Abstract
Purpose.
To evaluate cell survival and tumorigenicity of human embryonic stem cell–derived retinal pigment epithelium (hESC-RPE) transplantation in immunocompromised nude rats. Cells were transplanted as a cell suspension (CS) or as a polarized monolayer plated on a parylene membrane (PM).
Methods.
Sixty-nine rats (38 male, 31 female) were surgically implanted with CS (n = 33) or PM (n = 36). Cohort subsets were killed at 1, 6, and 12 months after surgery. Both ocular tissues and systemic organs (brain, liver, kidneys, spleen, heart, and lungs) were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Every fifth section was stained with hematoxylin and eosin and analyzed histologically. Adjacent sections were processed for immunohistochemical analysis (as needed) using the following antibodies: anti-RPE65 (RPE-specific marker), anti-TRA-1-85 (human cell marker), anti-Ki67 (proliferation marker), anti-CD68 (macrophage), and anti-cytokeratin (epithelial marker).
Results.
The implanted cells were immunopositive for the RPE65 and TRA-1-85. Cell survival (P = 0.006) and the presence of a monolayer (P < 0.001) of hESC-RPE were significantly higher in eyes that received the PM. Gross morphological and histological analysis of the eye and the systemic organs after the surgery revealed no evidence of tumor or ectopic tissue formation in either group.
Conclusions.
hESC-RPE can survive for at least 12 months in an immunocompromised animal model. Polarized monolayers of hESC-RPE show improved survival compared to cell suspensions. The lack of teratoma or any ectopic tissue formation in the implanted rats bodes well for similar results with respect to safety in human subjects.
Keywords: age-related macular degeneration, retinal pigment epithelium, human embryonic stem cells
Growing RPE cells on biocompatible, nondegradable membranes that mimic Bruch's membrane is a promising approach to achieve intact RPE monolayers. A polarized monolayer of hESC-RPE grown on a parylene membrane in this study demonstrated improved survival compared to hESC-RPE cell suspensions.
Introduction
Retinal pigment epithelium (RPE) cells have important functions in the maintenance of homeostasis of the outer retina. These functions include regulating the transport of nutrients to the photoreceptors, phagocytosing shed outer segments, and absorbing stray light.1 Furthermore, as a part of the blood–ocular barrier, the monolayer of the RPE cells helps limit access of blood components to the retina, thereby maintaining a certain immune privilege.2
An RPE cell defect has been implicated in and is perhaps primary to some pathological conditions of the retina. Age-related macular degeneration (AMD), a major cause of blindness worldwide, is characterized, in its dry form, by local dysfunction and loss of the RPE and by disruption of the RPE–photoreceptor interface.3 Reestablishment of this normal interface by moving foveal photoreceptors to an adjacent area of intact RPE via macular translocation, or placing a patch of free peripheral RPE graft under the fovea, restored central vision in selected cases.4,5 However, autologous older RPE cells may not behave as robustly as those from young donors.6
An alternative to endogenous repair is to replace lost cells with cells derived from human embryonic stem cells (hESC). Recently, subretinal injection of hESC-RPE was tested in a clinical trial for safety and tolerability in patients with advanced-stage Stargardt's macular dystrophy and dry AMD without abnormal proliferation or teratoma formation.7 However, the replacement RPE should ideally mimic the physiologic state as a polarized monolayer precisely juxtaposed between photoreceptors and the basal lamina of Bruch's membrane (BM). The trial investigators yet lack clinical evidence that the RPE cell suspension injection led to a confluent RPE monolayer in AMD or to RPE polarization and RPE survival. Therefore, it seems prudent to transplant the substituted RPE on a basal membrane, imitating the BM, to achieve sustainable effects.3 We believe that growing RPE cells on biocompatible, nondegradable membranes that mimic BM, including its diffusion characteristics, is a promising approach to achieving an intact RPE monolayer graft.
Assessment of safety and efficacy is crucial before new hESC therapies can move into the clinic. Potential concerns with such therapies include the risk of teratoma formation and immune rejection of the implanted cells.8,9 The lack of tumor formation in immunocompetent animal models may be misleading in terms of safety, as it might reflect the ability of the host to reject tumorigenic cells before they can form tumors. Transplantation of the same cells in an immunocompromised recipient enhances cell survival, thereby increasing the chance of observing tumor or teratoma formation.10 Various immunodeficient models are used for that purpose, one being the athymic nude rat. Our purpose in conducting this study was to determine whether transplantation of a polarized monolayer of H9 hESC-RPE, grown on a nondegradable parylene membrane, into the subretinal space of athymic nude rats—an immunocompromised animal model—would lead to increased RPE cell survival and whether those cells had any tumorigenic effects.
Methods
Stem Cells and RPE Differentiation
The differentiation of hESC into functional RPE cells that express specific genes and characteristics similar to those of native RPE has been previously described.11 Briefly, the H9 hESC (WiCell, Madison, WI) were cultured in mTeSR1 medium (Stemcell Technologies, Vancouver, BC, Canada). The ES cells were allowed to spontaneously differentiate into RPE cells in XVIVO 10 medium (Lonza, Walkersville, MD) for 12 weeks. The pigmented RPE-like cells were enriched by mechanical isolation. The isolated RPE-like cells were dissociated by TrypLE (Life Technologies, Grand Island, NY) and cultured in human vitronectin (AMS Biotechnology, Lake Forest, CA)–coated plates with XVIVO 10 medium. Passage 3 cells were used for implantation.
