Abstract
Background
The earliest endoscopically-evident lesion in Crohn’s disease is the aphthous ulcer, which develops over ectopic lymphoid tissues (ie, inducible lymphoid follicles (ILF), tertiary lymphoid tissue (TLT)) in the chronically inflamed intestine. ILF/TLT are induced within effector sites by homeostatic lymphoid chemokines, but their role in the development of intestinal ILF/TLT and in the pathogenesis of Crohn’s disease is poorly understood.
Design
Using a mouse model of Crohn’s-like ileitis (TNFΔARE) which develops florid induction of ILF/TLT within its terminal ileum, the contribution of the CCR7/CCL19/CCL21 chemokine axis during the development of TLT and its role in disease pathogenesis were assessed.
Results
Both CCL19 and CCL21 were increased within the inflamed ileum of TNFΔARE mice, which resulted in CCR7 internalisation and impaired T cell chemotaxis. ILF/TLT were a major source of CCL19 and CCL21 and increased local synthesis, augmented recruitment/retention of effector, naïve and central memory T cell subsets within the inflamed ileum. Immunoblockade of CCR7 resulted in further effector T cell retention and exacerbation of ileitis.
Conclusions
Induction of ILF/TLT in the chronically inflamed intestine alters the homeostatic CCL19–CCL21 lymphoid-chemokine gradient and increases recruitment/retention of effector CCR7+ T cell subsets within the terminal ileum, contributing to the perpetuation of chronic inflammation. Thus, blockade of CCR7 or its ligands might result in deleterious consequences for subjects with chronic inflammatory diseases.
INTRODUCTION
Ectopic lymphoid tissues (tertiary lymphoid tissue (TLT) and inducible lymphoid follicles (ILF)) are invariably present at the base of aphthous ulcers, the earliest endoscopically-evident lesion in Crohn’s disease (CD).1 Furthermore, their appearance heralds recurrent disease within the neoterminal ileum after ilectomy.2,3 These structures have similar architecture to that of lymphoid organs and are induced from primordial developmentally-determined structures called cryptopatches.1,4 The chain of molecular events required for ectopic lymphoid tissue development under conditions of chronic inflammation and their role in the pathogenesis of CD are unclear.
The recirculation of T cells to the intestine, draining lymphatics and blood is critical for immunosurveillance and defence. While the trafficking determinants for intestinal homing have been characterised (eg, integrin α4β7, chemokine receptor 9), those that determine retention and egress of intestinal T cells are less well-defined. Chemokine receptor 7 (CCR7) is expressed by activated dendritic cells (DCs), naïve and central memory (TCM) T cells, all of which migrate to lymphoid organs where its ligands CCL19 and CCL21 are abundantly expressed. The expression of CCL19 and CCL21 is tightly regulated within lymphoid tissues, acting as chemotactic and retentive signals.5–7 CCL21 is also expressed by high endothelial venules (HEV), lymphatics and lymphoid stromal cells8 and overexpressed in a variety of autoimmune diseases.9,10 Recent evidence has highlighted a role for the ectopic expression of lymphoid chemokines CCL19 and CCL21 in the induction of ectopic lymphoid tissue at sites of chronic inflammation.11,12
While CCL19 and CCL21 are increased in the inflamed intestine of patients with CD,10,13 their role and that of their receptor CCR7 has not been evaluated in CD or in preclinical models. In this paper we assess the contribution of CCR7 and its ligands to the pathogenesis of CD using the TNFΔARE model of ileitis. We first examined the expression of CCL19 and CCL21 in the intestine, then explored whether chronic inflammation had an effect on ligand binding and function, and finally we investigated whether ectopic lymphoid tissues were a source of both ligands and whether their increased concentrations and antibody blockade of their receptor (ie, CCR7) had an effect on recruitment and/or retention of lymphocyte subsets and on disease severity.
MATERIALS AND METHODS
Mice
The B6.129S-Tnftm2Gkl/Jarn (TNFΔARE) strain has been described previously.14 Mice were heterozygous for the ΔARE mutation or wild-type (WT). CCR7-deficient mice (B6.129P2(C)-Ccr7tm1Rfor/J) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Animal procedures were approved by the Institutional Animal Care and Use Committee.
Tissue fixation, paraffin embedding and histological scoring
Terminal ilea were excised, opened longitudinally and fixed in 10% buffered formalin, embedded in paraffin, cut into 5 mm sections and stained with haematoxylin/eosin. Histological assessment of ileitis was performed in a blinded fashion, as described elsewhere.15
Immunofluorescence microscopy
Terminal ilea were snap-frozen in OCT, cut into sections (7 μm), fixed with ice-cold acetone/methanol (1:1) and incubated in TNB blocking buffer (PerkinElmer Life Sciences, Waltham, Massachusetts, USA). Fluorochrome-conjugated antibodies, anti-CD11c (HL3), anti-VCAM1 (429(MVCAM.A)), anti-B220 (RA3-6B2) (BD Biosciences, San Jose, California, USA), anti-CD33 (145-2C11), anti-IgD (11-26), anti-MHCII (M5/114.15.2) (eBioscience, San Diego, California, USA), anti-CD4 (RM4-5), anti-Thy1.2 (53-2.1) (Invitrogen, Carlsbad, California, USA) were diluted in TNB buffer and incubated at 258C for 1 h or at 4°C overnight. IgM was detected with either FITC-conjugated anti-IgM (11-26) or anti-mouse IgM (II/41) (eBioscience) followed by goat-anti-rat IgG Alexa-Fluor 555 (Invitrogen). Lyve.1 was detected using goat polyclonal anti-mouse Lyve.1 (R&D Systems, Minneapolis, Minnesota, USA) followed by rabbit-anti-goat IgG Alexa-Fluor 555. Images were acquired using a Zeiss AxioImage.A1 fluorescent microscope with Axio-vision software (Zeiss, Thornwood, New York, USA) or a Nikon Eclipse E400 fluorescent microscope with NIS-Elements software (Nikon, Tokyo, Japan).
Laser capture microdissection
Ectopic lymphoid aggregates were isolated from frozen 7 μm sections of ileum from mice aged > 12 weeks using an Olympus IX81 microscope and MMI Cellcut Microdissection System with UV-cut software. Tissues were mounted onto RNAse-free slides (MMI, Haslett, Michigan, USA), fixed with isopropanol and stained with haematoxylin/eosin. Samples were pooled into RNase-free collection tubes (MMI) and RNA-extracted using an RNeasy kit according to the manufacturer’s instructions (QIAGEN, Valencia, California, USA).
RNA extraction, cDNA synthesis and real-time RT-PCR
Total RNA was isolated from homogenised mouse ileum using the RNeasy Mini Kit. RNA (500 ng) was reverse-transcribed into cDNA with a high capacity cDNA archive kit (Applied Biosystems, Foster City, California, USA). A human cDNA array was used for assessment of human targets (TissueScan Crohn’s/Colitis cDNA Array I, Origene, Rockville, Maryland, USA). Realtime RT-PCR was performed using Taqman Gene Expression Assays (Applied Biosystems) containing forward and reverse primers and FAM-labelled MGB Taqman probes. PCR assays for mCCL19 (Mm00839967_g1), hCCL19 (Hs00171076_m1), mCCL21 (Mm03646971_gH), hCCL21 (Hs00171076_m1) and mCCR7 (Mm01301785_m1) were performed using Taqman Universal PCR Master Mix and 18 s as endogenous control. Relative gene expression was calculated using the ΔΔCT method with ABI RQ software (Applied BioSystems).
