Abstract
NMR relaxation experiments often require site-specific isotopic enrichment schemes in order to allow for quantitative interpretation. Here, we describe a new labeling scheme for site-specific 13C-1H enrichment of a single ortho position of aromatic amino acid side chains in an otherwise perdeuterated background by employing a combination of [4-13C]-erythrose and deuterated pyruvate during growth on deuterium oxide. This labeling scheme largely eliminates undesired contributions to 13C relaxation and greatly simplifies the fitting of relaxation data using Lipari-Szabo model-free formalism. This approach is illustrated with calcium-saturated vertebrate calmodulin and oxidized flavodoxin from Cyanobacterium anabaena. Analysis of 13C-relaxation aromatic groups of calcium-saturated calmodulin indicates a wide range of motion in the sub-nanosecond time regime.
Nuclear magnetic resonance (NMR) relaxation has proven to be a versatile probe of the link between fast internal protein motions and their relevance to function.1-4 The motions of the polypeptide chain or of the amino acid side chains in proteins of significant size are most often studied using 15N-relaxation of amide nitrogen and deuterium or carbon relaxation in methyl groups.2,3 This is generally due to restrictions arising from requirements of isotopic labeling and unfavorable relaxation properties of some sites within proteins. In other contexts, more specific and tailored enrichment schemes are often vital in order to eliminate unwanted dipolar and scalar interactions as well as to simplify data interpretation. Examples include use of [3-13C]-pyruvate5, [2-13C]-glycerol or [1,3-13C]-glycerol6,7 or mixtures of singly 13C-enriched acetates8 as carbon precursors to generate isolated 13C spins. Even more selective spin enrichment schemes are sometimes required to suppress unwanted spin interactions and often employ more complex biosynthetic precursors. Prominent examples include labeling schemes targeted for optimal relaxation in methyl groups.5,9,10 Comprehensive chemical synthesis, though relatively expensive, has also proven viable.11
Here we focus on the use of NMR relaxation phenomena to characterize the fast sub-nanosecond motion of aromatic residues. Aromatic amino acid side chains have a rich structural role within proteins12-15 and are often central to their biological function particularly in the context of molecular recognition16,17 and catalysis.18 Thus the motional character of aromatic residues would seem to be of central importance in a range of protein structure-function issues. In this context, NMR phenomena have long been used to characterize relatively slow motions that are manifested in line broadening or population exchange.19
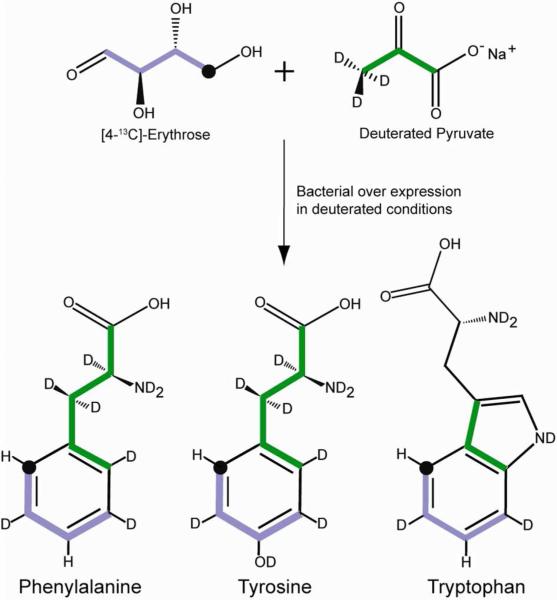
For classical relaxation phenomena used to probe fast sub-nanosecond motions, aromatic residues present a difficult situation. In addition to the concern about the isolation of the spin interaction of interest from extraneous contributions, aromatic ring systems suffer from extensive homo- and heteronuclear scalar (J) interactions that can also significantly complicate the quality and information content of obtained relaxation data. Previously, 1-13C- or 2-13C-glucose has been used to create isolated 13C sites in aromatic side chains and thereby eliminate one-bond 13C-13C interactions. However, this labeling scheme does not eliminate remote scalar or dipolar interactions with non-bonded 1H spins.20-22 In our hands, the 1-13C glucose labeling scheme, which is designed to place isolated 13C at the δ position, results in minor scrambling of label that confounds somewhat the subsequent analysis (see below). In addition, though anticipated to be less of an issue than for methyl 13C relaxation studies,9,23 the presence of remote 1H spins does present a complication to the analysis of aromatic 13C relaxation in proteins. Isolation of the 1H-13C pair in an otherwise perdeuterated background would eliminate potential complications from dipolar interactions with remote 1H spins as well as scalar couplings