Abstract
Pancreatic ductal adenocarcinoma (PDAC) carries a dismal prognosis and lacks a human cell model of early disease progression. When human PDAC cells are injected into immunodeficient mice, they generate advanced stage cancer. We hypothesized that if human PDAC cells were converted to pluripotency and then allowed to differentiate back into pancreatic tissue, they might undergo early stages of cancer. Although most induced pluripotent stem (iPS) cell lines were not of the expected cancer genotype, one PDAC line, 10-22 cells, when injected into immunodeficient mice, generates pancreatic intraepithelial neoplasia (PanIN) precursors to PDAC that progress to the invasive stage. The PanIN-like cells secrete or release proteins corresponding to genes and networks expressed in human pancreatic cancer progression and which predicted an HNF4α network also seen a mouse model. Thus, rare events allow iPS technology to provide a live human cell model of early pancreatic cancer and new insights into disease progression.
Keywords: induced pluripotent stem (iPS) cell, pancreas, PanIN, tumor, ductal epithelium, cancer, HNF4
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) carries a dismal prognosis, with less than a 5% survival rate (Hezel et al., 2006; Maitra and Hruban, 2008; Rustgi, 2006). Due to a paucity of biomarkers for early stage detection of the disease, PDAC is usually detected at advanced stages, with limited therapeutic options. Given the frequent occurrence of KRAS mutations in human PDAC, the prominent animal model of PDAC is based upon inducing a G12D mutant allele of Kras in the mouse pancreatic epithelium (Hingorani et al., 2003). The mice develop pancreatic intra-epithelial neoplasias (PanINs) with prolonged latency and incomplete penetrance of PDAC. PDAC and related tumors develop much more rapidly when KrasG12D/+ mice also contain mutations of Ink4a/Arf, Tgfbr2, p53, or PTEN (Morris et al., 2010), although these mutations alone do not efficiently cause PDAC. In an effort to develop human models of early pancreatic cancer, PDAC cells have been grafted into immunodeficient mice either as tumor fragments (Rubio-Viqueira et al., 2006), dispersed cells (Kim et al., 2009) or cells sorted for cancer stem cell markers (Hermann et al., 2007; Ishizawa et al., 2010; Li et al., 2007). In these contexts, tumors rapidly arise that resemble the advanced PDAC stages from which the cells were derived and do not undergo the slow growing, early PanIN stages of PDAC (Ding et al., 2010). Presently there is no dynamic, live human cellular model that undergoes the early stages of PDAC and disease progression.
Most of the secreted proteins from pancreatic cancers (Harsha et al., 2009) that could serve as biomarkers have been identified in advanced, invasive PDAC or cell lines thereof, and thus may not represent markers for early stages of the disease. Markers have been sought for precancerous lesions, such as PanINs and intraductal papillary mucinous neoplasms (IPMNs) (Brat et al., 1998; Hruban et al., 2001), but the markers are typically intracellular or cell surface proteins (Harsha et al., 2009) and not known to be secreted or released proteins that would provide the best opportunity for diagnosis.
Although irreversible mutations in oncogenic and tumor suppressor genes promote human cancers, potentially reversible epigenetic changes also play a role (Esteller, 2007). Indeed, the cancer phenotype can be suppressed in certain medulloblastoma cells, RAS-induced melanoma cells, and embryonal carcinoma cells and renal tumor cells when they are reprogrammed to pluripotency by nuclear transfer (Blelloch et al., 2004; Hochedlinger et al., 2004; Li et al., 2003; McKinnell et al., 1969). More significantly, the resultant pluripotent cells can then differentiate into multiple early developmental cell types of the embryo. Such embryos die partly through organogenesis, presumably due to re-expression of the cancer phenotype. Still, it is remarkable that, in certain circumstances, the pluripotency network can suppress the cancer phenotype sufficiently to allow early tissue differentiation. Using iPS cell technology (Takahashi and Yamanaka, 2006), cancer cell lines have been made into iPS cells (Carette et al., 2010; Miyoshi et al., 2010). However, no iPS cell lines from solid primary human cancers have been reported.
Based on the above considerations, we hypothesized that creating iPS cells from an epithelial tumor would allow the cells to be propagated indefinitely in the pluripotent state and that, upon differentiation, a subset of the cells would undergo early developmental stages of the human cancer. This could provide a live cell human model for unprecedented experimental access to early stages of the disease. We therefore sought to reprogram epithelial cells from human PDAC, along with apparently normal, isogenic cells beyond the tumor margins, and assess the reprogrammed cells’ developmental potential. From a variety of initial PDAC samples, only once were we able to reprogram a cell from a recurrent, late stage human pancreatic cancer to a near-pluripotent state. Yet the reprogrammed cells, when injected into immunodeficient mice, consistently generate PanIN lesions that can progress to invasive PDAC. We developed conditions for isolating the early stage lesions, culturing the cells, and performing proteomic studies on proteins that are secreted or released and stable. We discovered known networks and a previously unappreciated network that we find to be associated with early to invasive stages of pancreatic cancer. These studies provide an example of where a rare, new iPS cell line can be validated against known features of human cancer and provide potential biomarkers and novel insights into networks activated in early to intermediate stages of the disease.
RESULTS
Creating iPS-like cell lines from human pancreatic ductal adenocarcinoma
We obtained human pancreatic ductal adenocarcinoma samples immediately after resection (Table S1). Histologically normal pancreatic tissues at the margin of the specimens were used as controls. Epithelial cells were isolated and cultured in serum-free medium with cholera toxin, to impair the growth of fibroblasts. We performed two successive infections of the pancreatic cancer and margin cells with 5 lentiviruses separately encoding doxcycyclineinducible mouse Oct4, Sox2, Klf4, and c-Myc, and the rt-TA transactivator, while isolating genomic DNA from the pancreatic specimen margin and cancer epithelial cells that had been cultured separately. Pyrosequencing analysis revealed KRAS mutant cells in 7 of 9 of the initial tumor samples (Tables S1, S2). ES-like colonies began to arise after 7 days, with fewer colonies arising from cancer epithelial cells. ES-like clones from cancer or margins started to differentiate and disappear 4 days after withdrawal of doxycycline. Thus, we maintained colonies in the presence of a low doxycycline concentration (50 ng/ml) and called the resulting lines “iPS-like” because of their dependency upon doxycycline. We established iPS-like lines from 4 of the 9 tumor epithelial specimens and corresponding margin iPS-like lines from 3 of the specimens (Figure 1A, Table S1).