Quantification of CCL19 and CCL21 by ELISA
CCL19 and CCL21 concentrations were measured by ELISA from tissues using specific antibodies and standards (Duoset, R&D Systems) according to the manufacturer’s instructions. Tissue protein concentrations were equalised using a Bradford assay (BioRad, Hercules, California, USA).
Leucocyte isolation
Splenocytes, mesenteric lymph node (MLN) and ileal lamina propria mononuclear cells were isolated as previously described.14,16
Flow cytometry
Cells from indicated compartments were incubated with fluorescently-labelled antibodies against CD4-APC-Cy7(GK1.5), CD103-PerCp(2E7) (Biolegend, San Diego, California, USA), CD3-eFluor450(500A2), CD8-APC(53-6.7), CD62L-PE-Cy7 (MEL-14), CD44-FITC(IM7), CCR7-PE(4B12), MHCII-FITC (M5/114.15.2), CD11c(N418), CD11b-eFluor(M1/70), CD45-PerCp-Cy5.5(30-F11) (eBioscience) or corresponding isotype controls. CCR7-deficient cells were used as controls for all experiments. CCL19-Fc is a fusion protein bound to human Fcγ fragment (eBioscience). A PE-conjugated polyclonal anti-human IgG (Fcγ-specific) (eBioscience) served as secondary detection reagent. Analysis was performed using a BD FACSCanto (BD Biosciences). Further analyses were performed using FLOWJo software (Tree Star, Ashland, Oregon, USA).
Chemotaxis assay
Anti-mouse CCR7 antibody (4B12) and recombinant mouse CCL19 and CCL21 were from R&D Systems. Migration of splenocytes was assessed in transwell plates (24-well; 5 μm, Costar) according to the manufacturer’s protocol using RPMI 1640 with 5% fetal bovine serum, 2 mmol/l L-glutamine and 1% penicillin/streptomycin. Chemokines were added 30 min prior to loading 1 × 105 cells per transwell. Assays were run in duplicate for 60 min at 378C/5% CO2 with or without preincubation with pertussis toxin (PTX; 2 h, 500 ng/ml). Migrated cells were stained for flow cytometry and cell numbers counted with a BD FACS CantoII (BD Biosciences) and normalised using cell titration.
Treatment studies
Twelve-week-old TNFΔARE mice received four intraperitoneal doses (200 μg) of an isotype control (IgG2ak) or a rat/mouse chimeric-monoclonal antibody against TNF (clone CNTO5048) every 4 days for 2 weeks (Centocor Ortho Biotech, Horsham, Pennsylvania, USA) or five intraperitoneal doses (200 μg) of dexamethasone (BIMEDA-MTC Animal Health Inc, Ontario, Canada) or vehicle control (saline) every 2 days for 10 days. Ten-week-old TNFΔARE mice received four intraperitoneal doses (500 μg) of murine anti-CCR7 monoclonal antibody (4B12) or rat IgG2a isotype (Amgen, Thousand Oaks, California, USA) every 4 days for 2 weeks. Tissues were collected 24 h after the final injection.
Statistical analysis
Statistical analyses were performed using ANOVA or a two-tailed Student t test. Data were expressed as mean ± SEM. Statistical significance was set at p < 0.05.
RESULTS
CCL19 and CCL21 mRNA transcripts are increased in the ileum and MLN of TNFΔARE mice
mRNA transcripts in the colon, ileum, jejunum and MLN of TNFΔARE mice were assessed at 10 weeks of age and compared with age-matched WT mice. A significant increase in CCL19 and CCL21 transcripts was observed in the ileum (p < 0.01) and draining MLN (p < 0.05) of TNFΔARE mice compared with non-inflamed WT controls (figure 1A,B). Thus, CCL19 and CCL21 mRNA transcripts are increased in the inflamed ileum and MLN of TNFΔARE mice.
Figure 1.
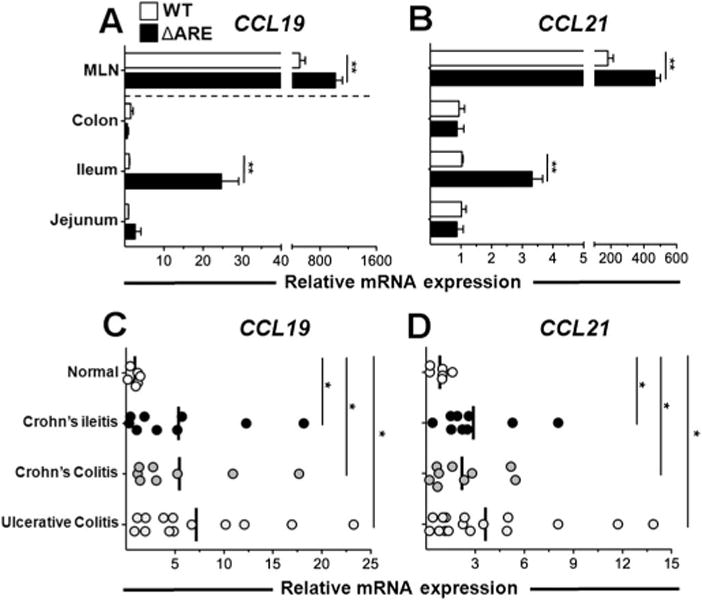
Increased CCL19 and CCL21 mRNA transcripts in TNFΔARE mice and inflammatory bowel disease. (A, B) CCL19 and CCL21 mRNA expression was assessed in indicated organs of TNFΔARE and wild-type (WT) mice at 10 weeks of age. *p < 0.05, **p < 0.01 vs WT (n = 6 mice/strain). (C, D) CCL19 and CCL21 mRNA expression was assessed using a cDNA array generated from human intestinal tissue. Data expressed as mean ± SEM. *p < 0.05 vs normal (n = 6–17). MLN, mesenteric lymph node.
CCL19 and CCL21 mRNA transcripts are increased in the intestine of patients with CD and ulcerative colitis
We then quantified their expression in intestine from patients with inflammatory bowel disease (IBD) and normal subjects. A significant increase in CCL19 and CCL21 transcripts was observed in tissue from patients with Crohn’s ileitis (p < 0.05), Crohn’s colitis (p < 0.05) and ulcerative colitis (p < 0.05) (figure 1C,D). Thus, as shown previously,9 increased CCL19 and CCL21 mRNA transcripts were observed in inflamed intestine of patients with IBD.
