Abstract
Oculodentodigital dysplasia (ODDD) is a rare developmental disease resulting from germline mutations in the GJA1 gene that encodes the gap junction protein connexin43 (Cx43). In addition to the classical ODDD symptoms that affect the eyes, teeth, bone and digits, in some cases ODDD patients have reported bladder impairments. Thus, we chose to characterize the bladder in mutant mouse models of ODDD that harbor two distinct Cx43 mutations, G60S and I130T. Histological assessment revealed no difference in bladder detrusor wall thickness in mutant compared to littermate control mice. The overall localization of Cx43 in the lamina propria and detrusor also appeared to be similar in the bladders of mutant mice with the exception that the G60S mice had more instances of intra-cellular Cx43. However, both mutant mouse lines exhibited a significant reduction in the phosphorylated P1 and P2 iso-forms of Cx43, while only the I130T mice exhibited a reduction in total Cx43 levels. Interestingly, Cx26 levels and distribution were not altered in mutant mice as it was localized to intracellular compartments and restricted to the basal cell layers of the urothelium. Our studies suggest that these two distinct genetically modified mouse models of ODDD probably mimic patients who lack bladder defects or other factors, such as aging or co-morbidities, are necessary to reveal a bladder phenotype.
Keywords: Connexin, Cx43, Mutant, Bladder, Mutant mouse, Gap junction
Introduction
Overactive bladder (OAB) is a syndrome that affects millions of men and women worldwide, generally manifesting itself in some degree of incontinence. Stress-related incontinence is caused by increased intra-abdominal pressure, resulting from physical activity or coughing/sneezing. Urge-related incontinence can be subdivided into neurogenic and idiopathic types. The neurogenic type is related to altered afferent and efferent neuronal bladder stimuli, which is common in conditions such as spinal cord injury, Parkinson disease and multiple sclerosis (Christ et al. 2003). Idiopathic incontinence has no known pathological cause (Christ et al. 2003; Miller and Hoffman 2006); however, there is a growing body of evidence to suggest a myogenic origin for at least some forms (Brading 1997; Haferkamp et al. 2004). It has been proposed that altered gap junctional intercellular communication (GJIC) or cell signaling may be the cause of idiopathic incontinence, resulting in an increased sensitivity to acetylcholine or changes in how cells communicate (Imamura et al. 2009; Kuhn et al. 2008).
Gap junctions are formed from specialized proteins known as connexins (Cxs). Six connexins that have oligomerized to form a connexon are delivered to the plasma membrane, where they can function as a hemichannel or dock to a connexon from an adjacent cell to form a gap junction channel (Laird 2006). Hemichannels and gap junction channels can selectively pass small molecules of <1 kDa in size to the extracellular matrix or between connected cytoplasms, respectively (Alexander and Goldberg 2003; Evans et al. 2006). The selectivity of a gap junction channel or hemichannel is highly dependent on its connexin constituents. The 21 human and 20 mouse connexins all share similar membrane topologies and can selectively intermix to create a plethora of channel variations that exhibit unique characteristics (Laird 2005, 2006; Sohl and Willecke 2003).
In the bladder, the urothelial layer is composed of transitional epithelium, which acts as a passive barrier and actively responds to urine composition and bladder distension (Apodaca 2004). ATP, which is known to be released from the urothelium, is thought to signal adjacent urothelial cells and/or bladder nerves through P2X receptor stimulation within the underlying lamina propria, therefore acting in both an autocrine and a paracrine fashion (Apodaca 2004; Cockayne et al. 2000). Both Cx26 and Cx43 have been localized to the urothelium, although their roles in bladder function have yet to be elucidated (Grossman et al. 1994; Haefliger et al. 2002). The lamina propria, composed of loose fibroelastic connective tissue, expresses Cx43, which is localized to fibroblasts and myofibroblasts (Fry et al. 2007; Neuhaus et al. 2007; Sui et al. 2002; Wiseman et al. 2003). Myofibroblasts have been shown to be in close proximity with both afferent and efferent neurons and are therefore thought to aid in bladder signaling (Sui et al. 2002; Wiseman et al. 2003). The detrusor layer of the bladder is composed of smooth muscle cells that express both Cx43 and Cx45, which collectively act to facilitate bladder contractions (Heinrich et al. 2011; Ikeda et al. 2007). Specifically, Cx43 has been localized to the border of smooth muscle cell bundles, suggesting its importance in the transmission of electrical stimulus from one muscle bundle to the next, while Cx45 has been localized to the plasma membrane of cells within the muscle bundles (Hashitani et al. 2004; John et al. 2003; Sui et al. 2003).
To date, over 65 mutations in the gene encoding Cx43 have been linked to the rare autosomal dominant disease oculodentodigital dysplasia (ODDD) (Paznekas et al. 2003, 2009). All mutants examined to date have been shown to have reduced channel function and to act as dominant-negatives to the function of coexpressed wild-type Cx43 (Gong et al. 2007; McLachlan et al. 2005; Roscoe et al. 2005). Patients harboring Cx43 mutants share common clinical characteristics, such as syndactyly and camptodactyly of the digits, microdontia, enamel loss, ophthalmic defects and craniofacial abnormalities (Paznekas et al. 2003, 2009). Bladder defects have been reported in ~12 % of ODDD patients, although the mechanism behind these defects remains poorly understood (Loddenkemper et al. 2002; Paznekas et al. 2003, 2009). Although the percentage of ODDD patients suffering from bladder defects appears lower than that in the general population at ~17 % (Abrams et al. 2003), many of these patients develop bladder defects early in life (Paznekas et al. 2003, 2009), a trait rarely seen in the general population. Since Cx43 is broadly distributed throughout the bladder and is thought to play a critical role in bladder contraction, we hypothesized that the reason ODDD patients suffer from bladder problems may be rooted in how specific ODDD-linked mutants affect the distribution and function of Cx43-based gap junctions within the bladder. To test this hypothesis, we employed two heterozygous Cx43 mutant mouse lines harboring the G60S or I130T mutant (Flenniken et al. 2005; Kalcheva et al. 2007). Upon examination of several cell types obtained from these mice, all were found to exhibit reduced Cx43-based GJIC and the mutant proteins were found to act as dominant-negatives to coexpressed wild-type Cx43 (Flenniken et al. 2005; Kalcheva et al. 2007; Lai et al. 2006; Manias et al. 2008; Shibayama et al. 2005). In the present study, we examined the distribution and spatial localization of Cx43 in the bladders of these two Cx43 mutant mouse lines to assess if Cx43 was perturbed in a manner that might suggest the bladder is functionally compromised.
