6 (20.3-24.8) | 43.3 (37.7-48.4) | 11.0 (8.3-13.6) |
| 10- y | 49.9 (45.9-53.7) | 76.8 (69.8-82.1) | 32.7 (26.6-38.3) |
| Patient-reported complication rate, % | 14.9 | 20.2 | 25.0 |
Abbreviation: LLE, limited life expectancy.
The 5-year mortality of the overall cohort was 22.6%; 43.3% of patients in the LLE subgroup died within 5 years compared with an 11.0% mortality for those without LLE (P < .001). The overall 10-year mortality of the cohort was 49.9%; 10-year mortality was also significantly higher in the LLE subgroup compared with the non-LLE subgroup (10-year mortality, 76.8% vs 32.7%; P < .001).
No patient died of a cause related to NMSC. The leading causes of death included ischemic heart disease and myocardial infarction, cerebrovascular disease, lung cancer, pneumonia and chronic respiratory diseases, prostate cancer, and Alzheimer disease.
TREATMENT PATTERNS
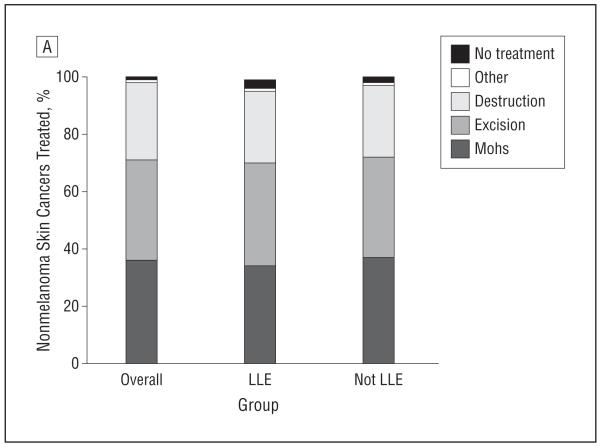
Overall, 68.7% of tumors underwent surgery; 34.2% underwent Mohs surgery, and 34.5% underwent simple excision. Another 26.7% were treated with destruction, which included cryotherapy, electrodessication and curettage, laser, and irradiation. A total of 3.1% received no treatment. Among patients with LLE, 70.1% underwent surgery (33.9% underwent Mohs surgery and 36.2% underwent simple excision). Of the patients with LLE, 25.2% were treated with destruction. Among patients with LLE, 3.3% received no treatment. No significant difference was noted in treatment rates according to patient life expectancy (Figure 2). These results did not differ in stratified analyses according to BCC or SCC. Treatment patterns differed between sites, with more surgery (Mohs plus excision) performed at the VAMC compared with private clinics (multivariable adjusted relative risk [RR], 0.87; 95% CI, 0.77-1.00; P = .04).
Figure 2.
Percent of nonmelanoma skin cancers treated with Mohs, excision, destruction, other, or no treatment. LLE indicates limited life expectancy.
We also examined treatment patterns in patients who died within 2 years of initial treatment. In this group, we also found high levels of surgical treatment (66.7% of all tumors underwent surgery: 28.7% underwent Mohs surgery and 38.0% underwent simple excision).
COMPLICATIONS
Data about patient-reported complications was obtained from the questionnaires. Overall, 671 patients and 212 patients with LLE responded to the questionnaire. A total of 203 unique patients (15% of the total cohort and 30% of 671 respondents to this item) reported a complication after treatment for NMSC. Sixty-seven complications occurred in patients with LLE (20% of the LLE subgroup and 32% of 212 respondents to this item in the LLE subgroup). The most common medical complications described by patients on the questionnaires related to poor wound healing and symptoms including numbness and itching and pain. No significant differences were noted in the types of complications reported by LLE compared with that of the overall cohort.
PREDICTORS OF SURGICAL TREATMENT
The probability of undergoing surgery for NMSC was the same in patients with LLE as in those with longer life expectancy (Table 3). Even after adjusting for multiple patient, tumor, and care setting characteristics, LLE was unrelated to the choice of surgery for NMSC, with relative risks close to 1 and nonsignificant P values. This finding remained true in univariate and multivariable models.
Table 3. Unadjusted and Multivariable Adjusted Relative Risk of Receiving Surgical Treatments (Excision and Mohs) According to Category of LLE.