Preparation of Polarized hESC-RPE Sheets on Parylene Membranes
Ultrathin membranes (0.3-μm thickness supported on a 6.0-μm-thick mesh frame) made from parylene C were specially designed for rat implantation (1.0 × 0.4 mm) and used successfully for RPE culture.12 These ultrathin membranes were coated with vitronectin (AMS Biotechnology), then seeded with hESC-RPE. The cells were allowed to grow to confluence for approximately 4 weeks before implantation, achieving a density of approximately 2700 cells/membrane.13
Preparation of Dissociated hESC-RPE for the Suspension Injection
Passage 3 hESC-RPE that had been cultured in vitronectin-coated culture dishes for 4 weeks were used for subretinal injections. To harvest cells from culture, cells were dissociated from the plastic culture dishes by TrypLE (Life Technologies). After neutralization with X-VIVO 10 medium, cells were pelleted by centrifugation, washed with Dulbecco's minimum essential medium (DMEM)/F12 medium (Life Technologies) once, and resuspended in DMEM/F12 medium to a final concentration of 5 × 107 cells/mL. Two microliters of cell mixture, containing approximately 105 hESC-RPE cells, was injected into the subretinal space.
Animals
All experiments were approved by the University of Southern California Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Sixty-nine athymic nude rats (Harlan Laboratories, Indianapolis, IN), male (38) and female (31), were used for the implantation surgery. Only the left eye was selected for experimentation in each animal. Animals underwent surgery at postnatal days 28 through 40. Anesthesia was induced by abdominal injection of ketamine (37.5 mg/kg) and xylazine (5 mg/kg) before each surgical procedure. In addition, topical anesthesia was administered with 0.5% proparacaine hydrochloride ophthalmic solution (Akorn, Inc., Lake Forest, IL). Pupils were dilated using ophthalmic solutions of 2.5% phenylephrine hydrochloride and 0.5% tropicamide (Akorn, Inc.).
The rats were kept in an aseptic and temperature-controlled environment and were euthanized with intracardiac injection of 0.5 mL pentobarbitol sodium 390 mg and phenytoin sodium 50 mg (Euthasol; Virbac AH, Inc., Fort Worth, TX). Cohort subsets were killed at 1, 6, and 12 months. Rats were imaged with a Spectralis HRA+OCT device (Heidelberg Engineering, Heildeberg, Germany) for infrared images immediately before killing.
Surgical Procedure
For both injection of RPE cell suspension and implantation of RPE cell–seeded parylene membranes, the rats were anesthetized and placed under a surgical microscope, and their pupils were pharmacologically dilated. A temporal peritomy was made, and the superior and lateral recti were isolated. A 4–0 silk suture was passed under these two muscles and used to mechanically hold the eye in the desired position. A 27-gauge needle was used to make a scleral incision of approximately 1.2 mm, approximately 1.5 mm posterior to the limbus at the temporal equator. An anterior chamber paracentesis was performed to lower the intraocular pressure. A 32-gauge needle was then inserted into the subretinal space through the aforementioned scleral incision, and 5 μL balanced salt solution (Alcon Laboratories, Inc., Fort Worth, TX) was injected to create a local retinal detachment.
Cell Parylene Membrane Insertion
The choroid was delicately cut while preserving the retinal integrity, and the hESC-RPE membrane substrate was inserted into the subretinal space with forceps.
Cell Suspension Injection
Cells were injected using a submicroliter injection system. A 32-gauge blunt-end injection cannula attached to the microsyringe pump was inserted through the sclerotomy, and 2 μL phosphate-buffered saline (Life Technologies) containing approximately 105 hESC-RPE was injected into the subretinal space.
Immediately after the surgical procedure, a fundus examination was performed to confirm the successful delivery of the cells. The sclera and conjunctiva were sutured using a 10/0 nylon surgical suture (S&T, Neuhausen, Switzerland). After surgery, the implanted eyes were administered neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment (Alcon Laboratories, Inc.). The rats were then allowed to recover from anesthesia in a thermal care incubator.
Histopathology
All implanted eyes were enucleated at 1, 6, or 12 months after the procedure and processed for histology. Twelve contralateral eyes were evaluated as controls. Whole eyes were fixed in Davidson's solution overnight. The cornea and lens were removed, and the eye cups (posterior poles) were soaked in 75% ethanol for 2 hours. After dehydration in progressive ethanol concentrations, the tissues were placed in xylene. Finally, the eye cups were embedded in paraffin and processed for sectioning (5-μm sections). Groups of consecutive slides were stained with hematoxylin and eosin (H&E) for light microscopy. Adjacent sections of the implanted eye were processed for immunohistochemical analysis using the following antibodies as needed: human-specific cell surface marker (anti-TRA-1-85; EMD Millipore, Darmstadt, Germany), a marker of differentiated RPE cells (anti-RPE65; Abcam, Cambridge, MA), a marker of cell proliferation (anti-Ki67; Abcam), a macrophage marker (anti-CD68; Abcam), an astrocyte/Müller cell marker (anti-GFAP; EMD Millipore), and an epithelial cell marker (anti-cytokeratin; Abcam).
The organs (brain, liver, kidneys, spleen, heart, and lungs) were removed from 12 rats at the different time points (1, 6, and 12 months), fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. A masked board-certified pathologist evaluated the specimens.