CCR7 expression on T cells is underestimated when assessed by monoclonal antibody reactivity
We then assessed the mean fluorescence intensities (MFI) for CCR7 on T cell subsets using a widely used monoclonal antibody (clone 4B12). To minimise receptor internalisation,17 staining was performed on freshly isolated cells maintained at 4°C. The MFI for CCR7 was not different between WT and TNFΔARE TCM (CD44+/CD62L+) and naïve (CD44−/CD62Lhigh) T cell subsets in spleen and MLN (p > 0.05, figure 2A,B). CCR7-deficient lymphocytes (grey histograms) and isotype monoclonal antibody controls (data not shown) were used as controls. Similarly TCM cells extracted from ileum showed no significant difference in CCR7 expression (figure 2A,B; p > 0.05). By contrast, naïve CD4+ and CD8+ T cells revealed a significant decrease in CCR7 MFI in TNFΔARE cells compared with age-matched WT controls (figure 2A,B; CD4+, p < 0.05; CD8+, p < 0.05). Thus, only major differences in CCR7 expression in certain cell subsets were detected by this approach.
Figure 2.
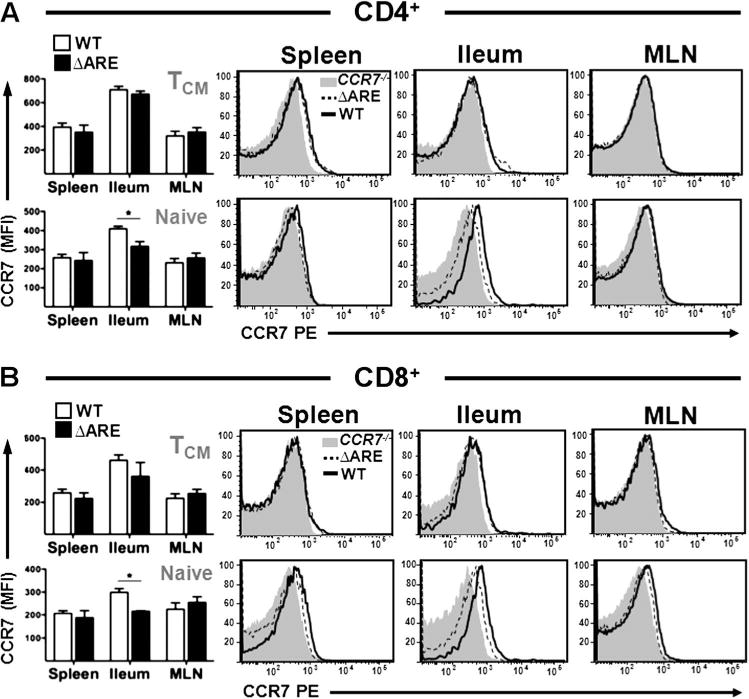
Decreased CCR7 expression in naïve TNFΔARE CD4+ and CD8+ T cells isolated from terminal ileum. (A, B) Flow cytometric analyses of CCR7 expression by central memory (CD62L+/CD44+) and naïve (CD62L+/CD44−) T cell subsets was performed on cells from 10-week-old wild-type (WT) and TNFΔARE mice. Data expressed as mean ± SEM. *p < 0.05 vs WT (n = 4 mice/strain from three independent experiments). MLN, mesenteric lymph node.
Increased CCR7 occupancy on lamina propria T cells of TNFΔARE mice
Chemokine receptors are internalised upon ligand binding and recycled back to the membrane.4,17 We hypothesised that increased ligand concentrations in inflammation might result in receptor internalisation as, in normal mice, ligand binding was inversely related to local ligand concentrations (see figure 1 in online supplement). Using CCL19 bound to human Fc followed by incubation with PE-conjugated anti-human IgG (Fcγ-specific) and CCR7-deficient cells as controls, we observed that most T cell populations from TNFΔARE mice showed significantly decreased ligand binding (less free CCR7) compared with WT mice. While no difference was observed in CD4+ populations isolated from MLN (figure 3A), there was a marked decrease in the CCL19-Fc MFI in TNFΔARE ileal cells compared with WT mice (CD4+ TCM; p < 0.001, CD4+ TNaive; p = 0.001, figure 3A). In addition, naïve CD4+ splenocytes from TNFΔARE mice also had significantly less CCL19-Fc binding than WT mice (figure 3A) (CD4+ TNaive; p = 0.004), whereas expression by the CD4+ TCM population was not significantly different (p = 0.07, figure 3A).
Figure 3.
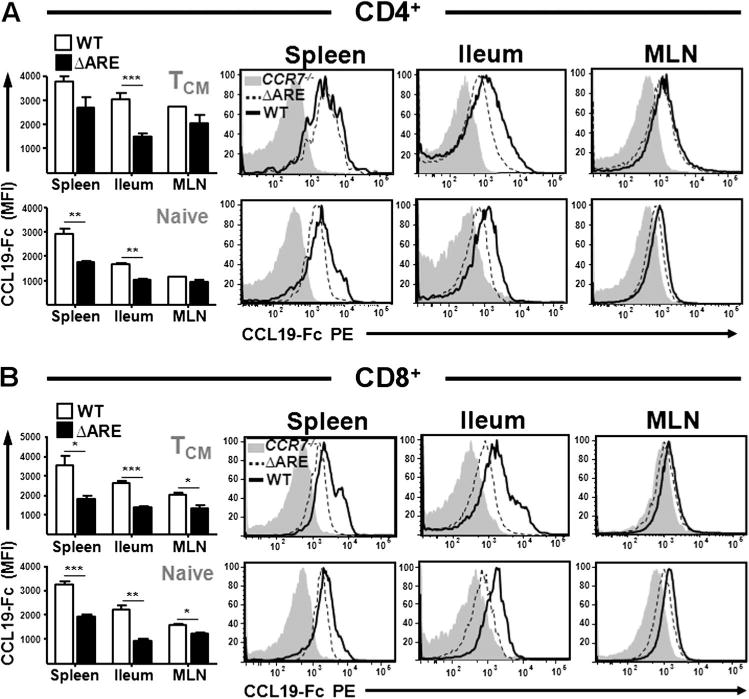
Impaired CCL19 binding by TNFΔARE CD4+ and CD8+ T cells. (A, B) Flow cytometric analyses of CCL19 binding by central memory TCM (CD62L+/CD44+) and naïve (CD62L+/CD44−) CD4+ (A) and CD8+ (B) T cells from 10-week-old wild-type (WT) and TNFΔARE mice. Data expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs WT (n = 4 mice/strain from four independent experiments). MLN, mesenteric lymph node.
Assessment of CCL19-Fc binding on CD8+ T cells revealed a similar pattern. TNFΔARE cells bound less ligand, both TCM (CD8+ TCM: spleen, p = 0.014, ileum; p < 0.001) and naïve subsets (CD8+ TNaive: spleen and ileum; p = 0.001, figure 3B). Unlike the CD4+ population, TCM and naïve CD8+ T cells isolated from MLN revealed a modest but significantly decreased binding of CCL19-Fc on TNFΔARE cells compared with WT cells (CD8+ TCM; p = 0.03: CD8+ TNaive; p = 0.005, figure 3B). This was also observed in MHCII+/CD11chigh antigen presenting cells (see figure 2 in online supplement). Thus, increased local chemokine concentrations under conditions of chronic inflammation result in decreased receptor-ligand reactivity.