Materials and Methods
Animals
Animal studies were conducted on two mutant mouse lines harboring heterozygous mutations, G60S or I130T, in the gene Gja1, which encodes Cx43. Therefore, the mice are expected to translate a 1:1 ratio of mutant to wild-type Cx43 protein and, thus, genetically match human ODDD patients. G60S, also known as Gja1Jrt/+, mice were supplied by the Centre for Modeling Human Disease, University of Toronto (Toronto, Canada), on a mixed C57BL/ 6J and C3H/HeJ background (Flenniken et al. 2005). After confirming 100 % penetrance of the syndactyly feature in all mutant mice as determined by PCR genotyping (Flenniken et al. 2005), genotype determination was completed by visual inspection of the pups. I130T, also known as Gja1I130T/+, mice were obtained from Dr. Glenn Fishman (New York University School of Medicine, New York, NY). These mice, which were on a mixed CD1/C57BL6 background, were further backcrossed onto a C57BL6 background for an additional one to three generations. A 100 % incidence rate of syndactyly (n = 203) in I130T mice was confirmed by PCR genotyping. However, while syndactyly was found on the front limbs of all mice, the back limbs did not always exhibit this phenotype.
Tissue Harvesting and Preparation
Whole bladder, heart and liver samples were collected from G60S, I130T and wild-type littermate mice at 3 months of age. All mice used in this study were killed using a carbon dioxide chamber followed by cervical dislocation, in accordance with the University of Western Ontario Guide for Care and Use of Laboratory Animals. Tissue samples assigned to hematoxylin and eosin (H and E) staining were fixed overnight at 4 °C in 10 % neutral buffered formalin (NFB) (EMD, Mississauga, Canada), dehydrated, embedded in paraffin blocks and sectioned longitudinally at 5-μm intervals. Tissue samples assigned to immunohistochemistry were snap-frozen in liquid nitrogen; embedded in 10.2 % polyvinyl alcohol, 4.3 % polyethylene glycol Optimal Cutting Temperature Compound (Sakura, Torrance, CA); sectioned longitudinally at 5 μm thickness; and stored at −80 °C. Tissue samples assigned to immunoblotting were snap-frozen in liquid nitrogen and stored at −80 °C for future use.
Histology
To assess the histology of the mouse bladders, 5-μm paraffin-embedded sections from G60S and I130T mice and their wild-type littermates were stained with H (0.4 %) and E (0.5 %). Briefly, paraffin-embedded bladder sections were deparaffinized in xylene, rehydrated in a descending gradient of ethanol baths and stained with hematoxylin (5 min), then washed and stained with eosin (5 min). Sections were then dehydrated in an ascending gradient of ethanol baths followed by xylene and mounted with Cytoseal (Thermo Scientific, Rockford, IL). Bladder sections were imaged on a Leica (Deerfield, IL) DM IRE2 inverted microscope equipped with a Micropublisher 5.0 RTV CCD color, cooled camera. Linear measurements were made perpendicularly through the detrusor layer using ImageJ software (http://rsbweb.nih.gov/ij/). In total, 405 measurements were made on each of four bladders from G60S and I130T mice and their wild-type littermates, corresponding to three slides per mouse, nine sections per slide and 15 measurements per section. Statistical analysis included standard error and comparison between G60S and I130T mice and their wild-type littermates.
Immunofluorescence
To assess the localization of Cx43 and Cx26 in mouse bladders, hearts and livers, 5-μm cryosections from G60S and I130T mice and their wild-type littermates were first fixed in 10 % NBF for 30 min at room temperature. Tissues sections were blocked with 3% BSA (Sigma-Aldrich, St. Louis, MO) and 0.02 % Triton X-100 in PBS for 45 min at room temperature. Cx43, Cx26 and actin were all detected using rabbit anti-Cx43 (2 μg/ml, Sigma C6219), rabbit anti-Cx26 (0.5 μg/ml; Invitrogen 51-2800; Invitrogen, Carlsbad, CA) and mouse anti-phalloidin (2 U/ml, Invitrogen A12379), respectively. All antibodies were diluted in PBS containing 3 % BSA and 0.02 % Tween-20 and allowed to incubate on tissue sections for 1 h. After repeated washings with PBS, tissues were incubated with secondary anti-rabbit AlexaFluor 555 antibody (4 μg/ml, Invitrogen A-21428), followed by repeated washings. Nuclear staining was performed using Hoechst 33342 (10 μg/ml; Molecular Probes, Eugene, OR) for 5 min at room temperature, followed by a 5-min wash with ddH2O. Tissues sections were mounted using glass coverslips and allowed to sit overnight at 4 °C. Tissue sections were imaged on a Zeiss (Thornwood, NY) LSM 510 Meta confocal microscope as previously described (Roscoe et al. 2005). Digital images were prepared using Zeiss LSM and CorelDraw 12 software.