Data Analysis
Histological sections of cell suspension and cell-seeded membranes were evaluated to assess hESC-RPE survival and localization and to look for any evidence of teratoma or tumor formation. Subretinal placement was considered correct in cases in which most of the substrate was located in the subretinal space. To confirm cell survival, the presence of hESC-RPE had to be observed in at least three sections, 125 μm apart, using light microscopy and confirmed by immunostaining. Cell migration was suspected when pigmented cells or aggregates were seen in locations other than the implanted position and confirmed with immunohistochemistry. Moreover, if no human/RPE marker was found, the specimen was classified as pigment migration. The retina outer nuclear layer (ONL) integrity was evaluated for loss of cells over the implants. Cellular reaction around the implants, observed by light microscopy, was assessed for the presence of macrophages or expression of astrocytes and for cytokeratin. Tumor formation was suspected if clumps of transplanted cells were observed and if the cells expressed a cell proliferation marker (anti-Ki67).
The nonimplanted eyes and the rats' organs were evaluated for the presence of pigmented cells or any tumor formation. Binary logistic regression, adjusted for the age at death, was used to identify the factors associated with the outcome of cells on the parylene membrane when compared with injections of cell suspension. SAS V9.2 (SAS Institute, Cary, NC) programming language was used for all analyses, and the accepted level of significance was <0.05.
Results
Sixty-nine rats implanted with hESC-RPE, either in suspension (n = 33) or plated on a parylene membrane (n = 36), were randomized for killing at 1, 6, or 12 months postimplantation. Light microscopy was performed on all eyes. On average, for all time points, 72% of the implanted cell-seeded membranes were found in the subretinal space. For the RPE cell suspension injection, 84% of the cells were found in the subretinal space (Table 1).
Table 1.
Histology Assessment, Number of Animals (%)
|
Surgical Procedure |
Cell Survival |
Monolayer* |
Subretinal Placement |
Pigment Migration |
Cell Reaction |
ONL Loss |
| hESC-RPE on membrane | ||||||
| 1 mo, n = 12 (%) | 12 (100) | 12 (100) | 8 (66.66) | 5 (41.66) | 11 (91.66) | 4 (33.33) |
| 6 mo, n = 12 (%) | 11 (91.66) | 9 (81.81) | 9 (75) | 8 (66.66) | 6 (50) | 11 (91.66) |
| 12 mo, n = 12 (%) | 6 (50) | 5 (83.33) | 9 (75) | 6 (50) | 7 (58.33) | 12 (100) |
| Total, n = 36 (%) | 29 (80.55) | 26 (89.65) | 26 (72.22) | 19 (52.77) | 24 (66.66) | 27 (75) |
| hESC-RPE suspension | ||||||
| 1 mo, n = 9 (%) | 7 (77.77) | 1 (14.28) | 6 (66.66) | 1 (11.11) | 3 (33.33) | 1 (11.11) |
| 6 mo, n = 12 (%) | 6 (50) | 1 (16.66) | 11 (91.66) | 5 (41.66) | 6 (50) | 10 (83.33) |
| 12 mo, n = 12 (%) | 3 (25) | 2 (66.66) | 11 (91.66) | 7 (58.33) | 7 (58.33) | 12 (100) |
| Total, n = 33 (%) | 16 (48.49) | 4 (22.22) | 28 (84.84) | 13 (39.39) | 16 (48.48) | 23 (69.69) |
hESC-RPE, human embryonic stem cell–derived retinal pigment epithelium.
Monolayer evaluation in the rats with cell survival (n = 45).
The presence of pigmented transplanted cells was confirmed by light microscopy in animals for up to 12 months after surgery. Cell survival, confirmed by robust anti-TRA-1-85 and anti-RPE65 staining, was observed at a greater percentage in rats that received hESC-RPE–coated membranes than in those that received only the RPE cell suspension. For the implanted hESC-RPE on membranes, the presence of cells was confirmed in 100% of the rats at 1 month, 91% at 6 months (Fig. 1), and 50% at 12 months (Table 1). For the injected hESC-RPE in suspension, cells were observed in 77% of the eyes at 1 month (Fig. 2), 50% at 6 months, and 25% at 12 months. Thus, cell survival was significantly higher in the rats that were implanted with hESC-RPE on parylene than in those injected with RPE cell suspensions (P = 0.006) (Table 2).
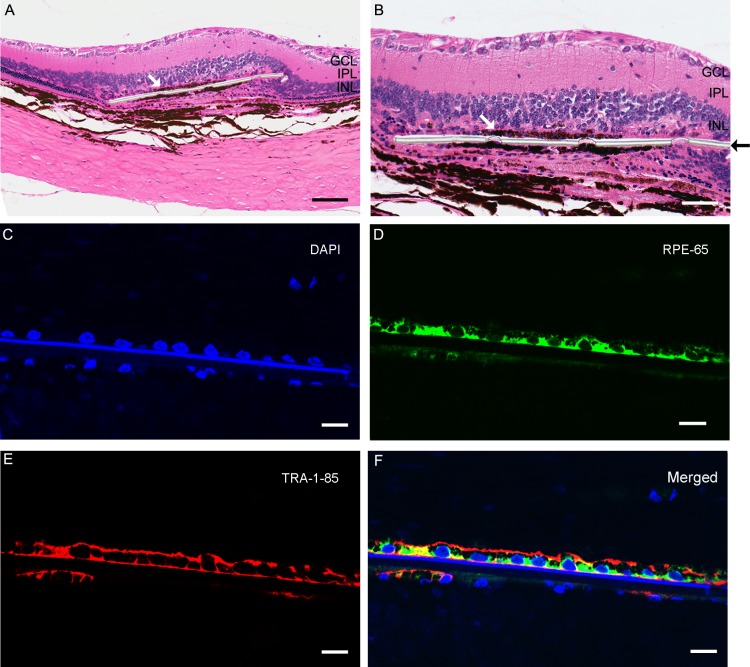
Figure 1. .