with other ring hydrogens. Labeling strategies based on glucose are unable to provide this labeling scheme. To largely overcome this limitation we have developed a biosynthetic strategy that takes advantage of the flow of carbon from erythrose 4-phosphate (E4P) to the biosynthesis of the Tyr, Phe and Trp through condensation with phosphoenolpyruvate24 (Figure 1). Consideration of this pathway suggests that use of [4-13C]-erythrose as a sole source of 13C in conjunction with deuterated 12C-pyruvate will lead to creation of an isolated bonded 1H-13C pair at a single delta position (C2 position) within the aromatic ring. Protein expression during growth on [4-13C]-erythrose and deuterated 12C-pyruvate and 99% D2O allows for exchange of all other non-aromatic hydrogens while preserving that bonded to the target 13C. In the case of phenylalanine and tryptophan, an additional 1H spin is predicted to remain at the ζ position (C4 position) of the benzoid ring. Fortunately, this spin is greater than 3 Å away from the sole 13C nucleus rendering its contribution to relaxation negligible. All other aromatic carbons remain NMR inactive 12C nuclei. This labeling pattern therefore largely eliminates the potential complications of extraneous intra-ring scalar or dipolar interactions (1H or 13C) with the isolated 13C spin. The low gyromagnetic ratio of the replacement deuterons will cause them to contribute insignificantly to relaxation of the isolated 13C nucleus. Similarly, the presence of random 13C at natural abundance will also have negligible contribution to the measured relaxation of the target 13C nucleus.
Figure 1.
Carbon inflow into the aromatic pathway through the condensation reaction of erythrose 4-phosphate and phosphoenolpyruvate. Green and purple highlighting indicates the carbon originating from pyruvate and erythrose, respectively. The position of the 13C label is indicated by ‘•’.
Vertebrate calmodulin was expressed in E. coli25 to test this strategy. 12C,2H-pyruvate was used to suppress scrambling of 13C to other amino acids and to aid in perdeuteration of the aromatic ring. One-dimensional 13C-filtered and unfiltered 1H spectra reveal essentially complete deuteration of the protein except at single delta-positions in Tyr and Phe residues and the ζ position (C4) of Phe (Figure 2). No protonation at the ε-position (C3, C5) of the aromatic ring is observed. 13C-labeling is restricted to the delta positions of Tyr and Phe. The enrichment of 13C-1H pairs at the delta position was determined by comparison of the unfiltered 1H spectrum and the 13C-filtered 1H spectrum with and without 13C decoupling, and was found to be uniformly 67% (Supplementary Fig. S1). Analysis of the one-dimensional 13C spectra with and without 1H coupling confirmed that the isolated 13Cδ-1H has not been diluted with 2H from solvent (Supplementary Fig. S3). The under-labeling of 13C is a consequence of using a ratio of 3:2 for 12C,2H-pyruvate to [4-13C]-erythrose, which is motivated by the need to suppress scrambling of 13C to other amino acids or other positions in aromatic rings. This is a reasonable price to pay to maintain the fidelity of the 13C nucleus for relaxation studies.
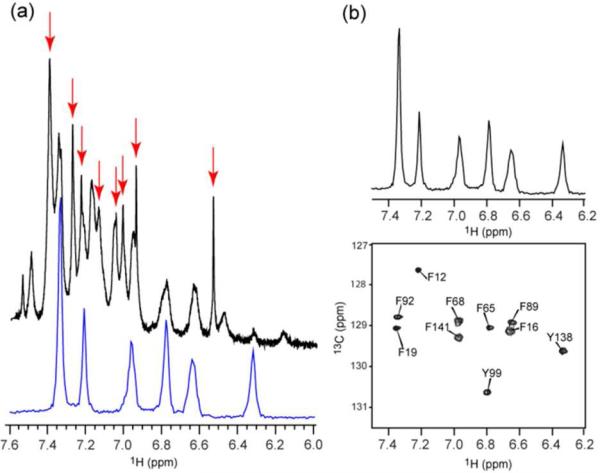
Figure 2.
(a) Overlay of the one-dimensional 1H spectrum of aromatic region from 13C filtered and decoupled (lower, blue) and unfiltered 1H spectrum (upper, black) of calcium-saturated calmodulin expressed during growth on [4-13C]-erythrose, deuterated pyruvate and D2O indicating a selective introduction of a 1H-13C pair at a single δ-carbon of Phe and Tyr. Also evident are resonances arising from hydrogen bonded to 12C at the ζ position of the aromatic ring of Phe (red arrows). These resonances have narrow line widths due to the absence of scalar coupling. A more detailed analysis of the 1H spectrum is presented in the supplementary (Fig. S1 and S2). (b) The two-dimensional 13C-HSQC spectrum of the aromatic region and the corresponding one-dimensional 1H spectrum (13C filtered) are shown. No significant 13C labeling of other amino acids was observed.