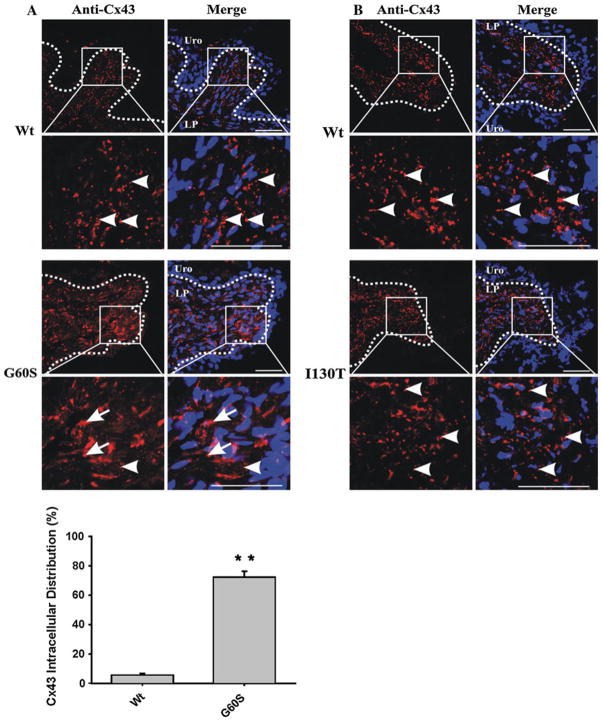
To quantify the percentage of cells in the detrusor layer of wild-type and G60S mice that exhibited intracellular Cx43, we randomly selected 10 fields (215 × 215 μm) from immunofluorescently labeled Cx43 bladder sections. The total number of Cx43-positive cells in the detrusor layer per field was counted, as was the number of cells that displayed primarily paranuclear Cx43 staining indicative of intracellular Cx43. The total number of Cx43-positive cells was divided by the number of intracellular Cx43-positive cells from wild-type and G60S mouse bladder sections. Data are presented as the mean percent of cells displaying intracellular Cx43 ± SEM (**P < 0.01).
Western Blot Analysis
Tissue samples stored at −80 °C were homogenized in RIPA lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1.0 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 1 mM sodium orthovanadate, 1 mM sodium fluoride), and homogenates were removed from cell debris via a 10-min, 6,000-rpm centrifugation at 4 °C.
Immunoblotting was performed as previously described (Gehi et al. 2011) using 10 or 12 % SDS-PAGE. Cx43, Cx26 and GAPDH were detected using polyclonal rabbit anti-Cx43 (0.02 μg/ml, Sigma C6219), polyclonal rabbit anti-Cx26 (0.05 μg/ml, Invitrogen 710500) and polyclonal mouse anti-GAPDH (1 μg/ml; Millipore, Temecula, CA), respectively. Primary antibodies were detected using either anti-rabbit AlexaFluor 680 (0.2 μg/ml, Invitrogen) or anti-mouse IRDye 800 (1:10,000; Rockland, Gilbertsville, PA) antibodies. Membranes were developed using an Odyssey infrared imaging system (LiCor, Lincoln, NE) and analyzed under unsaturated conditions using Odyssey 2.0.4 software (Licor).
Statistics
All results were analyzed using Student’s two-tailed independent sample t-test, using a P < 0.05 value to denote significance. All results were analyzed using GraphPad (San Diego, CA) Prism 4.03 software and are presented as mean ± standard error.
Results
G60S and I130T Bladders Have Similar Histology and Detrusor Thickness as Wild-Type Littermates
Increased detrusor thickness is a known indicator of OAB syndrome, a condition commonly seen in patients suffering from bladder abnormalities (Miyazato et al. 2009; Uvelius et al. 1984). H and E staining revealed that G60S and I130T mice display similar bladder histology as seen in wild-type littermate controls, with no disruption in any of the three bladders layers (Fig. 1a, b). Subsequent detrusor measurements revealed that detrusor thickness was similar between G60S and I130T mutant mice and their respective wild-type littermate controls (Fig. 1c). It is likely that the difference in detrusor thickness between wild-type mice used as controls is due, at least in part, to differences in the mouse strains.
Fig. 1.