Implanted eye with parylene C membrane with cells 12 months after surgery. Monolayer of pigmented cells (white arrow) placed in the subretinal space over the parylene membrane (black arrow) observed with hematoxylin and eosin staining (A, B). Scale bars: A, 100 μm; B, 50 μm. Nuclei of implanted cells and host cells are counterstained by 4′,6-diamidino-2-phenylindole (DAPI) ([C], blue). Immunofluorescent staining for RPE65 ([D], green) and TRA-1-85 human marker ([E], red) was found in the transplanted cells. (F) Merged image of RPE65, TRA-1-85, and DAPI. Scale bars for C, D, E, F: 10 μm.
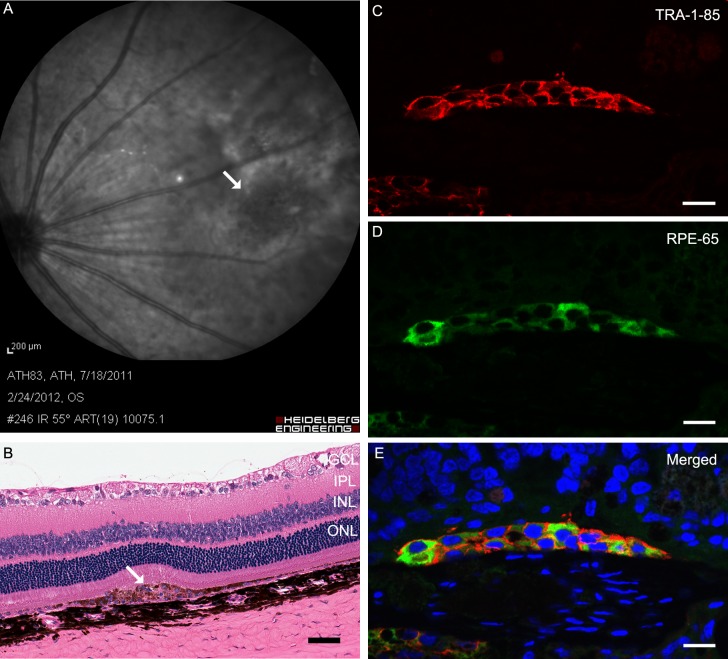
Figure 2. .
Implanted eye with cell suspension 1 month after surgery. The infrared image shows the placement of the injected cells immediately before killing ([A], white arrow). Two layers of pigmented cells (white arrow) in the subretinal space observed by hematoxylin and eosin staining ([B], scale bar: 50 μm). Transplanted cells expressed TRA-1-85 ([C], red) and RPE65 ([D], green) markers. (E) A merged image of RPE-65, TRA-1-85, and DAPI (blue, nuclear counterstain). Scale bars for C, D, E: 10 μm.
Table 2.
Logistic Regression, Adjusted for Age of Death, to Identify Factors Associated With Human Embryonic Stem Cell–Derived Retinal Pigment Epithelium on a Parylene Membrane When Compared to Cell Suspension Injection
|
P
Value |
OR |
95% CI |
|
| Cell survival | 0.006 | 6.18 | 1.70–22.53 |
| Monolayer | <0.001 | 61.86 | 8.77–436.23 |
| Subretinal placement | 0.238 | 0.48 | 0.14–1.62 |
| Pigment migration | 0.202 | 1.91 | 0.71–5.18 |
| Cell reaction | 0.141 | 2.09 | 0.78–5.58 |
OR, odds ratio; CI, confidence interval.
The presence of a viable hESC-RPE monolayer was observed more often (P < 0.001) in the rats implanted with hESC-RPE on membranes compared to those injected with RPE cell suspensions (Table 2). In the majority of the eyes that received the RPE cell suspension, the cells were observed as clumps, especially 1 month after surgery (Fig. 3).
Figure 3.