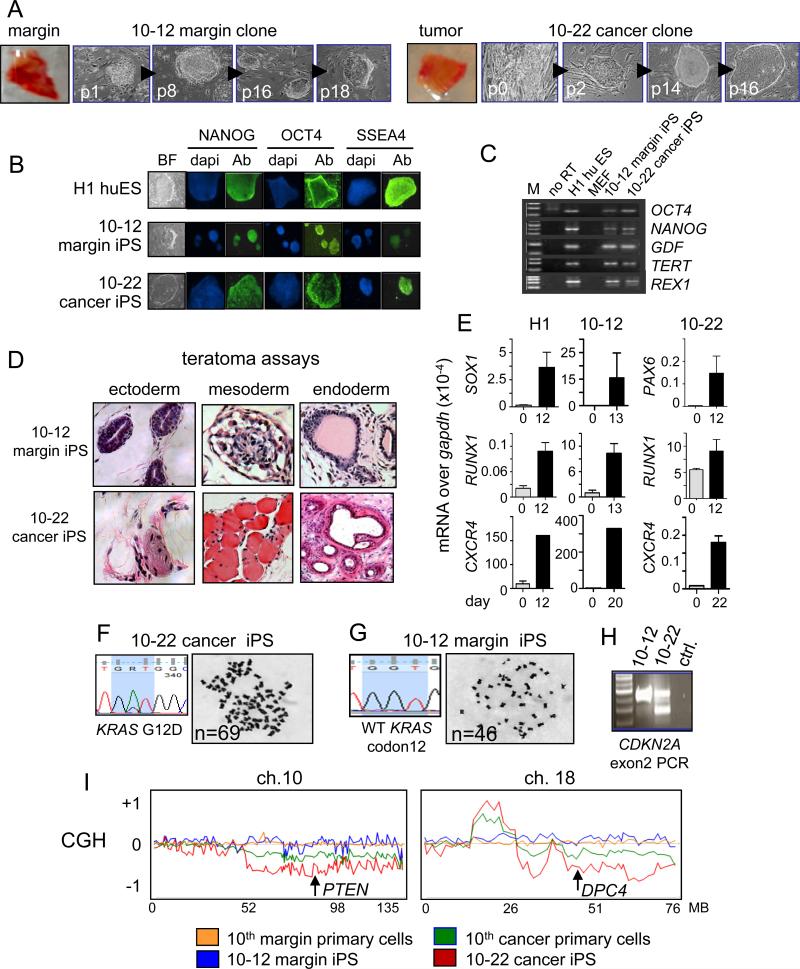
Figure 1. Establishing iPS-like lines from patient-matched margin and pancreatic ductal adenocarcinoma.
A. Cells from pancreatic cancer and margin tissues were reprogrammed and different passages of the 10-12 margin and 10-22 cancer iPS-like clones from patient #10 are shown. B, C. Expression of pluripotency markers in 10-12 margin and 10-22 cancer iPS-like lines by immunostaining (B) and RT-PCR (C). D. Three month teratomas in NSG mice from 10-12 margin and 10-22 cancer iPS-like lines showed differentiation into tissues of all three germ layer lineages (also see Figure S2A). E. Expression of differentiation markers for endoderm (CXCR4), mesoderm (RUNX1), and ectoderm (SOX1, PAX6) relative to GAPDH in embryoid bodies from H1 huES cells and 10-12 margin and 10-22 cancer iPS-like lines cultured for 12-14 days. Error bars, mean ± SD. F, G. DNA sequence tracks revealing KRAS mutation and karyotype showing subtetraploidy in the 10-22 cancer iPS-like line (F) and wild type KRAS and normal karyotype in the 10-12 margin iPS-like line (G). H. PCR analysis of CDKN2A (p16Ink4a) exon 2 reveals a heterozygous deletion in 10-22 cells. I. Comparative genomic hybridization showing normal profiles for the 10th primary margin cells (orange) and the 10-12 margin iPS-like line (blue). Gross chromosomal rearrangements are evident in the 10th primary cancer cell culture (green), which was mixed with normal stromal cells; the rearrangements are evident more clearly in the 10-22 cancer iPS-like line (red) (also see Figure S3B), demonstrating that the 10-22 line is clonally derived representative of the initial tumor epithelial cell population. The locations of PTEN and DPC4 (SMAD4) loci are shown in regions of chromosome loss in the 10th cancer and 10-22 cells.
We characterized the cells’ pluripotency by RT-PCR, immunostaining, embryoid body formation, teratoma assays, karyotyping, and a subset by CpG methylation analysis. Most iPS-like lines expressed endogenous pluripotency marker RNAs and protein (Figure 1B, C; Figure S1A, B, C, D). Teratoma assays in immunodeficient NSG mice and embryoid body assays revealed that the 10-12 pancreatic margin and 10-22 cancer iPS-like lines from the 10th patient (Figure 1D, E; Figure S2A) and the 14-24 pancreatic margin and 14-27 cancer iPS-like clones from the 14th patient (Figure S1E, F) could generate tissues of multiple germ layers, demonstrating pluripotency. The original tumor #10 was negative for NANOG expression and exhibited sporadic expression of POU5F1/OCT4 (Figure S2B). Detailed pyrosequencing analysis of CpG methylation showed that the 10-22 iPS-like line exhibited demethylation at 9 of 9 sites at the NANOG promoter, compared to the primary tumor, and at 3 of 6 sites at the OCT4 promoter; by comparison, the H1 huES line was similarly demethylated at NANOG and demethylated at 4 of 6 sites at POU5F1 (Figure S2C). Thus, the iPS-like lines exhibit diverse characteristics of reprogrammed pluripotent cells.
We screened all iPS-like lines for mutations in KRAS, CDKN2A, and BRAF, which are common genetic alterations in pancreatic cancer (Moskaluk et al., 1997) (Table S2). The 10-22 iPS-like line, derived from the recurrent, invasive, and poorly differentiated PDAC of the 10th patient, harbors the same KRAS G12D mutation seen in the initial tumor epithelial population (Figure 1F; Table S1, S2). 10-22 cells also possess a CDKN2A heterozygous deletion (Figure 1H; Table S2) and a comparative genomic hybridization (CGH) pattern with numerous chromosomal aberrations, more exaggerated than that of the primary cancer culture, and a correspondingly aberrant karyotype (Figure 1I; Figure S3). The exaggeration of the primary cancer CGH pattern in the 10-22 iPS-like line is expected because the primary cancer culture contained some stromal cells and thus was contaminated by cells of a normal genotype. Specifically, we detected 23 gross chromosomal aberrations in the PDAC epithelial cell population of the 10th tumor and 20 were represented in the 10-22 line (Fig. 1I, Supp. Fig. 3B). Decreased CGH signals in the primary tumor cells and the 10-22 line spanned PTEN and DPC4 (SMAD4), consistent with observations of allelic loss of these loci in human pancreatic cancers (Hahn et al., 1996; Hahn et al., 1995). By contrast, the isogenic 10-12 pancreatic margin iPS-like line had wild type KRAS, a flat CGH chromosomal profile, similar to the parental margin cell culture, and a normal karyotype (Figure 1G, H, I; Figure S3; Table S2). We conclude that the 10-12 and 10-22 iPS-like lines are derived from pancreatic margin and cancer epithelial cells, respectively, with the 10-22 line harboring the marked genomic rearrangements seen in the initial advanced tumor epithelial population.