Calmodulin does not contain tryptophan. To confirm that appropriate labeling of Trp is also achieved, flavodoxin C55A was similarly prepared.26 Flavodoxin has four Trp, eight Tyr, eight Phe and one His residue. The 22 anticipated aromatic 1H-13C correlations are seen in the 13C-HSQC spectrum (Supplementary Figure S4). No other significant 13C-labeling was observed. Thus the desired labeling pattern was observed for all four aromatic amino acid side chains in the context of a perdeuterated background.
13C R1 and R1ρ relaxation in calcium-saturated calmodulin prepared using this labeling strategy was measured at three magnetic fields (11.7, 14.0 and 17.6 T) using standard two-dimensional sampling pulse sequences.10 For comparison, similar measurements were done using calmodulin prepared with a labeling strategy based on [1-13C]-glucose27,28 (Figure 3). The anisotropy of global macromolecular tumbling was characterized in the usual way29 using the crystal structure of calcium-saturated calmodulin (pdb code: 3CLN) and assessing the two globular domains separately.30
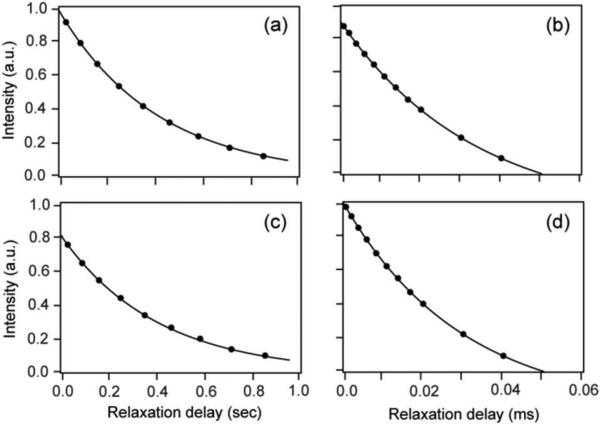
Figure 3.
Aromatic 13C R1 and R1ρ relaxation in calcium-saturated calmodulin prepared using [4-13C]-erythrose/deuterated pyruvate/D2O strategy (Panels a and b) or a [1-13C]-glucose strategy (Panels c and d). Relaxation at the delta-2 position of F92 is shown.
Lipari-Szabo model-free squared generalized order parameters (O2) and effective correlation times (τe) were determined using a grid search approach35 and employed an effective bond length of 1.09 Å and residue-type specific chemical shift anisotropy tensors32 with axially symmetric and anisotropic36 CSA values for Phe and Tyr, respectively. The analysis was carried out with an updated version (Relxn2A) of our in-house software.23,35 Standard statistical F-tests were used to determine if Rex terms were justified. None were found. The contributions from 13C-2H remote dipolar coupling and 13C-13C dipolar interaction due to natural abundance 13C amounted to less than 0.05% of 13C-1H direct bond dipolar interaction. Relaxation data obtained from the [4-13C]-erythrose labeling scheme sample fit well to the simple model-free spectra density (Table 1). Obtained squared generalized order parameters ranged from 0.47 to 0.96 indicating a rich spectrum of aromatic ring motion within calcium-saturated calmodulin on the sub-nanosecond time scale.
Table 1.
Lipari-Szabo model-free parameters for aromatic ring motion in calcium-saturated calmodulina
| Probe | O2 | τe (ps) | Probe | O2 | τe (ps) |
|---|---|---|---|---|---|
| F12b | 0.49 ± 0.03 | 236 ± 21 | F89c | 0.94 ± 0.02 | 900 ± 39 |
| F16b,d | 0.95 ± 0.01 | 127 ± 12 | F92c | 0.72 ± 0.03 | 624 ± 23 |
| F19b | 0.47 ± 0.01 | 232 ± 19 | Y99c | 0.79 ± 0.02 | 240 ± 21 |
| F65b | 0.70 ± 0.04 | 176 ± 23 | Y138c | 0.89 ± 0.02 | 604 ± 36 |
| F68b | 0.96 ± 0.01 | 292 ± 17 | F141c | 0.95 ± 0.01 | 101 ± 11 |
Prepared using the [4-13C]-erythrose labeling scheme. Model-free squared generalized order parameters (O2) and effective correlation times (τe) determined using the simple model-free spectral density,31 an effective C-H bond length of 1.09 Å, and the axially symmetric and fully anisoanisotropic chemical shift anisotropy tensors for the δ-13C of Phe and Tyr, respectively, as determined by Ye et al.32 Macromolecular tumbling was characterized using 15N-relaxation with an effective N-H bond length33 of 1.04 Å and a simple uniform 15N chemical shift anisotropy tensor breadth34 of −170 p.p.m. The N- and C-terminal domains were treated separately. The precision of the squared generalized order parameters(O2) and effective correlation times (τe) were estimated by Monte Carlo sampling.