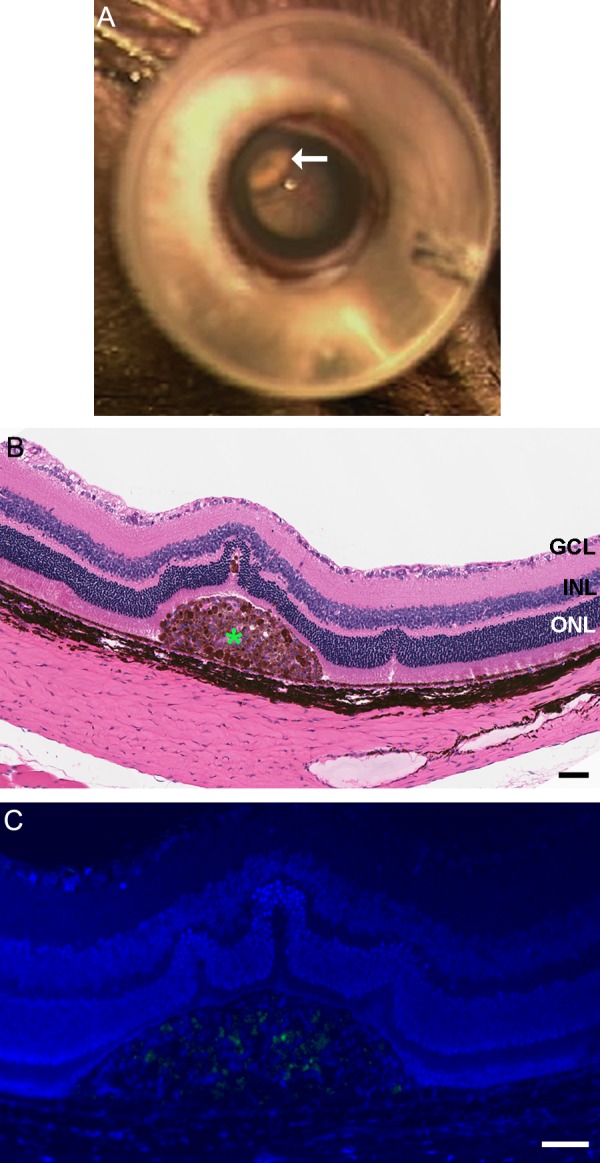
A localized retinal detachment (white arrow) after injection of RPE cells as seen through operating microscope (A). Hematoxylin and eosin–stained section of rat eye with cell suspension injection 1 month after surgery ([B], scale bar: 50 μm). Clump of human embryonic stem cell–derived retinal pigment epithelium cells was observed in the subretinal space. Immunostaining for proliferation marker ant-Ki67 was not observed in the clump of cells found in the subretinal space ([C], scale bar: 10 μm).
Based on anti-RPE65 and anti-TRA-1-85 staining, no migration of transplanted RPE cells into the neural retina was observed in any of the implanted animals. However, large macrophages (confirmed by anti-CD68 staining) that contained pigmented material were observed in all implanted eyes. Such macrophages were found as single cells or cell clusters (Fig. 4). Dispersion of pigmented material (possibly the degraded hESC-RPE) into the neurosensory retina was observed in some of the implanted eyes in both groups (substrate implant and cell suspension injection, P = 0.202). H&E staining confirmed the absence of any pigment migration or transplanted cells near the optic disc.
Figure 4.
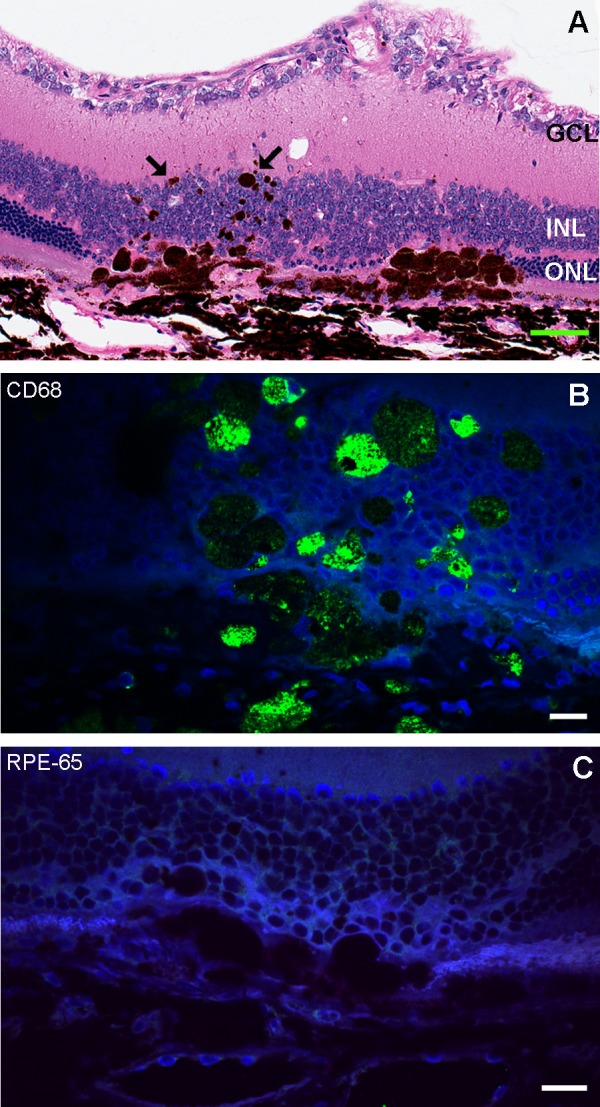
Pigment migration 6 months after cell suspension injection. (A) Pigment is shown by the arrows in hematoxylin and eosin staining (scale bar: 50 μm). (B) Confocal image of CD68 immunofluorescence (green) in large pigmented cells. (C) Confocal image of RPE65 (red). The pigmented cells show CD68 positivity and are negative for RPE65, which indicates that these are macrophages. Scale bars for B, C: 10 μm.