The iPS-like lines from the 14th patient (Figure S1A, B), with moderately differentiated PDAC containing scattered 1 mm foci, showed point mutations in CDKN2A and BRAF but a wild type KRAS, as with the parental epithelial culture (Tables S1, S2), and flat CGH profiles (data not shown). A pair of margin and cancer derived iPS-like lines from the 19th patient (Figure S1C, D) also had somatic mutations in CDKN2A, with wild type KRAS and BRAF (Table S2). Because the 10-22 cancer iPS-like line from the 10th patient contained the KRAS mutant background, typical of PDAC, we performed a detailed study of that line and its isogenic 10-12, KRAS wt margin iPS-like clone.
10-22 iPS-like cells from advanced human PDAC, upon differentiation, undergo the early stages of pancreatic cancer
Despite being derived from cancer, the 10-22 cancer iPS-like line as well as its companion 10-12 margin line gave rise to much smaller subcutaneous teratomas, at three months, compared to those seen from the control H1 human ES cells. More striking, there was a much higher proportion of endodermal, DBA lectin-positive ductal structures in teratomas from the 10-12 and 10-22 iPS-like lines (Figures 2A; Figure S4A), whereas H1 cells generated mostly neuronal lineages, as seen previously (Bock et al., 2011). These results are in concordance with reports on the propensity of other iPS cell lines to differentiate into the lineage from which they were derived (Bar-Nur et al., 2011; Kim et al., 2011).
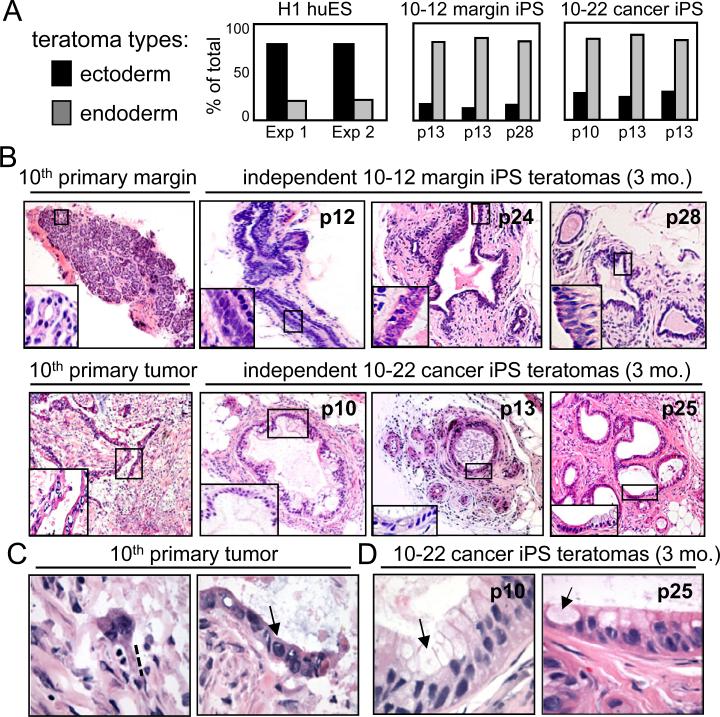
Figure 2. The 10-22 iPS-like cells preferentially generate ductal structures in teratomas.
A. Summaries of teratoma tissue types arising in 3 months in immunodeficient mice based on the number of histologic structures seen. Teratomas from huES cells are mostly ectodermal in appearance, whereas teratomas from 10-12 and 10-22 iPS-like lines are mostly endodermal/ductal. B. H&E staining of the margin and tumor tissue of patient #10 and of teratomas formed by 10-12 margin and 10-22 cancer iPS-like lines after 3 months. The latter lines create tubular, duct-like structures independent of passage number. Notably, the 10-22 cancer teratomas show a much a higher degree of differentiation than the primary tumor. C. The primary tumor shows an invasive phenotype (dotted line) and high nuclear to cytoplasmic ratio (arrow). D. 10-22 tumor iPS teratomas at 3 months show a low nuclear to cytoplasmic ratio, intracellular mucins (arrows), and a more differentiated phenotype.
We next compared the endodermal teratomas arising from the margin (10-12) and tumor (10-22) iPS lines with the original tumor of the 10th patient. The original tumor exhibited many areas of poorly differentiated foci and infiltration, although occasionally showed a more organized epithelium, but no PanINs (Figure 2B, C; Figure S4B). The tumor epithelium exhibited irregularly shaped and hyperchromatic nuclei and a high nuclear to cytoplasmic ratio (Figure 2C, arrow), along with cytoplasmic protrusions indicative of an invasive phenotype (dotted line in Figure 2C). By contrast, teratoma ductal tissues at 3 months from the 10-22 cancer iPS-like line had a high level of architectural organization, with abundant gland formation and a more differentiated cytology compared to the primary tumor (Figure 2B). This included an increased cytoplasm and smaller, less hyperchromatic nuclei with even nuclear membrane contours, a decreased nuclear to cytoplasmic ratio, and increased cell polarity, indicative of PanIN stages. Abundant mucin was present in the apical aspect of the cells (arrows in Figure 2D). There was no evidence of acinar differentiation or neuroendocrine neoplasia. PanINs are graded by the extent of dysmorphic structures compared to normal ducts (Hruban et al., 2001) and we observed a range of PanIN1-, PanIN2-, and PanIN3-like structures in the histology of the endodermal teratomas from the 10-22 iPS-like line at 3 months; though predominantly structures resembling the PanIN2 and PanIN3 stages (Figure S4C-G). Taken together, these findings suggested that the 10-22 cancer iPS-like line generates ductal structures that resemble PanIN (Maitra and Hruban, 2008).
PanIN-like teratomas formed in 9 of 10 teratoma experiments at 3 months, regardless of the passage number of the 10-22 cells. By contrast, the 10-12 pancreatic margin iPS-like line did not generate PanIN-like structures in teratomas (n=4) (Figure 2B). Also, iPS-like lines from the 14th and 19th tumors, containing predisposing mutations, but not of KRAS, did not generate PanIN-like lesions (n=5, data not shown), and therefore were not studied further.
PCR of DNA obtained by laser capture microdissection showed that the stromal cells surrounding the ductal epithelium in the PanIN-like teratomas of the 10-22 line contained the rt-TA lentiviral DNA, and thus were derived from the starting 10-22 cells (Figure S4H). This was confirmed by detecting rT-TA expression in the PanIN-like epithelium as well as throughout the local stroma, but not in the distal stromal portions of subcutaneous tissue (Figure S4I). Recently, stromal-like cells surrounding PDAC have been found to be derived by EMT from the pancreatic cancer epithelium in a mouse model (Rhim et al., 2012). However, the study observed very few EMT-derived stromal cells at the PanIN stages. Taken together, it seems that most but perhaps not all of the human stromal cells in the PanIN regions of the teratomas were derived by local co-differentiation of the pluripotent 10-22 cells into epithelial and mesenchymal tissues.