TNFΔARE T cells display impaired chemotaxis towards CCR7 ligands
To ascertain whether reduced reactivity towards CCL19 in TNFΔARE mice had a functional effect on cell migration, chemotaxis assays were performed. Splenocytes from WT and TNFΔARE mice were freshly isolated or incubated at 37°C for 16 h to allow maximum re-expression of surface CCR7. Migration of splenocytes towards CCL19 was impaired in TNFΔARE mice compared with WT mice at 1 h (0.1 μg/ml rCCL19; p < 0.05; 1 μg/ml rCCL19; p < 0.05). Chemotaxis towards rCCL21 revealed a similar deficit in TNFΔARE cells (0.1 μg/ml rCCL21; p < 0.05; 1 μg/ml rCCL21; p < 0.05; figure 4A). By contrast, incubation for 16 h at 37°C in vitro away from ligand fully restored the migratory capacity of TNFΔARE T cells (figure 4B). Preincubation with PTX inhibited chemotaxis of T cells isolated from both strains but did not affect viability (figure 4A). PTX from Bordetella pertussis irreversibly inhibits Gαi proteins, so it serves as a positive control for the G protein-coupled receptor mechanism.
Figure 4.
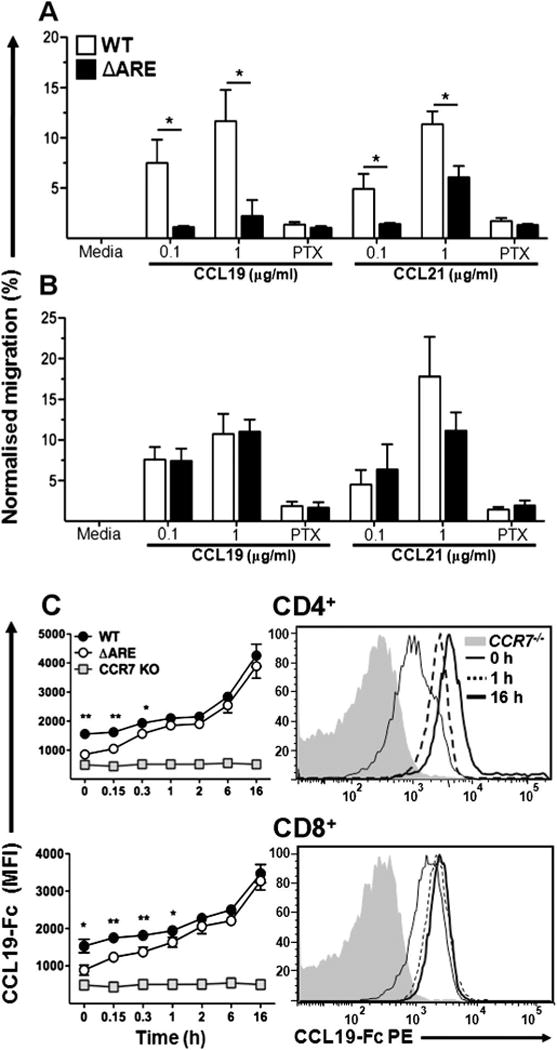
Impaired migratory capacity of TNFΔARE T cells is restored after ex vivo culture. (A) Chemotaxis of freshly isolated cells from TNFΔARE and wild-type (WT) mice T cells or (B) after 16 h ex vivo culture towards CCR7 ligands was performed in the presence or absence of pertussis toxin (PTX). *p < 0.05 versus WT. (C) Assessment of ligand binding kinetics following incubation at indicated time points up to 16 h. Data expressed as mean ± sem. *p < 0.05, **p < 0.01 vs TNFΔARE (n = 4 mice/strain from three independent experiments).
Furthermore, the time course of ligand binding confirmed an initial deficiency by freshly isolated TNFΔARE T cells to bind CCL19 but, between 15 min and 2 h after incubation at 37°C, ligand binding was the same in cells isolated from both strains. Cells continued to regain ligand binding for up to 16 h in both effector and naïve T cell subsets (see figure 3 in online supplement). Thus, CCR7 expression in T cells from TNFΔARE and WT mice is similar, but increased ligand concentrations in TNFΔARE tissues induce receptor internalisation. However, at the tissue level we observed increased CCR7 transcripts probably due to increased leucocyte infiltrates that express CCR7 (see figure 4 in online supplement).
Ectopic lymphoid tissues in the ileum of TNFΔARE mice result in increased local CCL19 and CCL21
Histological analysis of ileum revealed marked induction of ectopic lymphoid tissue in inflamed ileum by > 12 weeks of age in TNFΔARE mice, which correlated with chronic ileitis (figure 5 and supplemental figure 5). To investigate a role for CCR7 ligands in the induction of TLT, we assessed the kinetics of CCL19 and CCL21 ileal expression in TNFΔARE and WT mice by measuring their mRNA transcripts along the time course of the disease. Both CCL19 and CCL21 are induced in the inflamed terminal ileum at peak disease onset by 10 weeks of age (CCL19, p < 0.001; CCL21, p < 0.001) and remain elevated during chronic disease at 20 weeks of age (CCL19, p < 0.001; CCL21, p < 0.05; figure 5A,B). As the binding of CCL19-Fc decreased in ileal TNFΔARE T cells compared with that of cells from WT mice (figure 3), we hypothesised that the ectopic lymphoid tissues may be a major source of both chemokines. Indeed, both CCL19 and CCL21 protein concentrations were also increased in ileum lysates from TNFΔARE compared with WT mice (CCL19: 13.4 ± 2 vs 4.5 ± 1 pg/μg, p < 0.001; CCL21: 86 ± 12 vs 34 ± 6 pg/μg, p < 0.001), and regression analysis positively correlated this ectopic increase in CCR7 ligands with an increase in the number of ileal TLT during chronic disease (figure 5D–F).
Figure 5.