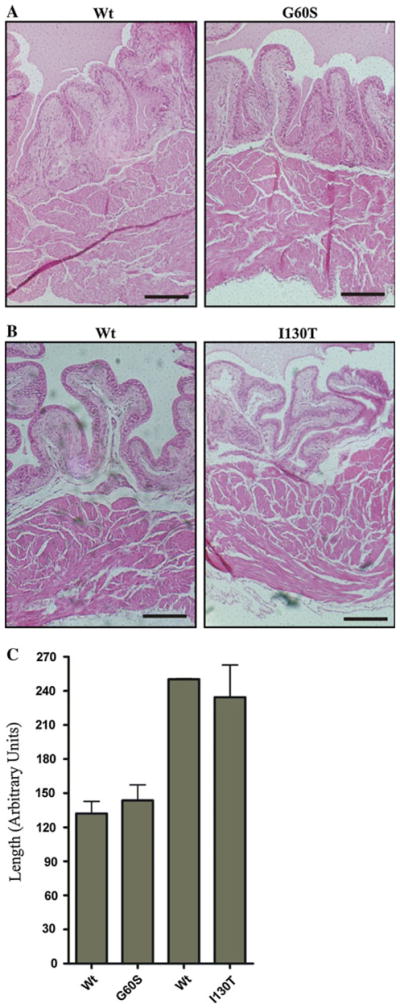
G60S and I130T mouse bladders have similar histology and c detrusor thickness as their wild-type littermate controls. Paraffin-embedded bladders were sectioned, stained with H and E and measured for detrusor thickness. In all mice, bladder histology was similar between mutant and matched wild-type (Wt) littermate controls (a, b). Subsequent detrusor measurements revealed that G60S and I130T mouse bladders have similar detrusor thicknesses as their respective wild-type littermates (c). Bars 200 μm (a, b), error bars ± SEM (c). n = 4 mice
G60S and I130T Bladders Have Reduced Levels of the Highly Phosphorylated Cx43 Species
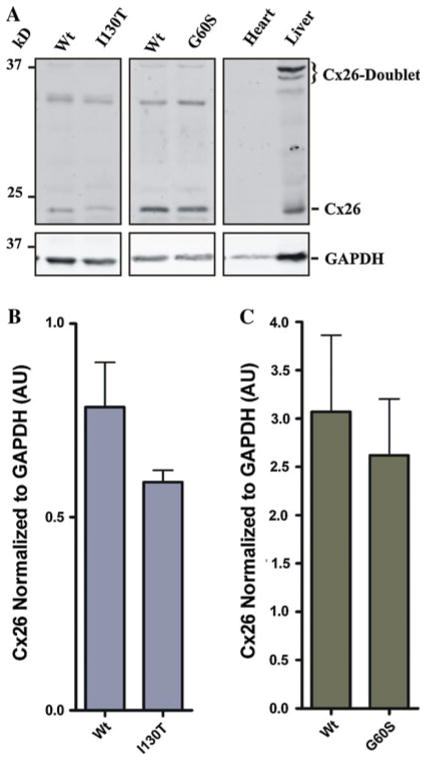
Previous studies have indicated that total Cx43 levels and levels of the highly phosphorylated Cx43 species are reduced in various tissues of the G60S mouse (Flenniken et al. 2005; Manias et al. 2008; Tong et al. 2009; Toth et al. 2010), while aside from heart, much less is known about the Cx43 species status in I130T mice. Western blots were used to assess the levels and phosphorylation status of Cx43 in the mutant mouse bladders. As positive and negative controls, Cx43 was examined in heart and liver lysates, respectively, from wild-type mice. Cx43 generally resolves in SDS-PAGE as multiple bands representing different phosphorylated species (P0, P1 and P2). Immu-noblotting for Cx43 in lysates from G60S and I130T mice and their wild-type littermates revealed low levels in the bladder samples of all mice (Fig. 2a–c). Quantification revealed that total Cx43 levels were reduced in I130T mice, while levels remained similar in G60S mice compared to their wild-type littermates (Fig. 2b, c). Further analysis revealed that the slower-migrating Cx43 P1 and P2 species were reduced in both G60S and I130T mice compared to their wild-type littermates (Fig. 2d, e). These phosphorylated Cx43 species have previously been correlated with fully assembled gap junction plaques and channel function. Immunoblotting also revealed nonspecific bands at ~47–50 and ~30 kDa in lysates from all bladder samples. Although these bands were present in only the bladder samples, it is unclear as to what they represent; therefore, they were not quantified.
Fig. 2.
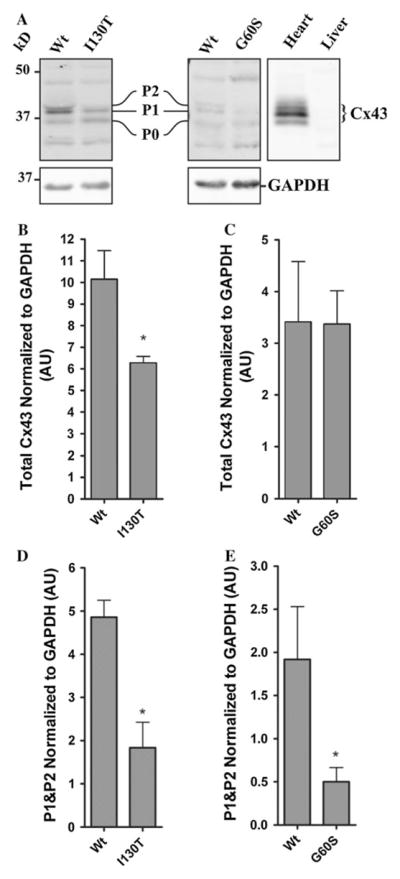
G60S and I130T mouse bladders exhibit a reduction in the c phosphorylated species of Cx43. Cx43 normally resolves as a triplet band representing different phosphorylated species of Cx43 (P0, P1 and P2), as seen in heart tissue (a). Cx43 was detected in the bladders of G60S and I130T mutant mice, as well as in wild-type (Wt) littermate mouse bladders but not in the mouse liver (a). Total Cx43 (P0, P1 and P2) expression was lower in I130T mouse bladders compared to littermate controls (b), a condition not observed in G60S mouse bladders (c). Lower levels of the phosphorylated Cx43 species (P1 and P2) were found in I130T (d) and G60S (e) mouse bladders compared to wild-type littermates. Nonspecific bands were noted at ~47–50 and ~30 kDa. Bars represent ±SEM (b–e). *P < 0.05. n = 5 (I130T mice) and 6 (G60S mice). AU arbitrary units
Cx43 Distribution in the Lamina Propria and Detrusor Layers of Wild-Type and Mutant Mice
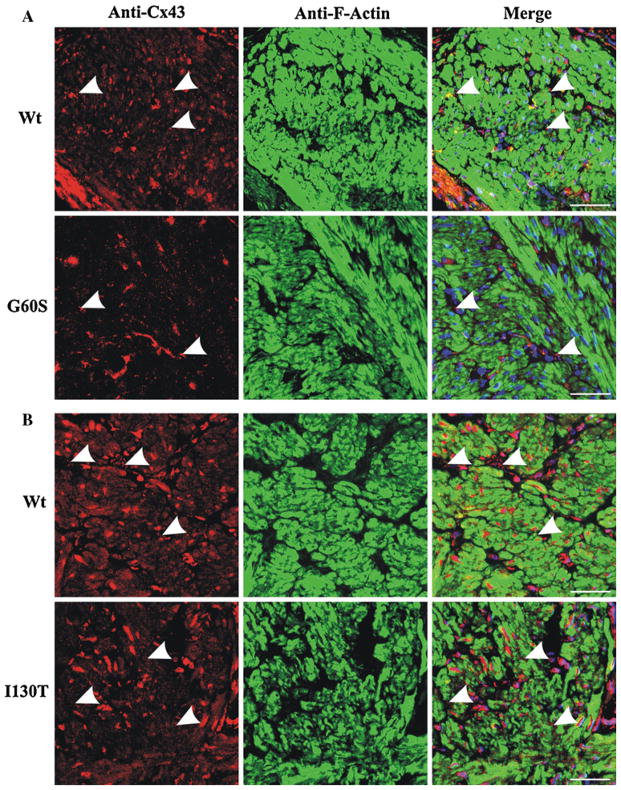
As a result of reduced Cx43 phosphorylated species detected in bladder lysates of G60S and I130T mice, we postulated that Cx43 localization and/or distribution may be disrupted in the bladder. Immunofluorescence revealed that Cx43 was highly expressed in the lamina propria of G60S and I130T mice, similar to their wild-type littermates (Fig. 3a, b). The Cx43 distribution at sites of cell to cell apposition in I130T and G60S mice appeared similar to that found in wild-type littermates (Fig. 3a, b, arrowheads). However, G60S mice exhibited significantly more intra-cellular Cx43 distribution, which was not readily detected in wild-type littermates (Fig. 3a, histogram). Immunofluorescence also revealed that Cx43 was localized to smooth muscle cells and fibroblasts surrounding smooth muscle bundles as revealed by F-actin localization in the detrusor layer of both mutant mice and their wild-type littermates (Fig. 4a, b). Thus, in both wild-type and mutant mice Cx43 was appropriately positioned in the muscle of the detrusor layer to allow for regulated bladder contraction.