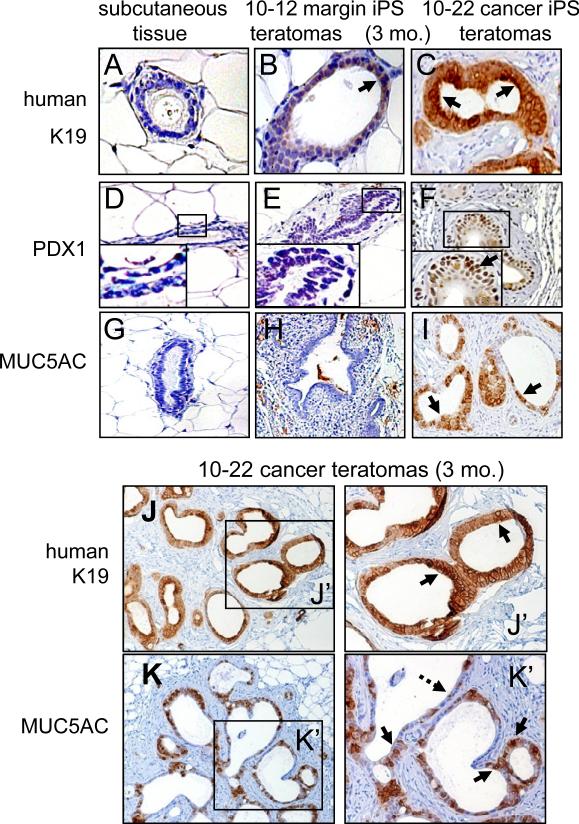
Characterizing the ductal structures in teratomas of the 10-22 cancer iPS-like line in further detail, we found that they expressed keratin 19 (K19) as well as nuclear PDX1, the pancreatic determination factor, and nuclear SOX9 (Figures 3A-F,J; Figure S5A-E). PDX1 is expressed at very low levels in adult pancreatic duct cells, it is not expressed in adult exocrine cells, and it is up-regulated in pancreatitis, PanIN, and PDAC (Miyatsuka et al., 2006). SOX9 is a coordinate effector, with mutant Kras, of precursor pancreatic lesions in a mouse model (Kopp et al., 2012). Teratomas from the 10-12 pancreatic margin iPS-like cells did not express the gastric mucin MUC5AC, whereas the PanIN-like structures of the teratomas from the 10-22 cells expressed abundant MUC5AC (Figures 3G-I,K, brown staining; Figure S5A), as observed in PanIN lesions and well differentiated PDAC (Kim et al., 2002). Considering the histology and CGH genomic profile of the parental pancreatic cancer tissue from which the 10-22 iPS-like line was derived, the data indicate that the 10-22 iPS-like line from poorly differentiated, late stage PDAC can differentiate into PanIN lesions associated with the early stage of the disease.
Figure 3. Teratomas at three months from 10-22 cancer iPS-like cells exhibit PanIN-like structures and marker expression.
A-C. No K19 staining in mouse subcutaneous tissue (negative control), weak K19 staining of 10-12 margin iPS-like teratomas at three months, and strong K19 staining of 10-22 cancer iPS-like teratomas at 3 months. D-I. Nuclear staining of PDX1 (arrow) and cytoplasmic staining of MUC5AC (arrow) only in teratomas of 10-22 cancer iPS-like teratomas at 3 months. J, K. Higher magnification shows uniform K19 staining (J, J’, arrow) and heterogeneous MUC5AC staining (K, K’) of teratomas from 10-22 cancer iPS-like cells.
The 10-22 iPS-like line PDAC progresses to the invasive stage of human pancreatic cancer
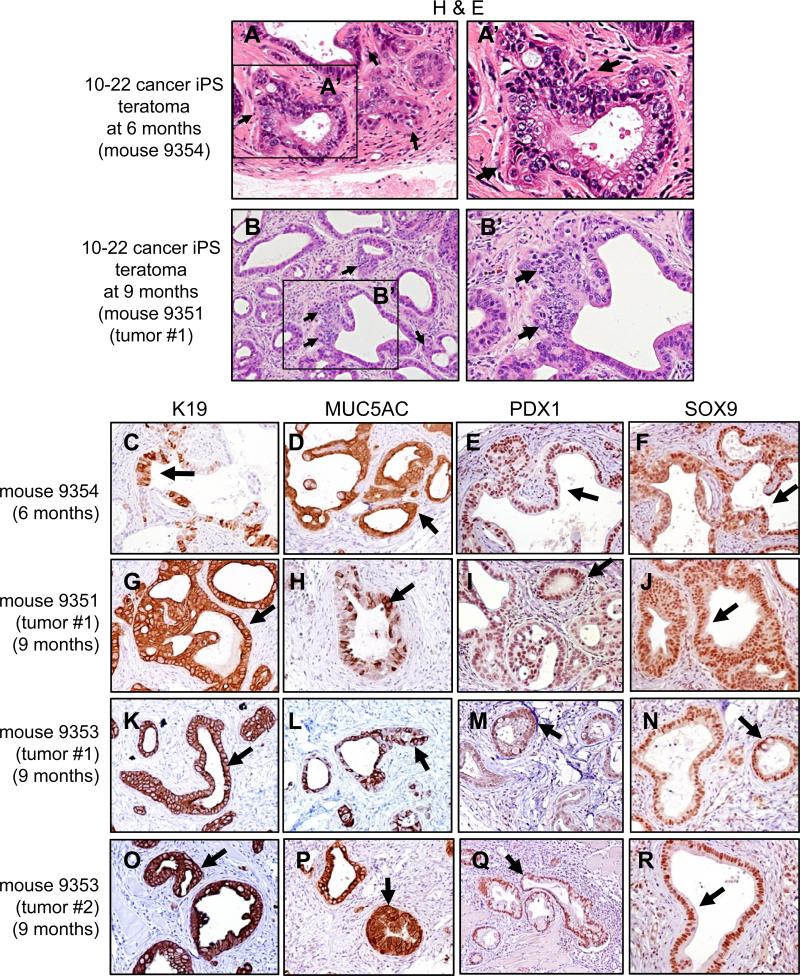
To assess whether PanIN-like structures from the 10-22 line could progress to later stages of PDAC, we investigated teratomas grown for 6-9 months in NSG mice. By 9 months, two solid, palpable tumors arose in each of two injected mice (3-6 mm diameter), all with a genotype characteristic of 10-22 cells (Figure S5F). While palpable tumors were not evident at 6 months, histological analysis showed highly glandular structures with nuclear heterotypia and hypochromia at both 6 and 9 months (Figure 4A, B). The epithelial cells were positive for K19, MUC5AC, PDX1, and SOX9 (Figure 4C-R, arrows). Notably, we observed structures indicative of a locally invasive phenotype (Figure 4A, B; arrows). We conclude that the PanIN-like stage of teratomas from the 10-22 iPS-like line is succeeded in vivo by the invasive stage of PDAC, indicating that the 10-22 iPS-like model undergoes a spectrum of pancreatic carcinogenesis.
Figure 4. Teratomas at 6 and 9 months from 10-22 cancer iPS-like cells exhibit invasive stages of pancreatic cancer.