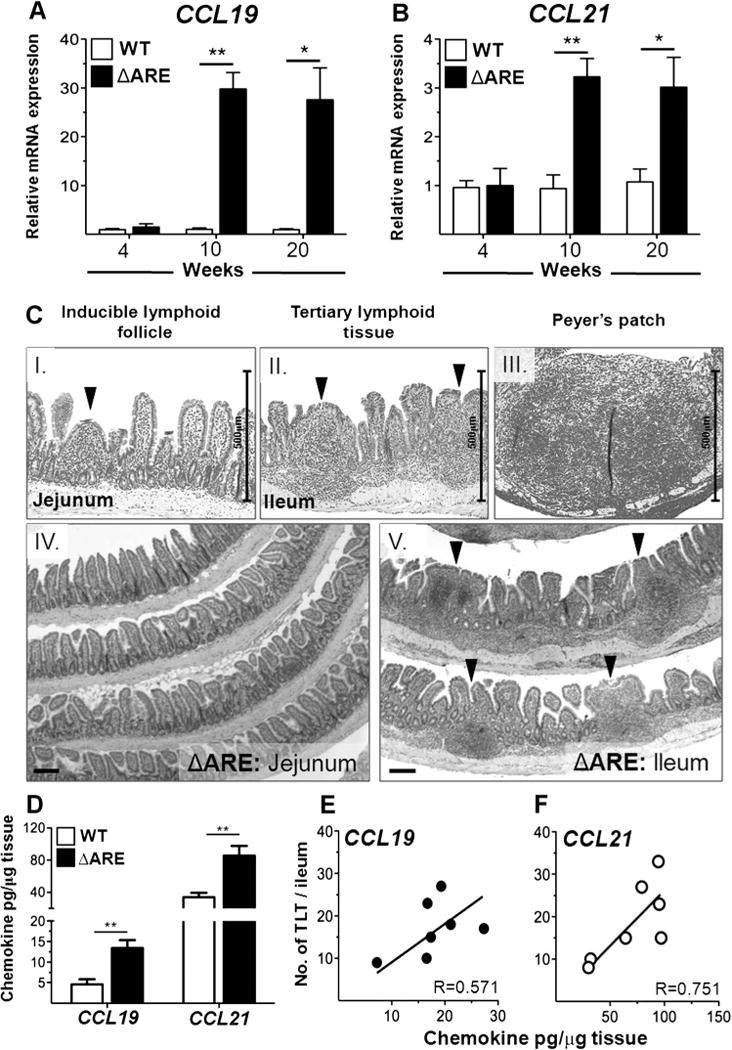
Ectopic expression of CCL19 and CCL21 correlates with widespread induction of ectopic lymphoid tissue in ileum of TNFΔARE mice. (A, B) CCL19 and CCL21 mRNA expression was assessed along the time course of ileitis in TNFΔARE and wild-type (WT) mice. (Ci–iii) Representative H&E micrographs showing the presence of inducible lymphoid follicle (arrowhead), tertiary lymphoid tissue (TLT) (arrowheads) and Peyer’s patch in TNFΔARE mice at 20 weeks of age; scale bar 500 μm. (Civ, v) Widespread ectopic TLT development is evident in the inflamed terminal ileum but not in non-inflamed jejunum of TNFΔARE mice (representative images at > 12 weeks of age); scale bar 100 μm. (D) CCR7 ligands assessed by ELISA in terminal ileum of TNFΔARE and WT mice. (E, F) Regression analysis correlating number of ileal TLT with CCL19 and CCL21 protein concentrations in TNFΔARE mice. Data expressed as mean ± SEM. *p < 0.05, **p < 0.01 vs WT-matched control normalised to corresponding WT tissue (n = 3–7 mice/strain). Scale bar 100 μm. This figure is produced in colour in the online journal—please visit the website to view the colour figure.
Ectopic lymphoid tissues are a main source of ectopic CCL19 and CCL21 in the ileum of TNFΔARE mice
Immunohistochemical characterisation of ectopic lymphoid tissues in TNFΔARE mice showed that they are composed of CD4+ and Thy1.2+ T cell clusters around B220+ IgD+ and IgM+ B cells. MHCII+ antigen presenting cells and CD11c+ DCs are also observed. In addition, these structures contain VCAM1+ stroma and Lyve.1+ lymphatics (figure 6A,C, i–vi). To further localise the primary source of CCL19 and CCL21 production, we compared mRNA transcripts from the ileum of WT and TNFΔARE mice to that of laser capture microdissected ectopic lymphoid aggregates. CCL19 and CCL21 mRNA transcripts were significantly increased in whole ileal homogenates of TNFΔARE mice (CCL19, p < 0.001; CCL21, p < 0.001). However, ectopic lymphoid aggregates contained more mRNA transcripts of both CCL19 (p < 0.05) and CCL21 (p < 0.01) than whole TNFΔARE ileum (figure 6B). Inducible lymphoid aggregates are therefore a major source of CCL19 and CCL21 within the ileum of TNFΔARE mice and their induction correlates with increased concentrations of these chemokines.
Figure 6.
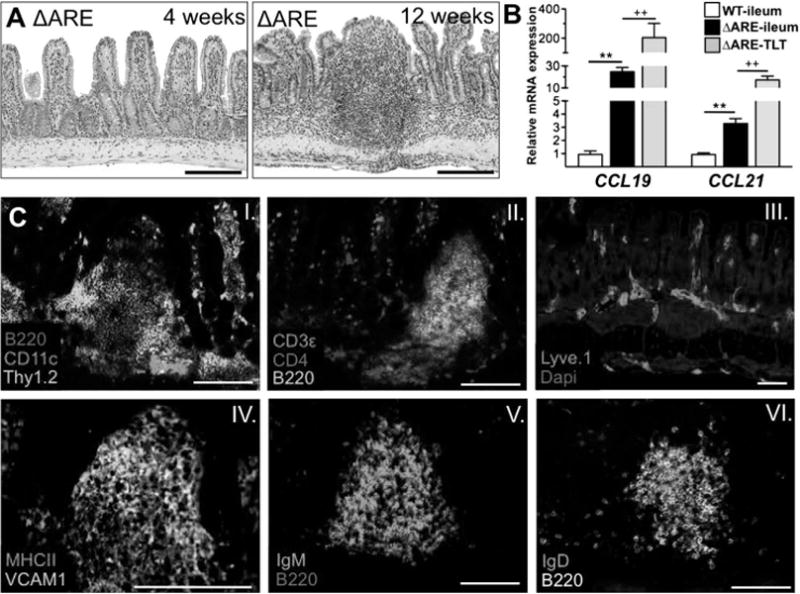
CCL19 and CCL21 mRNA transcripts in ileum of ΔARE mice localised to tertiary lymphoid tissue (TLT). (A) TNFΔARE mice had normal ileal architecture at 4 weeks of age. TLT develop between 10 and 12 weeks of age. (B) mRNA transcripts for CCR7 ligands from microdissected ileum and TLT. Data expressed as mean ± SEM, **p < 0.01 vs wild-type (WT) ileum; ††p < 0.01 vs ΔARE-ileum; normalised to corresponding WT tissue (n = 3–7 mice/strain). (Ci–vi) Immunofluorescence showed that these structures contain CD4+, Thy1.2+ T cells, CD11c+ dendritic cells, B220+ plasma cells producing IgM and IgD, VCAM1+ cells and Lyve.1+ lymphatics. Scale bar 100 μm. This figure is produced in colour in the online journal—please visit the website to view the colour figure.
Effector, central memory and naïve T cell subsets accumulate in the ileum and MLN of TNFΔARE mice
To assess whether the previous findings might play a role in the recruitment or retention of T cells within the chronically inflamed terminal ileum in vivo, we characterised the CD4+ and CD8+ effector (TEM; CD44+/CD62L−), central memory (TCM; CD44+/CD62L+) and naïve (TNaive; CD44−/CD62L+) T cell subsets in the ileum and MLN of WT and TNFΔARE mice. Histological analysis of TNFΔARE ileum revealed robust ileitis compared with age-matched WT mice (figure 7A). Chronic ileitis was characterised by an accumulation of T cells in the terminal ileum of TNFΔARE mice compared with WT mice, particularly CD4+ TEM (p < 0.001) (figure 7B), CD8+ TEM (p < 0.001) and CD8+ TCM (p < 0.001, figure 7C). Of note, while under homeostatic conditions naïve T cells preferentially migrate towards lymph nodes, in TNFΔARE mice naïve T cells were significantly increased in the ileum compared with WT mice (CD4+: p = 0.01, figure 7B; CD8+: p < 0.01, figure 7C). Thus, increased concentration of secondary lymphoid chemokines appears to result in increased recruitment of naïve and TCM T cells and retention of TEM cells within the effector site, the terminal ileum.
Figure 7.