Part of the N-terminal domain with an effective macromolecular tumbling time of 8.96 ± 0.14 ns.
Part of the C-terminal domain with an effective macromolecular tumbling time of 8.05 ± 0.10 ns.
Due to partial spectral overlap, only data obtained at 17.6 T was fitted.
In contrast, though the primary R1 and R1ρ relaxation time profiles derived from the calcium-saturated calmodulin obtained from the [1-13C]-glucose labeling scheme fitted reasonably to a single exponential decays (Figure 3), the obtained relaxation rates largely gave relatively poor fits to the L-S model-free interpretation (5-10% versus <1% residual error) (Supplementary Table S3). Inclusion of remote 1H spin dipolar interaction as well as dipolar interaction with 13Cε failed to recover the excellent statistics of the relaxation data derived from the more optimal [4-13C]-erythrose labeling scheme (Supplementary Fig. S5, Table. S3). It seems likely that unaccounted dipolar relaxation, intra-ring 1-bond 13C-13C J-coupling, intra-ring 2-bond 13Cδ-1Hε J-coupling effects contaminate the measurement and interpretation of 13C-relaxation in structured proteins in a protonated background. This is consistent with the presence of additional peaks in the 13C-1H HSQC spectrum of calmodulin derived from the [1-13C]-glucose labeling scheme (Supplementary Fig. S6). These additional peaks arise from partial 13C labeling (~8 - 15%) at the Cε of the aromatic aromatic ring and are a consequence of the scrambling of 13C label when using glucose as the carbon precursor. These considerations provide a plausible scenario where the relaxation profiles still fit reasonably to single exponential decays but fail to be fit reasonably by the L-S model-free formalism. Thus the [4-13C]-erythrose labeling strategy described here would seem to be highly advantageous in providing high fidelity relaxation for the study of fast aromatic ring dynamics.
In summary, we have demonstrated a strategy to produce perdeuterated proteins with isolated 1H-13C pairs in the aromatic ring systems. It is shown that the aromatic side chains of calmodulin have a wide range of motion on the sub-nanosecond time scale, the observation of which has thus far been restricted to detection at natural abundance using highly concentrated and relatively small proteins.37 The ability to investigate the fast dynamics of aromatic side chain will provide a highly complementary perspective to that accessed by 13C or 2H relaxation in methyl groups.
Supplementary Material
Acknowledgment
This work was supported by NIH grant GM 102447.
Footnotes
Supporting Information. Description of [4-13C]-erythrose labeling protocol. Carbon-13 relaxation decay rates for aromatic side chains in calcium-saturated calmodulin collected at 500 MHz, 600 MHz and 750 MHz (1H). 13C-HSQC spectrum of flavodoxin prepared with the [4-13C]-erythrose labeling strategy. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Mittermaier A, Kay LE. Science. 2006;312:224–228. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- 2.Jarymowycz VA, Stone MJ. Chem. Rev. 2006;106:1624–1671. doi: 10.1021/cr040421p. [DOI] [PubMed] [Google Scholar]
- 3.Igumenova TI, Frederick KK, Wand AJ. Chem. Rev. 2006;106:1672–1699. doi: 10.1021/cr040422h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marlow MS, Dogan J, Frederick KK, Valentine KG, Wand AJ. Nat. Chem. Biol. 2010;6:352–358. doi: 10.1038/nchembio.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AL, Urbauer JL, Wand AJ. j. Biomol. NMR. 1997;9:437–440. doi: 10.1023/a:1018311013338. [DOI] [PubMed] [Google Scholar]
- 6.LeMaster DM. Prog. NMR Spectr. 1994;26:371–419. [Google Scholar]