A-B, H&E staining of 10-22 teratomas in NSG mice at 6 months (A, A’) and 9 months (B, B’) . Arrows indicate epithelial cells with a more dysmorphic phenotype than at 3 months; nuclei are heterotypic and epithelia with hypochromic nuclei are invading the stroma (A, B, arrows). C-R immunohistochemistry showing epithelial cells positive for K19, MUC5AC, PDX1, and SOX9 in tumors arising at 6 and 9 months in NSG mice injected with 10-22 cells. See Figure S5F for genetic confirmation of 10-22 cells in the tumors.
Cultured organoids of PanIN-like cells from 10-22 line teratomas secrete or release proteins indicative of early stage pancreatic cancer
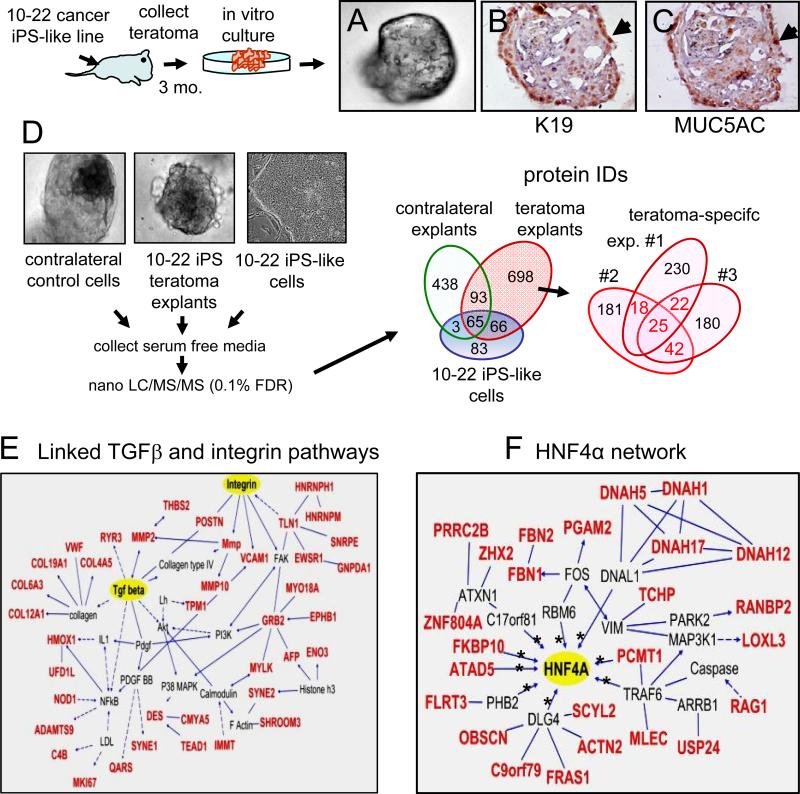
We sought to generate a system where the PanIN-like structures occurring within teratomas from the 10-22 cells could be studied as a live, in vitro model of early stage human pancreatic cancer. Accordingly, we harvested tissues from teratomas 3 months after injection, along with contralateral control tissue, and established conditions where the tissues were embedded separately into Matrigel and cultured in vitro (Figure S6A). PCR analysis of DNA from the resulting sphere-like organoids confirmed the 10-22 line genotype, which was absent from contralateral control explants (Figure S6B). The organoids from the 10-22 cancer iPS-like cells retained the expression of human K19 and MUC5AC (Figure 5A-C, sections).
Figure 5. Proteins secreted and HNF4 regulatory network within teratoma explants of 10-22 iPS-like cells at 3 months.
Scheme for in vitro explants of three month teratomas from 10-22 cancer iPS-like lines. A-C. Whole mount view of teratoma explant from 10-22 iPS-like cells (A), along with the explant sectioned and stained for K19 (B), and MUC5AC (C); arrows indicate positive (brown) domains. D. Proteomic analysis of conditioned media of 3 independent teratoma explants (#7761, 9223, and 9225) from 10-22 cancer iPS-like cells. Conditioned media was analyzed by nanoLC/MS/MS and protein candidates were identified by a Sequest search in the IPI DB. Using UniProKB TrEMBL (downloaded on Oct, 2011), we included peptides in our list that either consists of uniquely human sequences or sequences that are common to humans and mice. Mouse-specific sequences were excluded. Protein I.D.'s of conditioned media from teratoma explants were compared to those in conditioned media from explants of contralateral control tissue and of media from undifferentiated 10-22 ells (left Venn diagram). The total 698 secreted or released proteins specific to the teratomas are indicated in the three different experiments (right Venn diagram). Proteins secreted from at least two teratoma explants (red numbers) are summarized in Table S4A-D and were used for pathway analysis. E. Numerous proteins fall into linked pathways for TGFβ and integrin signaling. Lines and arrows connecting molecules indicate direct interactions and dashed lines and arrows indicate indirect interactions. F. All proteins in red are secreted or released specifically from the 10-22 teratoma explants and fall within a network controlled by the transcription factor HNF4α (Table S4F). Asterisks denote proteins whose genes are directly bound by HNF4α.
Given the poor prognosis of PDAC, we used the organoid system to identify biomarkers and pathways that could permit early detection and facilitate disease monitoring after therapy. We used nanoLC/MS/MS to examine the proteins that were secreted or released from explants of three independent teratomas that were cultured for 6 days (Figure 5D, left). The data were compared to proteins secreted or released from explants of contralateral control tissue cultured similarly and from the parent 10-22 iPS-like line cultured in the undifferentiated state. We then selected peptides with perfect matches to human peptides and that were specific to the human PanIN-like teratoma explants (Figure 5D, right). Of the 25 proteins that were secreted or released from all three 10-22 teratoma explants (Figure 5D, right; Table S4), 8 were previously reported to be expressed at the RNA or protein levels within PanIN, IPMN, and/or PDAC, whereas the remaining proteins have not been reported (Harsha et al., 2009) (Table S5). Of the 82 proteins that were identified from the intersection of pairs of 10-22 teratoma explants (Figure 5D, right; Tables S6-8), 15 proteins have been previously reported in PanIN/IPMN/PDAC (Harsha et al., 2009) (Table S5). While certain mRNAs were previously identified in PanIN, such as PGAM-M and VWF (Buchholz et al., 2005), we have determined that the corresponding proteins are secreted or released from cells and remain stable in the medium.
Of the total 107 proteins secreted or released from at least two 10-22 teratoma explants, Ingenuity pathway analysis revealed that 42 fall into interconnected TGFβ1 and integrin signaling networks (Figure 5E, for 38 proteins). These pathways have been previously reported in PanIN, IPMN, and PDAC (Bardeesy et al., 2006; Jones et al., 2008). In summary, we discovered numerous proteins that are secreted or released from the 10-22 live cell model of early stage human pancreatic cancer, as well as evidence of well-documented pathways involved in early cancer progression.