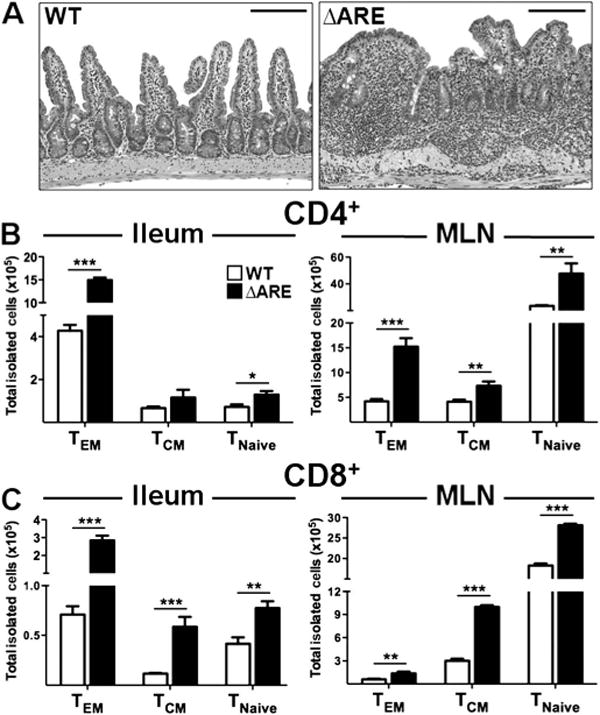
Effector, central memory and naïve T cells are increased in the ileal lamina propria and mesenteric lymph nodes (MLN) of TNFΔARE mice. (A) Histological evaluation of the ileum of TNFΔARE mice showed accumulation of leucocytes within the inflamed lamina propria. (B, C) Flow cytometric analyses showed that effector, TCM and naïve CD4+ and CD8+ T cells were recruited/retained more effectively to both compartments of TNFΔARE mice compared with wild-type (WT) mice. Data expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs WT (n = 0 mice/strain from three independent experiments). Scale bar 100 μm. This figure is produced in colour in the online journal—please visit the website to view the colour figure.
The MLN also displayed a marked increase in cell counts, predominantly with CD4+ TEM (p < 0.001) and CD8+ TCM subsets (p < 0.001). CD4+ TCM (p < 0.01) and CD8+ TEM (p < 0.01) were also increased (figure 7B,C). In addition, both CD4+ (p < 0.01) and CD8+ (p < 0.001) naïve T cell subsets were increased compared with WT mice. Thus, ileitis in TNFΔARE mice is characterised by accumulation of TEM cells and enhanced recruitment of TCM and naïve T cells into the terminal ileum and MLN.
Anti-CCR7 monoclonal antibody increases tissue retention, inhibits egress and exacerbates ileitis
To examine the effect of CCR7 antibody blockade on these cell subsets with ileum and MLN, we administered a neutralising monoclonal antibody (clone 4B12) or a corresponding isotype antibody to 8-week-old TNFΔARE mice. A doubling of the number of infiltrating cells within the ileum of anti-CCR7-treated mice occurred (10.5 ± 1.0 × 106 vs 4.8 ± 0.6 × 106, p < 0.001), with increased numbers of effector CD4+ (TEM, p < 0.001) and CD8+ (TEM, p = 0.02) populations (figure 8A,B). MHCII+/CD11chigh antigen presenting cells were similarly increased in the lamina propria of anti-CCR7-treated mice (see figure 6 in online supplement). Conversely there was a marked depletion of total cell counts in the MLN (p < 0.001), with corresponding decreases in CD4+ subpopulations (TEM: p < 0.001; TCM: p < 0.001; TNaive: p < 0.001) and naive CD8+ cells (TNaive: p < 0.001; figure 8C,D). In addition, anti-CCR7 monoclonal antibody treatment significantly exacerbated all histological parameters of disease: active (3.5 ± 0.1 vs 6.2 ± 1.0; p = 0.02), chronic (3.3 ± 0.3 vs 6.1 ± 0.6; p = 0.001), villus (3.7 ± 0.4 vs 6.7 ± 0.4; p < 0.001) and total histological indices (10.6 ± 0.7 vs 19.9 ± 1.8; p < 0.001) compared with controls (figure 8E). Our preliminary data also suggest that genetic deletion of CCR7 in TNFΔARE mice exacerbates ileitis (total ileal inflammatory index: ΔARE/CCR7+/+ 11.7 ± 3 vs ΔARE/CCR7−/− 21.9 ± 2; p = 0.02). Thus, blockade of CCR7 in TNFΔARE mice inhibited lymphocyte and dendritic cell egress from the ileum, promoted retention and exacerbated ileitis.
Figure 8.
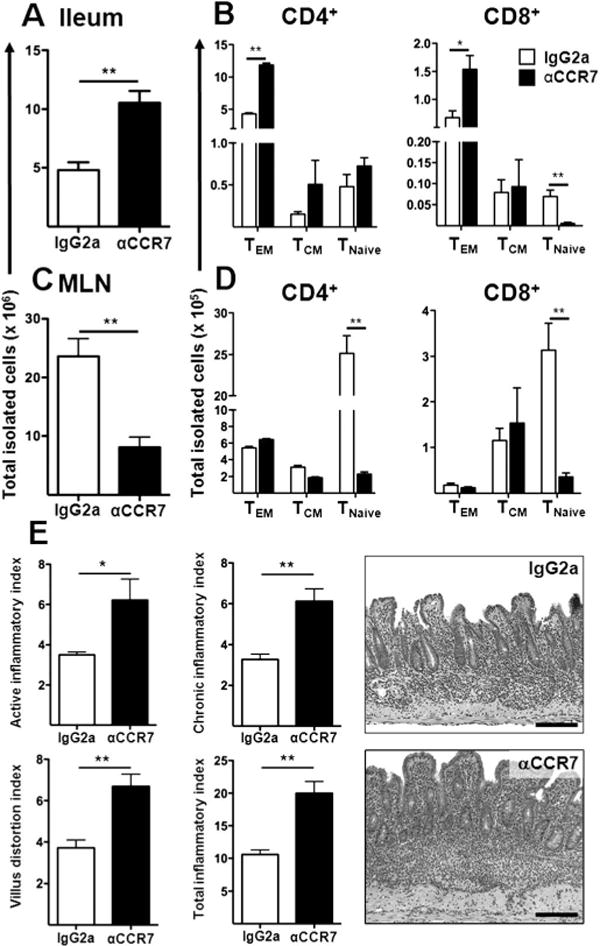
Antibody blockade of CCR7 increases lymphocyte numbers within the ileum, inhibits egress and exacerbates ileitis. (A–D) Eight-week-old TNFΔARE mice received isotype (IgG2a) or anti-CCR7 monoclonal antibody (αCCR7; clone 4B12). Their effect on the total cell counts and individual lymphocyte subsets was assessed in the ileum and mesenteric lymph nodes (MLN). (E) The severity of ileitis was assessed as described elsewhere.18 Data expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs IgG-treated mice (n = 6–7 mice/strain). Representative micrographs, scale bar 100 μm. This figure is produced in colour in the online journal—please visit the website to view the colour figure.