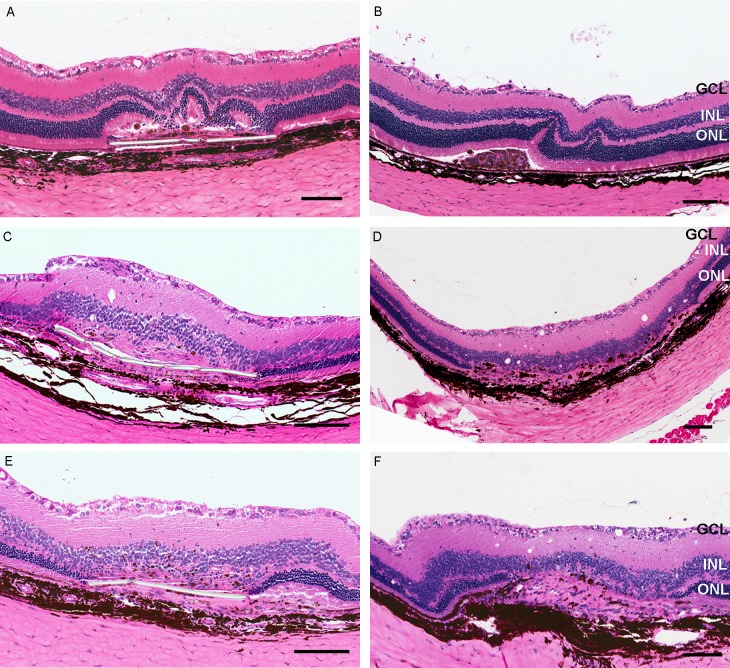
Cell reaction was frequently observed around the implants (Fig. 5) and was similar in the two groups (P = 0.141), except at 1 month (substrate 91% versus injection 33%). Those cells that did not stain with any of the markers used in this study were considered to be fibroblasts from scleral ingrowth, based on histological observation (Fig. 6). Neuroretinal damage was evident at various time points following both procedures. All the eyes examined had some degree of photoreceptor cell loss at 12 months over the implants. Rosette formation was initially observed at 1 month, with progression to loss of the ONL (Fig. 7).
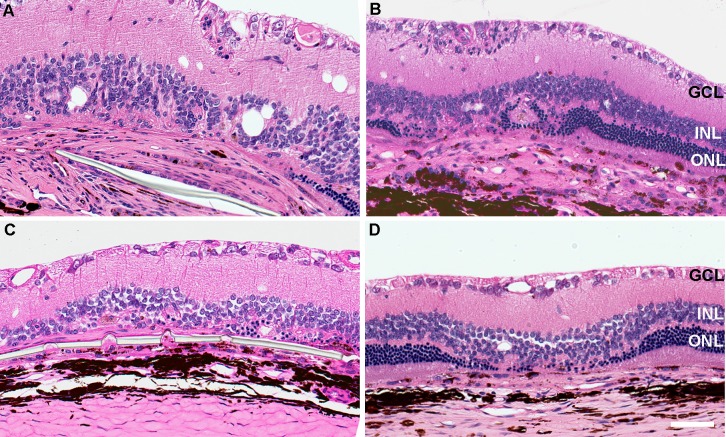
Figure 5.
Hematoxylin and eosin–stained sections of hESC-RPE implanted or hESC-RPE cell suspension–injected eyes 6 months after surgery. (A) Cell reaction around the parylene membrane. (B) At the cell suspension injection area. (C) Implanted eye without cell reaction and (D) cell suspension injection without cell reaction. Scale bar: 50 μm.
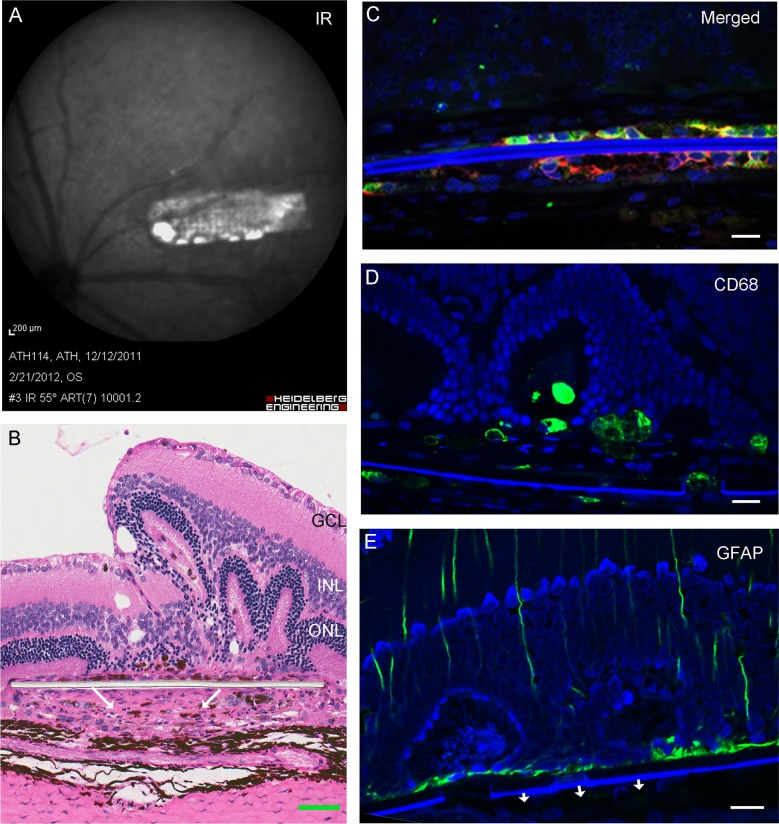
Figure 6.