HNF4α network activated in early to intermediate, but not late stage pancreatic cancer
Further Ingenuity analysis revealed that another 25 of the secreted or released proteins were in a network that we observed to be centered on the transcription factor HNF4α, including numerous direct gene targets (Odom et al., 2004) (Figure 5F, Table S9). Nine of the proteins in the HNF4α network were also secreted into the plasma during the PanIN stage of a mouse PDAC model (Table S9) (Taguchi et al., 2011). Although HNF4α has not been reported in the development or progression of PDAC, by searching databases, we noted the amplification of the HNF4α locus (Maser et al., 2007) and the up-regulation of HNF4α mRNA (Iacobuzio-Donahue et al., 2003; Logsdon et al., 2003) in human PDAC. Although some reports show HNF4α in pancreatic acinar cells as well as islets in adult mice (Gupta et al., 2007), we found HNF4a to be predominantly expressed in islets, with very low or no expression elsewhere in the normal pancreas (Figure S6C). Strikingly, while HNF4α was not expressed in normal human or mouse pancreatic ducts (Figure 6A, S6C), it was expressed in the nucleus of 10-22 teratoma PanIN-like cells at 3 months and invasive stage cells at 9 months (Figure 6B,C; Figure S6D). HNF4α was detected cytoplasmically in moderately differentiated domains of the original tumor of the 10th patient, from which 10-22 cells were derived, but not in undifferentiated portions of the tumor (Figure 6D).
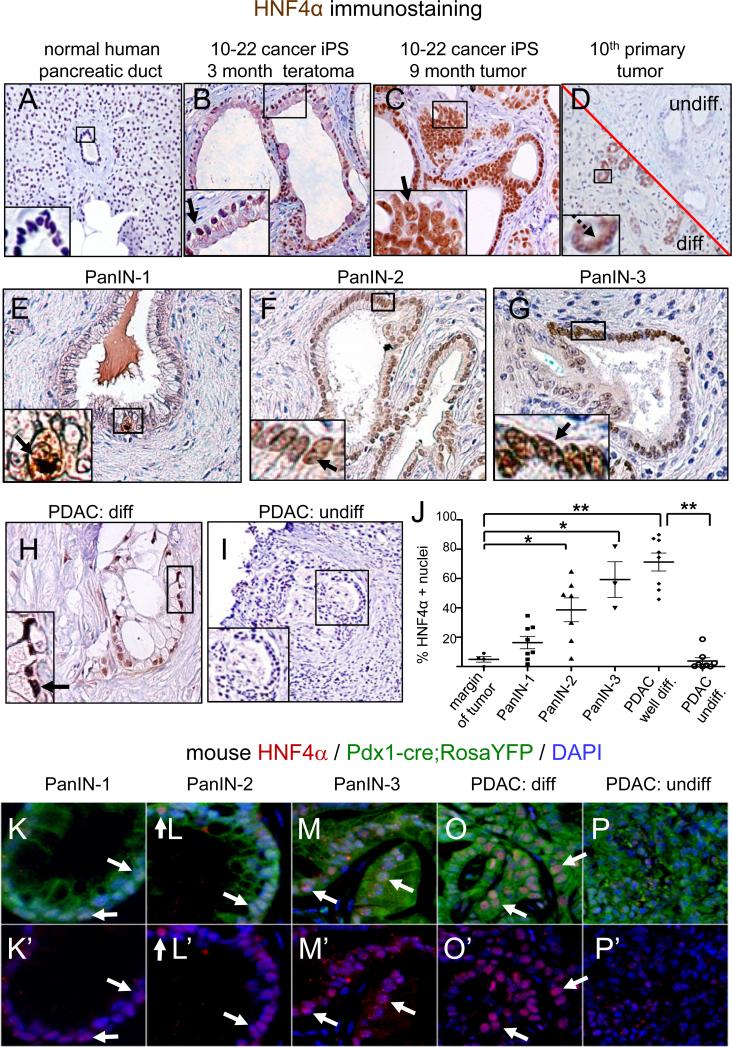
Figure 6. Activation of HNF4α in PanIN cells and well differentiated early pancreatic cancers in human clinical samples and a mouse model of human PDAC.
A-D. Immunohistochemistry for HNF4α showing the absence of staining in the main human pancreatic mass and ducts (A), strong nuclear staining in PanIN-like structures in a 3 month teratoma (B, arrow) and in invasive cells in a 9 month tumor (C, arrow) generated from NSG mice injected with 10-22 cells, and mostly cytoplasmic staining in small, moderately differentiated ducts (D, image section below the diagonal, brown, dashed arrow) and an absence of staining in undifferentiated ducts (D, image section above the diagonal) of the 10th patient's pancreatic tumor epithelium E-I. HNF4α is weakly labeled in human PanIN-1 cells (E, arrow, N=8), strongly in PanIN2 (F, arrow, N=7), PanIN3 (G, arrow, N=3), and mucinous well differentiated human PDAC (H, arrow, N=8) but decreased or not in undifferentiated/or poorly differentiated human PDAC (I, N=8). J, Percentage of HNF4α positive nuclei to total nuclei counted (See Table S4G) in multiple PanIN and PDAC samples on human tissue microarrays. Error bars, SEM. The differences in HNF4α expression is statistically significant between margin and PanIN-2 and -3 stages (* P<0.05); margin and well-differentiated PDAC (** P=5.46E-06); well differentiated PDAC and poorly/undifferentiated PDAC (**P=3.05E-06), as per two-tailed Student's t-tests. K-P. In a mouse model of PDAC, HNF4α is expressed weakly at the PanIN1 (K, K’), strongly at the PanIN2-3 stages (L, L’, M, M’) and in differentiated portions of tumors (PDAC) (O, O’), and weakly or not expressed in undifferentiated portions of the same tumor (P, P’).
To more quantitatively assess HNF4α expression at different stages of human pancreatic cancer, we used a tissue microarray to assess multiple samples of human PanINs at different stages as well as samples of pancreatic cancer. HNF4α was barely detected in the nuclei of normal pancreatic ducts and very weakly in the samples of PanIN-1 cells, but exhibited a statistically significant increase in nuclear expression in the samples of PanIN-2 (p<0.05) and stronger and more uniform expression in PanIN-3 epithelia (p<0.05) (Figure 6EG, J; Table S10). HNF4α was most frequently detected in well differentiated mucinous sections of PDAC (p<0.01) and barely or not detectable in undifferentiated or poorly differentiated epithelial structures of PDAC (Figure 6H-J), as seen in the original patient #10 tumor (Figure 6D). We also note that in the human protein atlas (Uhlen et al., 2010), HNF4α appeared positive in well differentiated epithelial structures of PDAC but either cytoplasmic or not expressed in poorly differentiated or undifferentiated epithelial structures of PDAC, and not expressed in metastatic PDAC.
We assessed HNF4α in a mouse model of PDAC arising in a KrasG12D;p53L/+; Pdx1-Cre; RosaLSL-YFP background (Rhim et al., 2012). As in humans, HNF4α was sporadically expressed at the PanIN-1 stage (Figure 6K), it was expressed in most nuclei of PanIN-2 (Figure 6L) and PanIN-3 lesions (Figure 6M), and observed in nuclei of the more differentiated portions of the murine tumors but not in the undifferentiated portions (Figure 6O, P; white arrows). In conclusion, we validated the ability of the 10-22 iPS-like cells to undergo early stages of human pancreatic cancer by their morphology, histology, secreted or released proteins, and using them to discover a previously unappreciated network associated with early to invasive stage pathology in human clinical samples and a mouse model of the disease.