Anti-TNF monoclonal antibody and dexamethasone treatment attenuates ileitis and decreases ectopic CCL19 and CCL21 transcripts in TNFΔARE mice
To examine whether CCL19/21 levels are correlated with disease severity, we administered a neutralising murine anti-TNF monoclonal antibody and corresponding isotype antibody, dexamethasone or vehicle control (saline) to 8–10-week-old TNFΔARE mice. Treatment with the anti-TNF monoclonal antibody and with dexamethasone attenuated the histological indices (anti-TNF, p < 0.001; dexamethasone, p < 0.001) (figure 9A,B) and significantly reduced CCL19 (p < 0.001) and CCL21 (p < 0.001; figure 9C) transcripts. Thus, ileal expression of CCL19 and CCL21 correlated with inflammation.
Figure 9.
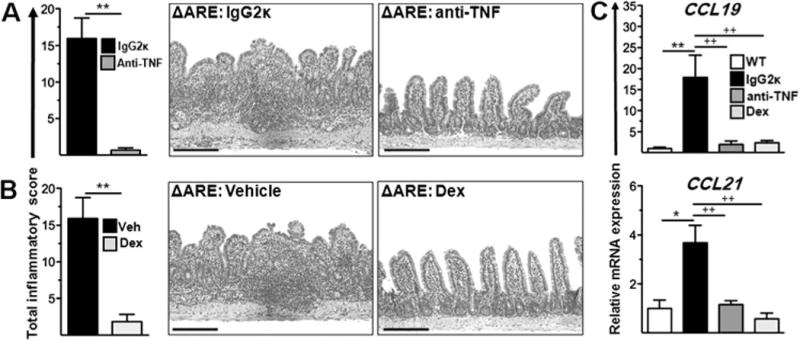
Anti-tumour necrosis factor (TNF) antibodies and corticosteroids attenuate ileitis and decrease CCL19 and CCL21 mRNA transcripts. (A) TNFΔARE mice aged > 12 weeks received isotype (IgG2ka) or anti-mouse anti-TNF monoclonal antibody (Centocor: clone CNTO5048; 200 μg) every 4 days for 2 weeks or (B) vehicle (saline) or dexamethasone (Dex) (200 μg) every 2 days for 10 days. The histological severity of ileitis was assessed as described.18 (C) mRNA transcripts for CCR7 ligands from terminal ileum of mice treated with vehicle, anti-TNF antibodies and Dex. Data expressed as mean ± SEM. *p < 0.05, **p < 0.001 vs IgG-treated mice (n = 6 mice/strain). Representative micrographs, scale bar 100 μm. This figure is produced in colour in the online journal—please visit the website to view the colour figure.
DISCUSSION
Aphthous ulcerations herald the onset of CD and its recurrence after surgical resection.2,3 These lesions uniformly localise to the luminal side of TLT which develop from cryptopatches, going through an intermediate state (ie, ILF).19 Currently, the molecular determinants required for their induction in the intestine of patients with CD are poorly understood, but the dysregulated expression of the chemokines CCL19 and CCL21 and their receptor CCR7 have been implicated.9
As there is widespread induction of ectopic lymphoid tissue in the chronically inflamed terminal ileum of TNFΔARE mice, we examined the expression of lymphoid chemokines and their receptor in this model. Transcripts for CCL19 and CCL21 were found to be increased. We then assessed CCR7 ligand binding using a chimeric ligand (CCL19-Fc) as a surrogate for free receptor. Available receptor was reduced in TNFΔARE mice within most compartments assessed. To ascertain whether the decreased ligand binding capacity had functional implications, chemotaxis assays were performed. These confirmed that T cells from TNFΔARE mice were impaired in their ability to migrate towards CCR7 ligands. However, ex vivo incubation at 37°C restored their migratory capacity as CCR7 was re-expressed. We further identified that the ectopic lymphoid tissues (which share multiple features with lymph nodes) were a major source of CCL19 and CCL21 in the chronically inflamed intestine, as mRNA transcripts were abundant within these structures and their numbers correlated with the local protein concentrations of both ligands. This had functional relevance for disease path-ogenesis as effector cells and also lymphoid-homing TCM and TNaïve were increased in the terminal ileum and MLN of TNFΔARE mice. Finally, treatment of TNFΔARE mice with an anti-CCR7 antibody resulted in T cell retention within the ileum and impaired egress to MLN.
CCL19 and CCL21 are produced by fibroblastic reticular cells in T cell-rich areas of lymphoid tissues and in HEV. DCs are an additional source of CCL19, promoting recruitment of CCR7-expressing cells to sites of antigen presentation. Both ligands are constitutively expressed under homeostatic conditions as they guide DCs, naïve and TCM migration into lymph nodes and egress of activated cells from effector sites. However, these classically homeostatic chemokines are increased in the chronically inflamed intestine of patients with CD,9,20 which suggests that they participate in induction and maintenance of chronic inflammation. As in CD, the expression of both CCR7 ligands was increased in TNFΔARE mice, highlighting a potential role for these molecules in disease pathogenesis.
CCR7 is expressed by cells that traffic to lymphoid organs (activated DCs, naïve T cells and TCM) where CCL21 is presented bound to glycosaminoglycans of HEV through its long basic C-terminus tail.21 In addition, effector T cells were not believed to express CCR7, a hypothesis challenged by simultaneously published studies.5,6 We have previously observed prominent effector T cell infiltrates within the terminal ileum and MLN of TNFΔARE mice as effector cells egress via afferent lymphatics.14,17 We therefore expected a correlative increase in the percentage of CCR7+ T cells, but there were no differences in most compartments and a counterintuitive decrease in CCR7 expression on T cell subsets isolated from the ileum and MLN (as measured by CCL19-Fc binding of free receptor) of inflamed mice. CCR7, like other chemokine receptors, is internalised upon ligand binding (CCL19) and incubation in a ligand-free environment promotes receptor re-expression back to the membrane.4,22 In support of receptor internalisation, chemo-taxis of freshly isolated TNFΔARE splenocytes was impaired. However, following ex vivo incubation, their ability to bind ligand and their migratory capacity was restored, supporting re-expression of the receptor. Furthermore, there did not appear to be inflammation-triggered differences in receptor synthesis between WT and TNFΔARE T cells as their ability to bind ligand equalised following ex vivo culture. Thus, receptor synthesis does not appear to be influenced by inflammation, yet increased concentration of ligand in the chronically inflamed terminal ileum of TNFΔARE mice clearly influenced receptor internalisation and recycling. These data suggest that, although there might be impaired egress of T cells from the inflamed terminal ileum due to increased local CCR7 ligand concentrations, T cells can recover from this deficit once they leave the ligand-rich effector site. Thus, increased availability of ligands in the inflamed ileum desensitised membrane-bound CCR7, acting as a retention signal. Increased retention of effector cells within chronically inflamed intestine probably contributes to the perpetuation of chronic inflammation.