Implanted eye with parylene C membrane with cells 1 month after surgery. The infrared image shows the placement of the implant immediately before killing (A). Arrows indicate cell reaction below the membrane in hematoxylin and eosin staining ([B], scale bar: 50 μm). Immunohistochemical localization of RPE65 (green), TRA-1-85 (red), and nuclear counterstain DAPI (blue) in the implanted hESC-RPE cells (C). Cells below the substrate in (B) did not show any positivity for anti-CD68 (D) and GFAP (E). Scale bars for C, D, E: 50 μm.
Figure 7.
Photoreceptor loss after the implantation. Rosette formation in retina 1 month after the implantation of cells above the parylene membrane (A) and suspension (B); the remaining ONL is shown away from the transplant site after 6 months (C, D) and after 12 months (E, F). Scale bar: 100 μm.
None of the 69 transplanted retinas examined at any time point showed any evidence of uncontrolled cell proliferation when evaluated by a board-certified pathologist. Cellular reaction and hESC-RPE aggregates did not stain with anti-Ki67 and were considered negative for cell division. No mitotic figures were observed in any animal. There was no evidence of teratoma or tumor formation. No sign of cell migration or tumor formation was observed in any of the nonimplanted eyes or systemic organs evaluated, based on H&E staining.
Discussion
Because the eye is an immunologically privileged site, both allogeneic and xenogeneic intraocular grafts can potentially enjoy a prolonged survival when compared with similar grafts implanted into other body sites.2 However, ocular immune privilege is not absolute, and as a consequence, immunologic recognition of transplanted tissues can result in rejection of this graft.14 In this study, immunohistochemical assessment with anti-TRA-1-85 and anti-RPE65 confirmed hESC-RPE survival at all time points for both injection of cell suspension and implantation of membranes with cells. Even though the suspension had 105 cells versus 2700 cells on substrate, the cells implanted as a suspension survived to a lesser extent and often developed into cell aggregates or clumps. In a recent clinical trial, hESC-RPE suspension was injected subretinally in two humans and, despite good intermediate safety (no teratoma formation), no confluent RPE monolayer could be observed clinically to prove sustainable efficacy. These investigators, however, reported seeing signs of RPE clumping in one of their patients.7 In our study, improved survival of hESC-RPE was observed when the cells were implanted as a polarized monolayer on a parylene membrane. This suggests that polarization of the transplanted RPE cells and the monolayer morphology might play an important role in the fate of these cells after implantation into the rats' subretinal space. But this conclusion might need further confirmation, as after the cell injection, some reflux was observed from the scleral incision, and the number of cells that effectively remained in the subretinal space is not precisely known. This quantitative factor might have contributed to the lower survival rate.
Normal RPE cells display a polarized morphology with apical microvilli between the rods and cones and with infoldings at the basal side. The RPE cells are connected to each other by tight junctions; as a result, the RPE monolayer is impermeable to macromolecules.1 Any substrate used must meet all the following conditions: First, it must support reasonable differentiation of the RPE monolayer; second, it must be compatible with the host immune and visual system; and finally, it must be surgically easy to transplant.15 Parylene C meets all of these requirements, hence is promising for consideration as an artificial BM. This biocompatible polymer has found numerous biomedical applications and is stable in the subretinal space of rabbits and pigs.16,17 Parylene C, with a thickness of 0.15 to 0.30 μm, has a permeability similar to that of human BM.12,18 In vitro cell culture on the parylene substrate shows that hESC-RPE are able to adhere, proliferate, and form epithelial monolayers with tight intracellular junctions and to become well polarized with microvilli, which exhibit characteristics similar to those of RPE cells in vivo.12 Hence it may be argued that implantation of hESC-RPE on a substrate is advantageous compared to delivery of dissociated hESC-RPE. On the other hand, the injection of RPE cell suspension in the subretinal space can be more easily performed with less trauma and no biocompatibility concerns.
Increased cell reaction at the implant area observed at 1 month when a substrate was used can be explained by the larger choroidal incision compared to that with the cell injection procedure. It is important to keep in mind that the exposure of the choroid and breakdown in the blood–ocular barrier (leading to invasion of macrophages and other innate response cells) that occur after the surgical procedure employed in this study is different than the pars plana approach to be used in humans.19 The ab externo surgical approach used in this animal model did lead to damaging some of the transplanted RPE cells and was associated with the increased melanin granules that were observed extracellularly and inside large macrophages in both surgical groups. This pigment migration was restricted to the adjacent neuroretina; that is, no migration to the optic disc, periocular structures, and systemic organs was observed. Reattachment of the retina in rats takes approximately 2 days after the induced detachment.20 The time for reattachment is likely to be an important parameter in the recovery of retinal structure and function and is likely responsible for the permanent structural and functional losses observed in rats and already reported by other authors.21 Some level of damage to the neuroretina above the implanted area was observed in almost all the eyes examined, but not in the adjacent retina; therefore, this loss of the ONL cannot be explained only as detachment consequences. Such loss was seen in animals that received the cell suspension or cells on substrate. Although this loss, evaluated by qualitative analysis, seemed to be similar in the two groups, a quantitative evaluation with cell count has to be done to confirm the results.