| Variable | Unadjusted RR (95% CI) |
P Value | Multivariable Adjusted RR (95% CI)a |
P Value |
|---|---|---|---|---|
| Prognosis | ||||
| LLE | 0.98 (0.89-1.08) | .73 | 0.95 (0.85-1.06) | .35 |
| Age ≥85 y | 1.05 (0.93-1.19) | .46 | 1.10 (0.90-1.33) | .35 |
| Multiple comorbidities | 0.98 (0.88-1.08) | .67 | 0.92 (0.82-1.03) | .16 |
| Patient | ||||
| Sex, male | 0.97 (0.89-1.06) | .49 | ||
| Income ≥$30 000/y | 0.91 (0.83-1.00) | .04 | 1.04 (0.91-1.18) | .59 |
| College education | 0.83 (0.76-0.91) | <.001 | 0.87 (0.78-0.97) | .009 |
| History of NMSC | 0.91 (0.85-0.99) | .02 | 1.00 (0.91-1.10) | .99 |
| ≥2 NMSCs at enrollment | 0.80 (0.72-0.88) | <.001 | 0.97 (0.87-1.09) | .70 |
| Bother | ||||
| Moderately: reference rare | 1.07 (0.95-1.21) | .30 | 1.08 (0.95-1.23) | .24 |
| Frequent: reference rare | 1.12 (0.98-1.27) | .09 | 1.16 (1.03-1.31) | .02 |
| Tumor | ||||
| Tumor diameter ≥10 cm | 1.03 (0.96-1.10) | .39 | 1.12 (1.00-1.26) | .09 |
| Tumor on central face | 1.50 (1.40-1.60) | <.001 | 1.42 (1.29-1.58) | <.001 |
| SCC: reference group BCC | 1.04 (0.96-1.12) | .39 | 1.12 (0.99-1.26) | .07 |
| High-risk histology10 | 1.47 (1.39-1.56) | <.001 | 1.37 (1.25-1.49) | <.001 |
| Care setting | ||||
| Private, reference group VAMC | 0.85 (0.79-0.92) | <.001 | 0.87 (0.77-1.00) | .04 |
| Nurse practitioner performed biopsy, reference group: attending | 1.1 1 (1.02-1.22) | .02 | NA | NA |
| Resident performed biopsy, reference group: attending | 1.11 (1.01-1.23) | .04 | 0.93 (0.82-1.06) | .27 |
Abbreviations: BBC, basal cell carcinoma; LLE, limited life expectancy; NA, not applicable; NMSC, nonmelanoma skin cancer; RR, relative risk; SCC, squamous cell carcinoma; VAMC, Veterans Affairs Medical Center.
Multivariable model adjusted for LLE status, income, education, history of and multiple NMSCs at enrollment, care setting (university-affiliated clinic vs VAMC), and practitioner performing biopsy (attending vs resident), tumor on the central face, tumor diameter greater than 10 mm, histologic subtype, and high-risk histologic characteristics. In addition to these variables, models of patient prognosis included either LLE alone, or age 85 years or older, and Charlson Comorbidity Index of 3 or higher without LLE status.
DISCUSSION
We found that regardless of patients’ life expectancy, most NMSCs were treated; and most were treated surgically. The decision to treat surgically was not influenced by patients’ relatively poor prognoses in multivariable models adjusted for tumor and patient characteristics. Most patients with LLE were not often bothered by their tumors, and among patients with LLE, approximately 1 in 5 reported a complication within 2 years after treatment. Finally, approximately half the patients with LLE died within the first 5 years after treatment, and all causes of death were unrelated to NMSC. These findings suggest that many patients may not live long enough to benefit from treatment of NMSC but are at risk for treatment-related complications.
A symptomatic or medically dangerous NMSC tumor (eg, tumor eroding into vital structure) should be treated, regardless of life expectancy.10,22 However, treatment of asymptomatic tumors—those that do not bother the patient—may not be indicated. The first questions the patient and clinician face are: Should we biopsy this lesion? And, If this biopsy is positive for NMSC, should we treat it? If the answers are yes, the question that follows is: Which treatment is best for this particular patient? considering his or her benefits, risks, and preference. Although data from this study cannot definitively answer these questions, opening this discussion is overdue.
Given their high mortality and low tumor recurrence rates, it is unclear if patients with LLE who receive a surgical procedure for NMSC will benefit significantly compared with patients who receive less invasive treatments. Mohs surgery can be time consuming, often requiring several hours to complete while patients are awake. In a randomized trial, Essers et al23 reported a mean of 3 hours to complete Mohs surgery, compared with 1 hour for excision. Mohs surgery may be especially problematic for frail patients with LLE who are unable to tolerate extended procedures. Additionally, because patients with LLE often require assistance with activities of daily living, wound care after surgery may be especially difficult for this group. This fact may explain the higher rates of patient-reported complications noted in the LLE subgroup.
Recent studies have highlighted the debate about appropriate use of medical procedures, including screening tests, and treatments at the end of life.2-5 The underlying argument of these studies is that at the end of life, benefits of screening and treatment may not outweigh potential risks because patients may not live long enough to benefit, and the complications of treatment may diminish quality of life. One important criticism of this premise is that death cannot be predicted accurately for any individual patient, and physicians do not want to deny potentially beneficial care. Our study provides useful evidence for clinicians facing this treatment choice dilemma with their patients, as it focuses on a cancer whose natural history is generally benign, where treatment itself may be discretionary. In addition, our study captured information about individual patient outcomes, including complications, recurrence, time to death, and cause of death. Therefore, we were able to contribute to this debate with both population and individual-level data.