Fig. 3.
The distribution of Cx43 in the lamina propria of wild-type and mutant mice. Cx43 was localized in the lamina propria of G60S (a) and I130T (b) mutant mice and compared to wild-type (Wt) littermate mouse bladder controls. G60S mouse bladders displayed significantly more intracellular Cx43 (arrows), as opposed to the punctate distribution (arrowheads) seen in wild-type littermates (a, histogram). I130T mouse bladders displayed a similar punctate Cx43 distribution pattern (arrowheads) as seen in wild-type littermates (b). Uro urothelium, LP lamina propria. Red indicates Cx43, blue indicates Hoechst nuclear stain. Bars 50 μm. Histogram error bars represent ±SEM. **P <0.01
Fig. 4.
Cx43 was localized to resident cell types of the detrusor in G60S and I130T mutant mice and their wild-type littermates. In the detrusor layer, immunolabeling revealed that Cx43 was predominantly localized to cells of connective tissue surrounding smooth muscle bundles and smooth muscle cells in G60S (a) and I130T (b) mice and their respective wild-type (Wt) littermates. Cx43 displays punctate structures (arrowheads) in G60S (a), I130T (b) and wild-type littermates. Red indicates Cx43, green indicates F-actin, and blue indicates Hoechst nuclear stain. Bar 50 μm
G60S and I130T Mouse Bladders Have Similar Cx26 Levels as Wild-Type Littermate Control Mice
The bladder urothelium has previously been shown to express Cx26, and it is thought to play a role in bladder signaling during filling (Ikeda et al. 2007). As controls, Cx26 was detected in liver lysates, while it was absent in heart lysates from wild-type mice (Fig. 5a). Cx26 generally resolves in SDS-PAGE at ~21 kDa, with a dimer species appearing in the liver at ~35–37 kDa. Immunoblotting of Cx26 in bladder lysates from G60S and I130T mice and their wild-type littermates revealed low levels in all mice (Fig. 5a). Quantification revealed similar Cx26 levels in the bladders of G60S and I130T mice compared to their wild-type littermates (Fig. 5b, c). Immunoblotting also revealed nonspecific bands located at ~32–34 kDa in lysates from all bladder samples. Although the band was present in only the bladder samples, it is unclear as to what this band represents; therefore, it was not quantified.
Fig. 5.
G60S and I130T mutant mouse bladders have similar Cx26 levels as wild-type littermates. Cx26 generally resolves at ~21 kDa, with the dimer resolving at ~37 kDa as seen in liver lysates (a). G60S, I130T and wild-type (Wt) littermate mouse bladders were all positive for Cx26 expression (a). Total Cx26 expression was similar in I130T and G60S mice and their wild-type littermates (b, c). A nonspecific band located at ~32–34 kDa was noted. Bars represent ±SEM (b, c). n = 5 (I130T mice) and 6 (G60S mice)
Cx26 Is Localized to Intracellular Compartments in the Basal Urothelium of Mutant Mice and Wild-Type Littermates
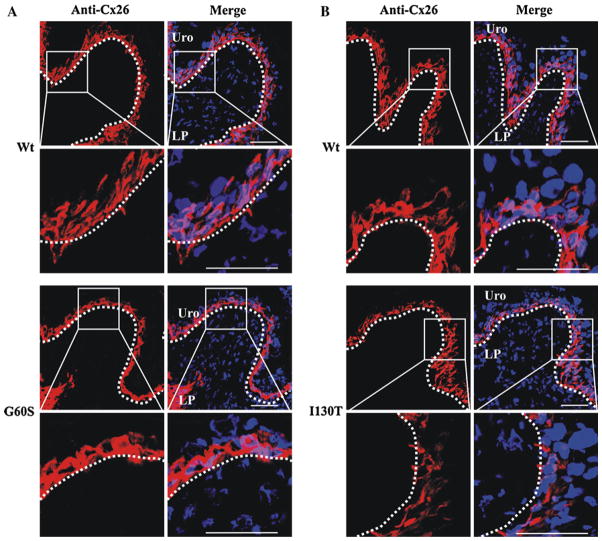
Immunoblotting revealed that Cx26 levels remain similar between wild-type and mutant mice; however, it was unclear if Cx26 localization and/or distribution were altered. As a positive control Cx26 was clearly detected at the cell–cell apposition of liver hepatocytes (data not shown). Immunofluorescence revealed that Cx26 was predominantly localized to the basal urothelium in G60S and I130T mutant mice and their wild-type littermate controls (Fig. 6a, b). Surprisingly, Cx26 was localized to intracellular locations and not, as expected, to intercellular boundaries.