- 7.LeMaster DM, Kushlan DM. J. Am. Chem. Soc. 1996;118:9255–9264. [Google Scholar]
- 8.Wand AJ, Bieber RJ, Urbauer JL, McEvoy RP, Gan ZH. J. Magn. Res. Ser B. 1995;108:173–175. doi: 10.1006/jmrb.1995.1119. [DOI] [PubMed] [Google Scholar]
- 9.Ishima R, Petkova AP, Louis JM, Torchia DA. J. Am. Chem. Soc. 2001;123:6164–6171. doi: 10.1021/ja0104711. [DOI] [PubMed] [Google Scholar]
- 10.Tugarinov V, Kay LE. Biochemistry. 2005;44:15970–15977. doi: 10.1021/bi0519809. [DOI] [PubMed] [Google Scholar]
- 11.Kainosho M, Guntert PQ. Rev. Biophys. 2009;42:247–300. doi: 10.1017/S0033583510000016. [DOI] [PubMed] [Google Scholar]
- 12.Thomas KA, Smith GM, Thomas TB, Feldmann RJ. Proc. Nat. Acad. Sci. USA. 1982;79:4843–4847. doi: 10.1073/pnas.79.16.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burley SK, Petsko GA. Science. 1985;229:23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- 14.Burley SK, Petsko GA. FEBS Lett. 1986;203:139–143. doi: 10.1016/0014-5793(86)80730-x. [DOI] [PubMed] [Google Scholar]
- 15.Dill KA. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 16.Birtalan S, Fisher RD, Sidhu SS. Mol. Biosystems. 2010;6:1186–1194. doi: 10.1039/b927393j. [DOI] [PubMed] [Google Scholar]
- 17.Bogan AA, Thorn KS. J. Mol. Biol. 1998;280:1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett GJ, Porter CT, Borkakoti N, Thornton JM. J. Mol. Biol. 2002;324:105–121. doi: 10.1016/s0022-2836(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 19.Wüthrich K, Wagner G. FEBS Lett. 1975;50:265–268. doi: 10.1016/0014-5793(75)80504-7. [DOI] [PubMed] [Google Scholar]
- 20.Boyer JA, Lee AL. Biochemistry. 2008;47:4876–4886. doi: 10.1021/bi702330t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teilum K, Brath U, Lundstrom P, Akke M. J. Am. Chem. Soc. 2006;128:2506–2507. doi: 10.1021/ja055660o. [DOI] [PubMed] [Google Scholar]
- 22.Lundstrom P, Teilum K, Carstensen T, Bezsonova I, Wiesner S, Hansen DF, Religa TL, Akke M, Kay LE. j. Biomol. NMR. 2007;38:199–212. doi: 10.1007/s10858-007-9158-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee AL, Flynn PF, Wand AJ. J. Am. Chem. Soc. 1999;121:2891–2902. [Google Scholar]
- 24.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 6th ed. W.H. Freeman; New York: 2007. [Google Scholar]
- 25.Kranz JK, Flynn PF, Fuentes EJ, Wand AJ. Biochemistry. 2002;41:2599–2608. doi: 10.1021/bi011818f. [DOI] [PubMed] [Google Scholar]
- 26.Liu WX, Flynn PF, Fuentes EJ, Kranz JK, McCormick M, Wand AJ. Biochemistry. 2001;40:14744–14753. doi: 10.1021/bi011073d. [DOI] [PubMed] [Google Scholar]
- 27.Kalan EB, Davis BD, Srinivasan PR, Sprinson DB. J. Biol. Chem. 1956;223:907–912. [PubMed] [Google Scholar]
- 28.Srinivasan PR, Shigeura HT, Sprecher M, Sprinson DB, Davis BD. J. Biol. Chem. 1956;220:477–497. [PubMed] [Google Scholar]
- 29.Tjandra N, Feller SE, Pastor RW, Bax A. J. Am. Chem. Soc. 1995;117:12562–12566. [Google Scholar]
- 30.Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A. Nat. Struct. Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- 31.Lipari G, Szabo A. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]
- 32.Ye CH, Fu RQ, Hu JZ, Hou L, Ding SW. Magn. Reson. Chem. 1993;31:699–704. [Google Scholar]
- 33.Ottiger M, Bax A. J. Am. Chem. Soc. 1998;120:12334–12341. [Google Scholar]
- 34.Yao LS, Grishaev A, Cornilescu G, Bax A. J. Am. Chem. Soc. 2010;132:4295–4309. doi: 10.1021/ja910186u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellwo MJ, Wand AJ. J. Am. Chem. Soc. 1989;111:4571–4578. [Google Scholar]
- 36.Goldman M. J. Magn. Reson. 1984;60:437–452. [Google Scholar]
- 37.Palmer AG, Hochstrasser RA, Millar DP, Rance M, Wright PE. J. Am. Chem. Soc. 1993;115:6333–6345. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.