The major limitation of this study is its observational design. Because treatment was not assigned randomly, it is possible that unmeasured factors could have influenced treatment choices in the LLE subgroup. For example, we did not measure patient preference for a particular treatment. However, we did measure tumor burden and quality-of-life measures that did not suggest that NMSCs are more bothersome in patients with LLE. Our study is limited to a single city, and about half of our cohort was from the VAMC (which differed significantly in treatment patterns compared with private clinics).11 Therefore, our findings may not be generalizable to the United States as a whole. However, recent publications highlighting the rapid rise in procedures for NMSC suggest a similar treatment distributionnationally.24 Furthermore, our study observed treatment as part of regular patient care, and not in the context of a trial, which makes our findings more likely to be representative of routine clinical practice. We only included patients who were biopsied, although in clinical practice it is possible that some patients are not biopsied even if NMSC is suspected. Finally, although our definitions of LLE were highly correlated with mortality, a significant proportion of patients with LLE lived past 5 years, confirming that prognostication for any individual patient is difficult.
In conclusion, most NMSCs were treated surgically in patients with LLE, and treatment choices were unaffected by patient prognosis. These results persisted even after adjusting for multiple tumor and patient characteristics. One in 5 patients with LLE reported a treatment-related complication. Given the very low tumor recurrence rates and high mortality rates in this patient population, we believe consideration of life expectancy should enter into treatment decisions. No guidelines address treatment of NMSC for the specific group of patients with LLE. We hope that this study will open the debate to optimal treatment decisions for NMSC, facilitating shared decision making and informed discussion about patient prognosis.
Acknowledgments
Funding/Support: This study was supported in part by Award KL2RR024130 from the National Center for Research Resources (Dr Linos), by grants R01 AR 054983 and K24 AR052667 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (Dr Chren), and by a Career Development Award from the American Skin Association and Dermatology Foundation (Dr Linos).
Footnotes
Author Contributions: Study concept and design: Linos, Parvataneni, and Chren. Acquisition of data: Stuart and Chren. Analysis and interpretation of data: Linos, Parvataneni, Boscardin, Landefeld, and Chren. Drafting of the manuscript: Linos. Critical revision of the manuscript for important intellectual content: All the authors. Statistical analysis: Linos, Parvataneni, and Boscardin. Obtained funding: Linos and Chren. Administrative, technical, and material support: Linos and Stuart. Study supervision: Linos.
Conflict of Interest Disclosures: Dr Chren is a consultant for Genentech, Inc.
Previous Presentation: This study was presented at the Sixth International Dermato-Epidemiology Association Congress; August 27, 2012; Malmo, Sweden.
Contributor Information
Dr Eleni Linos, Department of Dermatology, University of California, San Francisco.
Ms Rupa Parvataneni, Department of Dermatology, University of California, San Francisco.
Ms Sarah E. Stuart, Department of Dermatology, University of California, San Francisco.
Dr W. John Boscardin, Department of Medicine, University of California, San Francisco; Department of Epidemiology and Biostatistics, University of California, San Francisco.
Dr C. Seth Landefeld, Department of Medicine, University of Alabama, Birmingham.
Dr Mary-Margaret Chren, Department of Dermatology, University of California, San Francisco; San Francisco Veterans Affairs Medical Center, San Francisco, California.
REFERENCES
- 1.Fuchs VR. The doctor’s dilemma—what is “appropriate” care? N Engl J Med. 2011;365(7):585–587. doi: 10.1056/NEJMp1107283. [DOI] [PubMed] [Google Scholar]
- 2.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 3.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 4.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304(14):1584–1591. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378(9800):1408–1413. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5, pt 1):774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2007;143(11):1386–1392. doi: 10.1001/archderm.143.11.1386. [DOI] [PubMed] [Google Scholar]
- 8.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48(3):425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 9.Mudigonda T, Pearce DJ, Yentzer BA, Williford P, Feldman SR. The economic impact of non-melanoma skin cancer: a review. J Natl Compr Canc Netw. 2010;8(8):888–896. doi: 10.6004/jnccn.2010.0066. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed May 18, 2010];Basal cell and squamous cell skin cancers [updated 2012] 2010 http://www.nccn.org
- 11.Chren MM, Sahay AP, Sands LP, et al. Variation in care for nonmelanoma skin cancer in a private practice and a Veterans Affairs clinic. Med Care. 2004;42(10):1019–1026. doi: 10.1097/00005650-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Chren M-M, Torres JS, Stuart SE, Bertenthal D, Labrador RJ, Boscardin WJ. Recurrence after treatment of nonmelanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147(5):540–546. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Arias E. United States Life Tables, 2000. National Vital Statistics Reports. National Center for Health Statistics; Hyattsville, MD: 2002. [PubMed] [Google Scholar]
- 15.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783–791. doi: 10.1007/s10654-008-9290-y. [DOI] [PubMed] [Google Scholar]
- 17.Swanson NA, Grekin RC, Baker SR. Mohs surgery: techniques, indications, and applications in head and neck surgery. Head Neck Surg. 1983;6(2):683–692. doi: 10.1002/hed.2890060209. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed April 5, 2012];Data Access—National Death Index. http://www.cdc.gov/nchs/ndi.htm
- 21.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Connolly SM, Baker DR, Coldiron BM, et al. Ad Hoc Task Force. Ratings Panel AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Essers BA, Dirksen CD, Nieman FH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142(2):187–194. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- 24.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]