Abstract
Morphologic and biochemical studies indicate that the initial action of insulin is binding to a cell surface receptor. Whether further translocation of the hormone, or a product of the hormone, occurs is unclear and has not been investigated by direct means. To determine the fate of 125I-insulin bound to its receptor, we have examined the distribution of radioactivity by quantitative electron microscopic autoradiography. Cultured lymphocytes of the IM-9 cell line were incubated with 0.1 nM 125I-insulin at 15 degrees and 37 degreesC for incubation periods extending from 2 to 90 min. At 15 degreesC, grains localize to the plasma membane and there is no translocation as a function of time. At 37 degreesC, grains predominantly localize to the plasma membrane but there is a small shift in distribution to a distance of 300-700 nm from the plasma membrane. This small additional band component of irradiation extends to approximately to10--15% of the cell radius. When a morphometric analysis is applied to grains extending 300 nm and beyond from the plasma membrane, we find no preferential localization to any intracellular organelle. We interpret these data to indicate that in the cultured lymphocyte, labeled insulin initially localizes to the plasma membrane but as fuanction of time and increasing temperature there is a small but definite translocation of the hormone or a product of the hormone to a hihgly limited aea of the cell periphery.
Full text
PDF

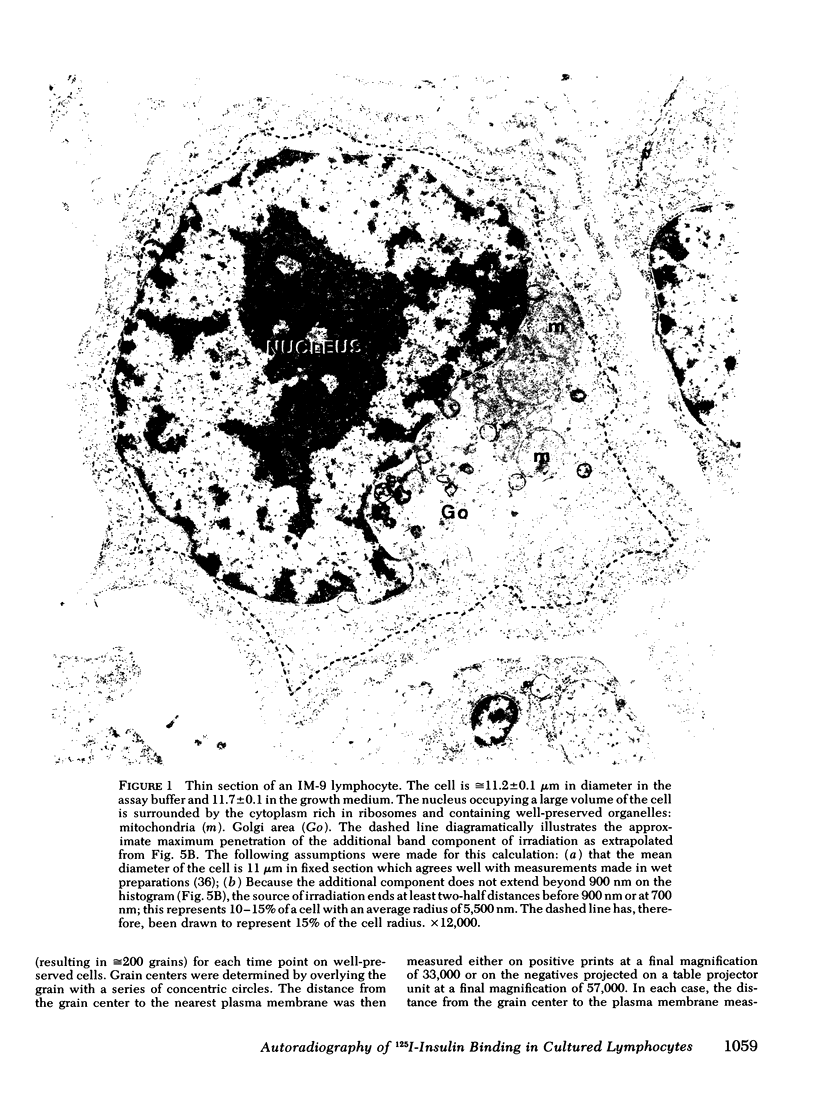











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Preparation of inverted plasma membrane vesicles from isolated adipocytes. Biochim Biophys Acta. 1973 Jul 6;311(3):362–380. doi: 10.1016/0005-2736(73)90316-7. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Evans W. H., Geschwind I. I. Insulin binding to rat liver Golgi fractions. J Cell Biol. 1973 Dec;59(3):771–776. doi: 10.1083/jcb.59.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford O. B. The uptake and inactivation of native insulin by isolated fat cells. J Biol Chem. 1968 Jan 25;243(2):362–369. [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Autoantibodies to the insulin receptor. Effect on the insulin-receptor interaction in IM-9 lymphocytes. J Clin Invest. 1977 Oct;60(4):784–794. doi: 10.1172/JCI108832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Gonatas J. O., Stieber A., Antoine J. C., Avrameas S. Quantitative ultrastructural autoradiographic studies of iodinated plasma membranes of lymphocytes during segregation and internalization of surface immunoglobulins. J Cell Biol. 1976 Sep;70(3):477–493. doi: 10.1083/jcb.70.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The natural occurrence of insulin receptors in groups on adipocyte plasma membranes as demonstrated with monomeric ferritin-insulin. J Supramol Struct. 1977;6(1):45–59. doi: 10.1002/jss.400060104. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Ultrastructural localization of insulin receptors on adipocytes. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3526–3530. doi: 10.1073/pnas.72.9.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Kono T. Destruction of insulin effector system of adipose tissue cells by proteolytic enzymes. J Biol Chem. 1969 Apr 10;244(7):1772–1778. [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Moore G. E., Kitamura H., Toshima S. Morphology of cultured hematopoietic cells. Cancer. 1968 Aug;22(2):245–267. doi: 10.1002/1097-0142(196808)22:2<245::aid-cncr2820220202>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Orci L., Rufener C., Malaisse-Lagae F., Blondel B., Amherdt M., Bataille D., Freychet P., Perrelet A. Symposium III: Hormones and hormone receptors. A morphological approach to surface receptors in islet and liver cells. Isr J Med Sci. 1975 Jul;11(7):639–655. [PubMed] [Google Scholar]
- Pastan I., Roth J., Macchia V. Binding of hormone to tissue: the first step in polypeptide hormone action. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1802–1809. doi: 10.1073/pnas.56.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Fertuck H. C., Salpeter E. E. Resolution in electron microscope autoradiography. III. Iodine-125, the effect of heavy metal staining, and a reassessment of critical parameters. J Cell Biol. 1977 Jan;72(1):161–173. doi: 10.1083/jcb.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen E., De Meyts P., Roth J. Cell surface receptors for insulin and human growth hormone. Effect of microtubule and microfilament modifiers. J Biol Chem. 1976 Nov 10;251(21):6844–6851. [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]