DISCUSSION
There has been an absence of live human cell models of PDAC progression and consequently little information about proteins that could serve as released biomarkers and pathway indicators for early stages of the disease. As noted in the Introduction, when human PDAC or pancreatic cancer stem cells are grafted into immunodeficient mice, tumors rapidly arise that resemble the advanced PDAC stages from which the cells were derived and they do not undergo the slow growing phenotype of PDAC precursors. We hypothesized that, based on the ability of certain cancer cells to be reprogrammed to pluripotency by nuclear transfer and then to undergo early mammalian development, pluripotent stem cell lines from human pancreatic tumors might have the capacity to progress through early developmental stages of the cancer. This would provide an opportunity for discovering intrinsic processes and secreted protein biomarkers of live, early-stage human cells for a devastating cancer. Indeed we showed that a rare, single pancreatic cancer iPS-like line, 10-22 cells, can provide novel insights into human cancer progression.
How does the ectopic expression of Oct4, Sox2, Klf4, and c-Myc and a pluripotent-like state suppress the cancer phenotype? Apparently the pluripotency epigenetic environment can dominate over certain oncogenic states (Lonardo E, 2011). In nuclear transfer studies, only certain cancer cells are amenable to reprogramming (Blelloch et al., 2004; Hochedlinger et al., 2004; Li et al., 2003) and, similarly, we only obtained one iPS-like line from pancreatic cancer harboring a KRAS mutation, the predominant driver of PDAC. While the KRAS mutation induces MAPK signaling, which can trigger mouse ES cells to differentiate (Kunath et al., 2007), in human ES cells, MAPK signaling can promote self-renewal (Eiselleova et al., 2009). Oncogenic RAS induces cellular senescence by the accumulation of p53 or CDKN2A (Serrano et al., 1997) and the expression of the four reprogramming factors also triggers senescence by inducing p53 and CDKN2A, thereby impairing reprogramming (Banito et al., 2009). Only patient #10 in our study had a deletion in exon2 of CDKN2A, possibly explaining how the 10-22 cells could escape a senescent phenotype. Additional mutations could have arisen in the 10-22 cells that made the cells particularly amenable to iPS formation. Further work will determine what kind of mutations predict whether a cancer cell can be reprogrammed to pluripotency.
How does release from pluripotency allow the cancer genome to be expressed in a stage-specific fashion, as opposed to undergoing an immediate regression to the late stage phenotype? While the answers to these questions are not in hand, release from pluripotency is normally accompanied by the development of germ layer cells and then specialized tissues, which may continue to dominate, epigenetically, over the resident cancer genome (Blelloch et al., 2004; Hochedlinger et al., 2004; Li et al., 2003). The 10-22 cells from PDAC generated diverse tissue types in teratomas as well as pancreatic ductal tissue that exhibited PanIN lesions and later progression. The apparent preference for pluripotent cells to regenerate the cancer type from which they were derived reflects the tendency of iPS cell lines in general to preferentially differentiate into their lineages of origin (Bar-Nur et al., 2011; Kim et al., 2011).
Several lines of evidence indicate that the 10-22 iPS-like line is derived from PDAC. First, the pathology of the original, recurrent tumor was that of PDAC and the CGH profile of the bulk population of cultured cells, which had a highly disrupted genome, was represented in the CGH profile of the 10-22 iPS-like line (Figs. 1I, S3B). While the tumor harbored pockets of more differentiated epithelial cells amidst a vast majority of undifferentiated cells, and therefore it is not certain which type of cell was immortalized in the 10-22 line, the 10-22 cells’ disrupted genome does reflect that of a typical epithelial cell in the recurrent tumor. Second, the PanIN-like structures from the 10-22 cells’ teratomas expressed SOX9 (Figure S5B-E), which is required for early, KrasG12D-dependent pancreatic precursor lesions in a mouse model (Kopp et al., 2012), as well as PDX1, a definitive pancreatic cancer epithelial marker. Thus the ductal lesions from the 10-22 cells are of a pancreatic type. Third, teratomas at 9 months from 10-22 cells progressed to the histology and locally invasive characteristics of later stage PDAC (Fig. 4). Thus, the 10-22 line was not from an early stage cell that would solely undergo an early stage phenotype. Taken together, the evidence indicates that the 10-22 iPS-like line is from PDAC cells in the original tumor and that, upon re-differentiation in teratomas, it undergoes progression of the disease. This is unlike other human PDAC lines, which exhibit late stages of cancer (Lieber et al., 1975; Yunis et al., 1977).
Could the 10-22 iPS-like line be derived from cancer stem cells within the original tumor? Pluripotency genes such as NANOG are expressed in sphere cultures of pancreatic cancer stem cells (CSCs), suggesting that such cells might be more susceptible to reprogramming (Lonardo et al., 2011). However, CD133+CXCR4+ pancreatic CSCs are not enriched and the expression of pluripotent genes is not observed in the adherent culture conditions we used to derive the 10-22 cells (Hermann et al., 2007). Also, the OCT4 and NANOG pluripotency genes were highly methylated in the parental tumor #10 epithelium cultures, in contrast to the 10-22 cell line and the huES H1 control, and NANOG itself was not expressed in the primary tumor, although OCT4 was expressed sporadically (Fig. S2B, C). Taken together, it seems unlikely that 10-22 cells were derived from pancreatic CSCs. In addition, pancreatic CSCs (Hermann et al., 2007; Ishizawa et al., 2010; Li et al., 2007) rapidly generate aggressive tumors that represent the primary tumors; whereas the 10-22 cells generate slow growing PanINs (Figs. 3, S4). Finally, tumors generated with pancreatic CSCs give rise to both cytokeratin negative and positive cells in the resultant tumors, in contrast to the homogenous K19 positive staining in PanIN-like ducts at 3 month teratomas (Figs. 3, S2A). Thus, 10-22 cells appear not to exhibit properties of pancreatic cancer stem cells.
The proteins released or secreted from the PanIN-like teratomas fell into 3 major networks, including inter-connected networks for TGFβ and integrin signaling that suppress PDAC progression (Hezel et al., 2012). We also report here for the first time the activation of an HNF4α network distinctive for the late PanIN stages. HNF4α is not or barely expressed in normal pancreatic ductal cells, poorly expressed in the PanIN1 stage, but is activated in PanIN2 and PanIN3 stages, invasive stages, and in early well-differentiated human pancreatic cancer. HNF4α levels then decrease markedly in advanced or undifferentiated PDAC. We found that these expression states also occur in a mouse model of PDAC progression. Dynamics in HNF4α expression affect the oncogenic transformation of liver cells (Hatziapostolou et al., 2011). It remains to be determined whether the expression of HNF4α and its target genes is a cause or consequence of pancreatic cancer progression. Yet considering that pancreatic cancer is typically discovered in advanced or metastatic stages, activation of HNF4α and the release or secretion of proteins from the factor's target genes specifically in the late PanIN stages should provide useful diagnostics.