Lymphoid aggregates within the intestine predate the appearance of the adaptive immune system and span the entire spectrum of vertebrates.23 The human intestine contains the entire continuum, from primordial cryptopatches to the intermediate ILF and the fully developed TLT observed in CD.24 Although the role of these structures in the pathogenesis of the disease is unknown, they may perpetuate dysregulated immune responses.20,25,26 It is now established that the CCR7/CCL19/CCL21 axis is critical for ILF/TLT development and maintenance and, in previous studies, ectopic expression of CCL21 in the thyroid and pancreas was sufficient to induce ectopic lymphoid tissues.12,27 While the clinical evidence points to their involvement during the onset and recurrence of CD, their role has not been formally addressed in preclinical models.1 In TNFΔARE ileitis, ILF/TLT are omnipresent by 10e12 weeks of age as ileitis becomes chronic. Unlike cryptopatches and ILF which are present in normal mouse intestine and contain small clusters of ckit+ lymphoid tissue inducer cells, DCs and B cells,19,28,29 the fully developed TLT in TNFΔARE mice spanned the full width of the ileum and were composed of B220+ B cells, CD11c+ DCs and clusters of CD4+ T cells, which is highly reminiscent of the architecture of Peyer’s patch and lymph nodes. TLT contained markedly higher levels of CCL19 (eightfold) and CCL21 (fivefold) transcripts than whole ileum, and the concentrations of both ligands correlated with the number of TLT. Increased levels of these chemokines disrupt the homeostatic lymphoid chemokine gradient, promoting retention of effector/memory T cell subsets within the terminal ileum and exacerbated disease. Furthermore, antibody blockade of CCR7 in TNFΔARE mice further enhances effector T cell accumulation, decreases egress and exacerbates ileitis.
Taken together, our studies show that, in chronic ileitis, there is widespread induction of ectopic lymphoid tissue within the chronically inflamed terminal ileum. The proliferation of these structures results in increased local production of CCL19 and CCL21 and disruption of the physiological chemokine-receptor gradient. This desensitises infiltrating CCR7+ effector memory T cells and augments their retention in the inflamed intestine,30 which contributes to the perpetuation of ileitis. Our work highlights a critical role of the CCR7/CCL19/CCL21 chemokine axis during T cell recirculation under conditions of chronic inflammation in the intestine and brings into question the utility of antagonising CCR7 as a strategy for the treatment of IBD or other chronic inflammatory diseases.
Supplementary Material
Significance of this study.
What is already known about this subject?
► The earliest endoscopically-evident lesion in Crohn’s disease is the aphthous ulcer, which develops over ectopic lymphoid tissue.
► Molecular mechanisms underlying induction of ectopic lymphoid tissue and their role in the pathogenesis of inflammatory bowel disease are poorly understood.
► While the CCR7/CCL19/CCL21 chemokine axis regulates T cell and dendritic lymphatic homing, their ectopic expression in non-lymphoid tissues has been associated with lymphoid neogenesis.
What are the new findings?
► Homeostatic lymphoid chemokines CCL19/CCL21 are upregulated in the chronically inflamed ileum of TNFΔARE mice and their mRNA transcripts localised to inducible lymphoid tissue.
► CCR7 is internalised by T cells infiltrating the inflamed ileum and are thus retained within the inflamed terminal ileum.
► Antibody blockade of CCR7 increased inhibition of T cell egress from the inflamed intestine to draining mesenteric lymph nodes, retention within the ileum and exacerbation of disease.
How might it impact on clinical practice in the foreseeable future?
► The use of CCR7-blocking reagents may prove deleterious as therapeutics for chronic inflammatory diseases.
► Our data highlight the TNFΔARE model of chronic murine ileitis as a novel and unique tool to examine further the role of ectopic lymphoid tissue in the pathogenesis of inflammatory bowel disease.
Acknowledgments
The authors thank Matthew D P Lebsack and Erin Walsh for technical assistance. Anti-mouse CCR7 was a gift from Dr Fergus Byrne (Amgen, Thousand Oaks, California, USA) and anti-TNF from Dr David Shealy (Centocor, Malvern, Pennsylvania, USA).
Funding This work was supported by National Institutes of Health (USPHS DK080212) and Crohn’s and Colitis Foundation of America Grants (CCFA # 2826) to JR-N; NIH/NCRR Colorado CTSI: UL1 RR025780 and Crohn’s and Colitis Foundation of America Grants (CCFA # 3332) to ENM.
Footnotes
Competing interests None.
Contributors All authors contributed to data generation, data analysis and interpretation as well as writing of the manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–32. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–72. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olaison G, Smedh K, Sjodahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–5. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers MA, Calloway PA, Shannon L, et al. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J Immunol. 2008;181:4723–32. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 6.Debes GF, Arnold CN, Young AJ, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luther SA, Tang HL, Hyman PL, et al. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn MD, Tangemann K, Tam C, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawashima D, Oshitani N, Jinno Y, et al. Augmented expression of secondary lymphoid tissue chemokine and EBI1 ligand chemokine in Crohn’s disease. J Clin Pathol. 2005;58:1057–63. doi: 10.1136/jcp.2004.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelmstrom P, Fjell J, Nakagawa T, et al. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–8. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Vassileva G, Kinsley D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–8. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 13.Otero C, Eisele PS, Schaeuble K, et al. Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking and chemotaxis. J Cell Sci. 2008;121:2759–67. doi: 10.1242/jcs.029074. [DOI] [PubMed] [Google Scholar]
- 14.Collins CB, Ho J, Wilson TE, et al. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology. 2008;135:1993–2002. doi: 10.1053/j.gastro.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamee EN, Wermers JD, Masterson JC, et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm Bowel Dis. 2010;16:743–52. doi: 10.1002/ibd.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–66. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 17.Britschgi MR, Link A, Lissandrin TK, et al. Dynamic modulation of CCR7 expression and function on naive T lymphocytes in vivo. J Immunol. 2008;181:7681–8. doi: 10.4049/jimmunol.181.11.7681. [DOI] [PubMed] [Google Scholar]
- 18.Burns RC, Rivera-Nieves J, Moskaluk CA, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz RG, Chaplin DD, McDonald KG, et al. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–82. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 20.Newberry RD. Intestinal lymphoid tissues: is variety an asset or a liability? Curr Opin Gastroenterol. 2008;24:121–8. doi: 10.1097/MOG.0b013e3282f4906d. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida R, Nagira M, Kitaura M, et al. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998;273:7118–22. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- 22.Otero C, Groettrup M, Legler DF. Opposite fate of endocytosed CCR7 and its ligands: recycling versus degradation. J Immunol. 2006;177:2314–23. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga T, Rahman A. What brought the adaptive immune system to vertebrates?—The jaw hypothesis and the seahorse. Immunol Rev. 1998;166:177–86. doi: 10.1111/j.1600-065x.1998.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 24.Drayton DL, Liao S, Mounzer RH, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 25.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 26.Lochner M, Ohnmacht C, Presley L, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–34. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin AP, Coronel EC, Sano G, et al. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173:4791–8. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, McDonough JS, McDonald KG, et al. Alpha4beta7/MAdCAM-1 interactions play an essential role in transitioning cryptopatches into isolated lymphoid follicles and a nonessential role in cryptopatch formation. J Immunol. 2008;181:4052–61. doi: 10.4049/jimmunol.181.6.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finke D. Fate and function of lymphoid tissue inducer cells. Curr Opin Immunol. 2005;17:144–50. doi: 10.1016/j.coi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Lira SA. A passport into the lymph node. Nat Immunol. 2005;6:866–8. doi: 10.1038/ni0905-866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