Fig. 6.
Cx26 was localized to intracellular compartments of basal urothelial cells of mutant and wild-type (Wt) mice. Immunolabeling revealed that Cx26 was localized to intracellular compartments of the basal urothelium cells in all mice (a, b). Uro urothelium, LP lamina propria. Red indicates Cx26, and blue indicates Hoechst nuclear stain. Bar 50 μm
Discussion
GJIC is thought to contribute to normal bladder function by allowing for coordinated contraction and ejection of urine from the bladder lumen (Fry et al. 2007; Hashitani et al. 2004; Neuhaus et al. 2007; Sui et al. 2002). Cx43 is the most prevalent connexin expressed in the bladder as it is found in both the lamina propria and the detrusor layer (Fry et al. 2007; Neuhaus et al. 2007; Sui et al. 2002, 2003; Wang et al. 2006). Therefore, any alteration in Cx43 function may ultimately lead to bladder defects. ODDD patients are prime candidates for dysfunctional bladders as Cx43 function in the bladder is expected to be well below 50 % given that these patients harbor loss-of-function Cx43 mutants that dominantly inhibit coexpressed wild-type Cx43 (Churko et al. 2011; Flenniken et al. 2005; Manias et al. 2008; Tong et al. 2009; Toth et al. 2010). Bladder impairments in ODDD patients often do not manifest until the second half of life (Goepel et al. 2010), long after the original diagnosis of ODDD. However, other ODDD patients suffer from bladder defects at a young age, a condition rarely found in the general population (Paznekas et al. 2003, 2009). It remains possible that the number of ODDD patients with bladder impairment is under-reported as symptoms may not be archived as an ODDD-associated disease. It is also not clear if only some ODDD-associated Cx43 mutations result in bladder impairments and whether the location of the mutation, the resulting amino acid change and/or the patient’s genetic background all contribute to the development of bladder impairment. Adding to the complexity, it is further unclear if bladder abnormalities reported by ODDD patients are due to neurogenic or myogenic defects (Christ et al. 2003; Haefliger et al. 2002). In the present study we assessed connexin status in the bladders of two genetically distinct Cx43 mutant mouse models of ODDD (G60S and I130T) and found that mice harboring systemic Cx43 mutants have an anatomically and histologically normal bladder even though Cx43 exists primarily as a species that is incompletely phosphorylated.
Characterization of Cx43 in the Bladder of G60S and I130T Mutant Mice
Bladder abnormalities have been reported in ~12 % of ODDD patients and, in a couple of cases, have been putatively linked to neurological defects (Paznekas et al. 2003, 2009). However, there is increasing evidence that suggests some bladder abnormalities are the result of myogenic defects (Christ et al. 2003; Haefliger et al. 2002). Past reports have localized Cx43 within the lamina propria and detrusor layers of the bladder, and other studies have linked Cx43-based gap junctions to various bladder syndromes (Christ et al. 2003; Mori et al. 2005; Neuhaus et al. 2007). It therefore remains possible that ODDD mutants which disrupt Cx43 function (Churko et al. 2011; Dobrowolski et al. 2007; Flenniken et al. 2005; Manias et al. 2008; Shibayama et al. 2005; Tong et al. 2009; Toth et al. 2010) may ultimately affect bladder function. In order to assess this possibility, we examined two Cx43 mutant mouse lines, G60S and I130T, both of which have been shown to phenotypically resemble ODDD patients (Flenniken et al. 2005; Kalcheva et al. 2007; Langlois et al. 2007; Toth et al. 2010); but it is unknown if they suffer from incontinence or any bladder abnormalities. Importantly, both mutant mice are predicted to possess a 1:1 ratio of mutant to wild-type Cx43, matching the genotype of human ODDD patients. As well, the two mutant mouse lines were generated on different genetic backgrounds, more readily mimicking the diverse genetic background of ODDD patients. Previous studies revealed that both mutant mouse lines have altered tissue function and/or development (Flenniken et al. 2005; Kalcheva et al. 2007; Langlois et al. 2007; Toth et al. 2010); however, no studies have assessed the connexin status within the bladder. Here, we aimed to determine the connexin localization and expression profile as possible indicators of altered bladder function.