Of 107 proteins reproducibly released or secreted from PanIN-like cells derived from the 10-22 line, a total of 68 proteins overlap with genes, proteins, and networks expressed in human PanIN and PDAC, further validating the origin of the cells. In addition, we show that such proteins are released from the cells and stable, thus serving as potential biomarkers of early PDAC. A subset of the secreted proteins could be from locally activated stromal cells in the explants. We suggest that the combined detection of released proteins that are the products of the HNF4α, TGFβ, and integrin networks within PanIN and invasive PDAC cells may provide the best means for noninvasively detecting the progression of pancreatic cancer in humans.
Based on our extensive efforts with diverse initial PDAC samples (see Tables S1, S2), iPS cells arising from epithelial cells of solid tumors appear to be rare events, possibly involving secondary changes that allow iPS formation. It is therefore unclear whether the iPS approach will work with other solid tumor samples. Despite these caveats, the 10-22 cells behaved in a highly consistent fashion and this study revealed novel information about human PDAC. It is hoped that a better understanding of how to create iPS cells from human epithelial cancers will provide opportunities in the future with other types of solid tumors.
EXPERIMENTAL PROCEDURES
Tissue (1-2 cc) was taken from the center of pancreatic ductal adenocarcinoma samples and sample margin furthest from the cancers, under the patient's informed consent. Tissue was dissociated and cells were cultured in defined K-SFM supplemented with EGF, cholera toxin, and bovine pituitary extract on dishes coated with collagen at 37°C for 3-4 days. The resultant epithelial cells were infected twice with TetO mouse Oct4, Sox2, Klf4, and c-Myc, and PWPT-rtTA lentiviruses for 72 hr. About 105 cells surviving per starting tumor or margin tissue were detached, resuspended in ES media supplemented with Y27632, and plated onto irradiated mouse embryonic fibroblasts (MEFs). ES-like colonies were picked 12 to 36 days post-infection and passaged mechanically every 5-7 days onto irradiated MEFs, with daily feedings. The iPS-like lines were characterized by RT-PCR and immunostaining for pluripotency markers, embryoid body and teratoma assays for pluripotency, and CGH, karyotyping, PCR for KRAS, CDKN2A, and BRAF mutations, and pyrosequencing for KRAS mutations. Female 4-6 week old NOD-SCID-IL2Rgc null (NSG) mice (Shultz et al., 2005) were used for subcutaneous injection of the iPS-like lines. The injection area was isolated after 3-9 months and analyzed by histology or dissociated in liberase T-flex (1.3 W/ml), embedded into Matrigel, and cultured in SFM. Conditioned media was collected and proteins were processed for analysis by nanoLC/MS/MS. See the Supplemental Information on-line for details.
Supplementary Material
Highlights.
An iPS-like line was created from human pancreatic ductal adenocarcinoma (PDAC)
Teratomas from PDAC iPS-like cells undergo early to invasive stages of human cancer
Stages of human pancreatic cancer can be studied in live iPS teratomas and explants
iPS teratoma cultures reveal biomarkers and pathways of early human pancreatic cancer
ACKNOWLEDGMENTS
We thank the patients who donated their tissue for this study and T. Jiang, G. Swan, T. Secreto, and C. Keeper for technical assistance, A. Rustgi, J. Gearhart, and D. Metzger for comments on the manuscript, and E. Hulme for help with the manuscript. We thank the UPenn Stem Cell and Xenograft Core, Proteomics Core (NIH P30CA016520, AFCRE and ES013508-04, CEET) and MPI Core of the Center for Molecular Studies in Digestive and Liver Diseases (NIH/NIDDK P30-DK050306). The work was funded by NIH Merit award R37GM36477 to K.S.Z. In memory of J.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet Beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blelloch RH, Hochedlinger K, Yamada Y, Brennan C, Kim M, Mintz B, Chin L, Jaenisch R. Nuclear cloning of embryonal carcinoma cells. Proc Natl Acad Sci U S A. 2004;101:13985–13990. doi: 10.1073/pnas.0405015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–169. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]
- Buchholz M, Braun M, Heidenblut A, Kestler HA, Kloppel G, Schmiegel W, Hahn SA, Luttges J, Gress TM. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626–6636. doi: 10.1038/sj.onc.1208804. [DOI] [PubMed] [Google Scholar]
- Carette JE, Pruszak J, Varadarajan M, Blomen VA, Gokhale S, Camargo FD, Wernig M, Jaenisch R, Brummelkamp TR. Generation of iPSCs from cultured human malignant cells. Blood. 2010;115:4039–4042. doi: 10.1182/blood-2009-07-231845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Cravero JD, Adrian K, Grippo P. Modeling pancreatic cancer in vivo: from xenograft and carcinogen-induced systems to genetically engineered mice. Pancreas. 2010;39:283–292. doi: 10.1097/MPA.0b013e3181c15619. [DOI] [PubMed] [Google Scholar]
- Eiselleova L, Matulka K, Kriz V, Kunova M, Schmidtova Z, Neradil J, Tichy B, Dvorakova D, Pospisilova S, Hampl A, et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells. 2009;27:1847–1857. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr., Kaestner KH. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, Karin M, Struhl K, Hadzopoulou-Cladaras M, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147:1233–1247. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O'Dell MR, Rivera LB, Harper J, Lonning S, Brekken RA, et al. TGF-beta and alphavbeta6 Integrin Act in a Common Pathway to Suppress Pancreatic Cancer Progression. Cancer Res. 2012;72:4840–4845. doi: 10.1158/0008-5472.CAN-12-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, Jaenisch R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq R, et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris J.P.t., Pan FC, Akiyama H, Wright CV, Jensen K, et al. Identification of Sox9-Dependent Acinarto-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li L, Connelly MC, Wetmore C, Curran T, Morgan JI. Mouse embryos cloned from brain tumors. Cancer Res. 2003;63:2733–2736. [PubMed] [Google Scholar]
- Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Lonardo E HP, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, Torres-Ruíz R, Garcia E, Hidalgo M, Cebrián DÁ, Heuchel R, Löhr M, Berger F, Bartenstein P, Aicher A, Heeschen C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell RG, Deggins BA, Labat DD. Transplantation of pluripotential nuclei from triploid frog tumors. Science. 1969;165:394–396. doi: 10.1126/science.165.3891.394. [DOI] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, Mori M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci U S A. 2010;107:40–45. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.P.t., Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–2143. [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- Rustgi AK. The molecular pathogenesis of pancreatic cancer: clarifying a complex circuitry. Genes Dev. 2006;20:3049–3053. doi: 10.1101/gad.1501106. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Politi K, Pitteri SJ, Lockwood WW, Faca VM, Kelly-Spratt K, Wong CH, Zhang Q, Chin A, Park KS, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell. 2011;20:289–299. doi: 10.1016/j.ccr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Yunis AA, Arimura GK, Russin DJ. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase. Int J Cancer. 1977;19:128–135. doi: 10.1002/ijc.2910190118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.