One of the simplest indicators of OAB syndrome is detrusor hypertrophy (Miyazato et al. 2009; Uvelius et al. 1984). Assessment of mutant mice and their wild-type counterparts revealed anatomically similar bladders as no differences were observed in the appearance or thickness of the detrusor layer. Within the lamina propria, we found Cx43-positive staining in all mice localized to a combination of fibroblasts and myofibroblasts. Myofibroblasts, in particular, are important in forming a functional syncytium for the rapid transmission of chemical and electrical stimuli, required for normal bladder function (Fry et al. 2007; Sui et al. 2002; Wiseman et al. 2003). Cx43 in the detrusor is thought to mediate the propagation of action potentials from one smooth muscle bundle to the next, allowing for a uniform bladder contraction (Hashitani et al. 2004). Our results revealed that while wild-type and all mutant mice displayed punctate, gap junction–like structures, G60S mutant mice frequently displayed a more diffuse intracellular Cx43 expression profile. This localization profile correlated with a reduction in the highly phosphorylated Cx43 species (P1 and P2), known to be important in gap junction plaque formation and GJIC (Solan and Lampe 2009). Interestingly, the I130T mouse bladders also exhibited a reduction in the highly phosphorylated Cx43 species, as well as reduced total Cx43 protein levels. Thus, we conclude that in both mutant mouse lines Cx43 does not likely form a complete complement of fully assembled functional gap junctions in the resident cells of the bladder, not unlike what is seen in other cell types that express ODDD-linked Cx43 mutants (Churko et al. 2011; Dobrowolski et al. 2007; Flenniken et al. 2005; Manias et al. 2008; Shibayama et al. 2005; Tong et al. 2009; Toth et al. 2010).
Even though bladder abnormalities are most commonly linked to increases in Cx43 (Christ et al. 2003; Imamura et al. 2009; Mori et al. 2005), it remains possible that a reduction in Cx43 levels and/or a resulting decrease in GJIC may also alter bladder function. For instance, reduced GJIC may alter electrical and chemical stimulus propagation within the bladder, resulting in incomplete bladder contractions and increased residual urine levels, ultimately leading to increased urinary frequency. Additional behavioral studies are necessary to see if Cx43 mutant mice suffer from frequent urination and/or bladder leakage.
While our study revealed no Cx43 staining in the bladder urothelium, other groups have localized both Cx43 and Cx26 within this tissue layer (Grossman et al. 1994; Haefliger et al. 2002). Therefore, as a result of Cx26 being localized to the urothelial layer (Grossman et al. 1994; Haefliger et al. 2002; Ikeda et al. 2007), we speculated that Cx26 may be upregulated in the urothelium through potential signaling cross-talk mechanisms to compensate for the overall reduction in Cx43 function. The function of Cx26 within the urothelium is still unknown, although it has been proposed to play a role in signal transduction during bladder filling (Ikeda et al. 2007). Cx26 localization in the urothelium has been reported to be typically at sites of cell to cell apposition (Haefliger et al. 2002; Ikeda et al. 2007); however, similar to our findings, one group has reported the intracellular localization of Cx26 (Gee et al. 2003). Other reports have also indicated that Cx26 hemi-channels can release ATP into the extracellular environment (Huckstepp et al. 2010; Majumder et al. 2010), which may alter bladder function. In our study, the levels of Cx26 were similar between the mutant and control groups. Interestingly, Cx26 displayed a unique intracellular urothelium expression profile localized to only the basal one or two cell layers of the urothelium, suggesting that few, if any, Cx26 gap junction channels or hemichannels are formed in these cell layers. The lack of Cx26 in the more luminally localized epithelial cells would suggest that Cx26 serves no role in the more differentiated cells of the urothelium. Nevertheless, we found no evidence that Cx26 was upregulated to compensate for the proposed reduction of Cx43 function in the bladder. Future studies will require the inclusion of additional mutant mice that harbor specific mutations that have strong linkages to patients with bladder abnormalities.
Acknowledgments
The authors thank Kevin Barr for breeding and maintaining the genetically modified mice used in the current study. D.W.L. was funded by the Canadian Institutes of Health Research and the Canada Research Chair Program.
Contributor Information
R. Lorentz, Department of Anatomy and Cell Biology, Western University, London, ON, Canada
Q. Shao, Department of Anatomy and Cell Biology, Western University, London, ON, Canada
T. Huang, Department of Anatomy and Cell Biology, Western University, London, ON, Canada
G. I. Fishman, Leon H. Charney Division of Cardiology, New York University School of Medicine, New York, NY, USA
D. W. Laird, Email: dale.laird@schulich.uwo.ca, Department of Anatomy and Cell Biology, Western University, London, ON, Canada
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–73. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, Andersson KE. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1241–R1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- Churko JM, Shao Q, Gong XQ, Swoboda KJ, Bai D, Sampson J, Laird DW. Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects. Hum Mutat. 2011;32:456–466. doi: 10.1002/humu.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sommershof A, Willecke K. Some oculodentodigital dysplasia–associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GI, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914–919. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- Gee J, Tanaka M, Grossman HB. Connexin 26 is abnormally expressed in bladder cancer. J Urol. 2003;169:1135–1137. doi: 10.1097/01.ju.0000041954.91331.df. [DOI] [PubMed] [Google Scholar]
- Gehi R, Shao Q, Laird DW. Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J Biol Chem. 2011;286:27639–27653. doi: 10.1074/jbc.M111.260711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepel M, Kirschner-Hermanns R, Welz-Barth A, Steinwachs KC, Rubben H. Urinary incontinence in the elderly: part 3 of a series of articles on incontinence. Dtsch Arztebl Int. 2010;107:531–536. doi: 10.3238/arztebl.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XQ, Shao Q, Langlois S, Bai D, Laird DW. Differential potency of dominant negative connexin43 mutants in oculodentodigital dysplasia. J Biol Chem. 2007;282:19190–19202. doi: 10.1074/jbc.M609653200. [DOI] [PubMed] [Google Scholar]
- Grossman HB, Liebert M, Lee IW, Lee SW. Decreased connexin expression and intercellular communication in human bladder cancer cells. Cancer Res. 1994;54:3062–3065. [PubMed] [Google Scholar]
- Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, Frey P, Meda P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res. 2002;274:216–225. doi: 10.1006/excr.2001.5465. [DOI] [PubMed] [Google Scholar]
- Haferkamp A, Mundhenk J, Bastian PJ, Reitz A, Dorsam J, Pannek J, Schumacher S, Schurch B, Buttner R, Muller SC. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004;46:799–805. doi: 10.1016/j.eururo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Oberbach A, Schlichting N, Stolzenburg JU, Neuhaus J. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS ONE. 2011;6:e20792. doi: 10.1371/journal.pone.0020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–F1025. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Negoro H, Kanematsu A, Yamamoto S, Kimura Y, Nagane K, Yamasaki T, Kanatani I, Ito N, Tabata Y, Ogawa O. Basic fibroblast growth factor causes urinary bladder overactivity through gap junction generation in the smooth muscle. Am J Physiol Renal Physiol. 2009;297:F46–F54. doi: 10.1152/ajprenal.90207.2008. [DOI] [PubMed] [Google Scholar]
- John H, Wang X, Hauri D, Maake C. Gap junctions in the human urinary bladder [in German] Aktuel Urol. 2003;34:328–332. doi: 10.1055/s-2003-42001. [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci USA. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A, Stadlmayr W, Monga A, Cameron I, Anthony F. A pilot study of connexin 43 (Cx43) in human bladder tissue in patients with idiopathic detrusor overactivity. Eur J Obstet Gynecol Reprod Biol. 2008;141:83–86. doi: 10.1016/j.ejogrb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Lai A, Le DN, Paznekas WA, Gifford WD, Jabs EW, Charles AC. Oculodentodigital dysplasia connexin43 mutations result in non-functional connexin hemichannels and gap junctions in C6 glioma cells. J Cell Sci. 2006;119:532–541. doi: 10.1242/jcs.02770. [DOI] [PubMed] [Google Scholar]
- Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Maher AC, Manias JL, Shao Q, Kidder GM, Laird DW. Connexin levels regulate keratinocyte differentiation in the epidermis. J Biol Chem. 2007;282:30171–30180. doi: 10.1074/jbc.M703623200. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol. 2002;249:584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. ATP-mediated cell–cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manias JL, Plante I, Gong XQ, Shao Q, Churko J, Bai D, Laird DW. Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant. Cardiovasc Res. 2008;80:385–395. doi: 10.1093/cvr/cvn203. [DOI] [PubMed] [Google Scholar]
- McLachlan E, Manias JL, Gong XQ, Lounsbury CS, Shao Q, Bernier SM, Bai D, Laird DW. Functional characterization of oculodentodigital dysplasia-associated Cx43 mutants. Cell Commun Adhes. 2005;12:279–292. doi: 10.1080/15419060500514143. [DOI] [PubMed] [Google Scholar]
- Miller J, Hoffman E. The causes and consequences of overactive bladder. J Womens Health. 2006;15:251–260. doi: 10.1089/jwh.2006.15.251. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Sugaya K, Nishijima S, Kadekawa K, Machida N, Oshiro Y, Saito S. Changes of bladder activity and connexin 43-derived gap junctions after partial bladder-outlet obstruction in rats. Int Urol Nephrol. 2009;41:815–821. doi: 10.1007/s11255-008-9516-7. [DOI] [PubMed] [Google Scholar]
- Mori K, Noguchi M, Matsuo M, Nomata K, Suematsu T, Kanetake H. Decreased cellular membrane expression of gap junctional protein, connexin 43, in rat detrusor muscle with chronic partial bladder outlet obstruction. Urology. 2005;65:1254–1258. doi: 10.1016/j.urology.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Neuhaus J, Heinrich M, Schlichting N, Oberbach A, Fitzl G, Schwalenberg T, Horn LC, Stolzenburg JU. Structure and function of suburothelial myofibroblasts in the human urinary bladder under normal and pathological conditions [in German] Urologe A. 2007;46:1197–1202. doi: 10.1007/s00120-007-1480-9. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder GM, Laird DW. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem. 2005;280:11458–11466. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Cir Res. 2005;96:e83–e91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- Sui GP, Coppen SR, Dupont E, Rothery S, Gillespie J, Newgreen D, Severs NJ, Fry CH. Impedance measurements and connexin expression in human detrusor muscle from stable and unstable bladders. BJU Int. 2003;92:297–305. doi: 10.1046/j.1464-410x.2003.04342.x. [DOI] [PubMed] [Google Scholar]
- Tong D, Lu X, Wang HX, Plante I, Lui E, Laird DW, Bai D, Kidder GM. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod. 2009;80:1099–1106. doi: 10.1095/biolreprod.108.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Shao Q, Lorentz R, Laird DW. Decreased levels of Cx43 gap junctions result in ameloblast dysregulation and enamel hypoplasia in Gja1Jrt/+ mice. J Cell Physiol. 2010;223:601–609. doi: 10.1002/jcp.22046. [DOI] [PubMed] [Google Scholar]
- Uvelius B, Persson L, Mattiasson A. Smooth muscle cell hypertrophy and hyperplasia in the rat detrusor after short-time infravesical outflow obstruction. J Urol. 1984;131:173–176. doi: 10.1016/s0022-5347(17)50253-0. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Brink PR, Christ GJ. Gap junction channel activity in short-term cultured human detrusor myocyte cell pairs: gating and unitary conductances. Am J Physiol Cell Physiol. 2006;291:C1366–C1376. doi: 10.1152/ajpcell.00027.2006. [DOI] [PubMed] [Google Scholar]
- Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]