One possibility is that transplanted cells in an otherwise healthy eye lead to some level of trauma that in turn leads to damage to the overlying neural retina. For example, the normal outer limiting membrane present in these eyes may act as a barrier, and less photoreceptor–RPE connectivity is expected after the transplant.22,23 In contrast, RPE transplantation in animal models of retinal degeneration rescues photoreceptors and preserves visual acuity.24,25 Additional experiments using the Royal College of Surgeons rat, an animal model of RPE dystrophy, are being performed also to determine the rejection potential to hESC-RPE xenografts, and whether this transplant will also damage the ONL or whether it can provide any rescue effect of the photoreceptors (Thomas B, et al. IOVS 2012;53:ARVO E-Abstract 313).
Although this therapeutic approach holds great promise, possible side effects such as tumor formation need to be considered. Pluripotent stem cells can, in principle, differentiate into all cell types of the human body. However, pluripotency and tumorigenicity are closely linked. In fact, the ability to form teratomas is one of the most rigorous criteria for defining pluripotency.26 Teratomas are tumors that contain tissues of ectodermal, mesodermal, and endodermal origin; thus the teratoma assay is a relatively easy tool to use to demonstrate the ability of stem cells to differentiate into tissues derived from all three embryonic germinal layers.27,28 Importantly, teratomas and teratocarcinomas are not the only tumors that can form out of stem cell–derived grafts. Transplantation of grafts containing differentiated stem cells might lead to growth of tumors that are more restricted to their tissue compostion10,29,30; however, this was not evident in AMD and Stargardt's dystrophy in a first clinical pilot trial with hESC-RPE.7
The stem cells used in the proposed therapy had to be differentiated into RPE cells. It is therefore important to guarantee that all the cells are no longer pluripotent and are well differentiated toward the RPE lineage. Despite efforts to remove tumorigenic cells from grafts by prolonged differentiation and cell sorting, all grafts derived from pluripotent stem cells are considered at risk of containing tumor-forming cells.10,31 In this study, the hESC-RPE cells were well differentiated and did not form tumors/teratomas for the 12-month period of observation. Aggregates of pigmented cells were found, mainly 1 month after cell suspension injection; but those cells did not exhibit positive staining for a cell proliferation/division marker.
Tumorigenicity also depends on the inability of the recipient to reject the tumorigenic cells.10 Athymic nude rats lack a normal thymus and functionally mature T cells.32 They have thus been useful in the study of mechanisms of tumor growth or graft rejection in immunocompromised hosts, since they can accept organ allografts or xenografts for several months.19,33 However, other leukocytes in nude rats, such as macrophages, natural killer cells, and B lymphocytes, may contribute to the innate immune response; they also may play a role in the rejection of the transplanted cells and, importantly, may kill tumor cells.34 Some investigators reported spontaneous regression of human cancers transplanted subcutaneously in nude rats,35,36 whereas others report high take rates for several human tumors.37 There seems to be a correlation between the age of the nude rats and tumor metastasis; higher metastasis rates were observed in 4-week-old rats than in 6- to 10-week-old animals.38 In the present study, no apparent tumor formation was observed even at early time points after transplantation. Although the nude rat is not a perfect animal model for evaluating tumor formation, the implanted cells survived for at least 12 months, providing a chance for tumor formation. Rats were chosen due to their larger eyes, improving the surgical success and visualization of the implants in comparison to mice. The absence of tumor in an immunodeficient animal model supports the hypothesis that the risk of tumor formation will be very low in an immunocompetent recipient.
Although animal studies support the use of stem cells in human RPE cell transplantation, there are many important differences between humans with AMD and laboratory animals, and these differences may have a significant effect on the RPE graft survival in humans. Such differences include but are not limited to age differences, AMD-related modifications of Bruch's membrane, the possible presence of neovascular membranes, and RPE detachments. In summary, we have found it possible to transplant hESC-RPE in the subretinal space of athymic rats and achieve long-term survival and differentiation and, more importantly, avoid tumor formation. Moreover, the polarized monolayer of hESC-RPE demonstrated improved survival compared to hESC-RPE cell suspensions.
Acknowledgments
Supported by the California Institute of Regenerative Medicine (DR1-01444), Research to Prevent Blindness, National Eye Institute Core Grant EY03040, and CAPES Foundation (BEX 2326-11-6 [BD]).
Disclosure: B. Diniz, None; P. Thomas, None; B. Thomas, None; R. Ribeiro, None; Y. Hu, None; R. Brant, None; A. Ahuja, None; D. Zhu, None; L. Liu, None; M. Koss, None; M. Maia, None; G. Chader, None; D.R. Hinton, P; M.S. Humayun, P
References
- 1. Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001; 20: 29–48 [DOI] [PubMed] [Google Scholar]
- 2. Taylor AW. Ocular immune privilege. Eye (Lond). 2009; 23: 1885–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tezel TH, Del Priore LV, Berger AS, Kaplan HJ. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. 2007; 143: 584–595 [DOI] [PubMed] [Google Scholar]
- 4. Mruthyunjaya P, Stinnett SS, Toth CA. Impact of fluorescein angiographic characteristics of macular lesions on outcomes after macular translocation 360 degree surgery in eyes with age-related macular degeneration. Retina. 2005; 25: 597–607 [DOI] [PubMed] [Google Scholar]
- 5. Joussen AM, Heussen FM, Joeres S, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006; 142: 17–30 [DOI] [PubMed] [Google Scholar]
- 6. Sugino IK, Sun Q, Wang J, et al. Comparison of FRPE and human embryonic stem cell-derived RPE behavior on aged human Bruch's membrane. Invest Ophthalmol Vis Sci. 2011; 52: 4979–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012; 379: 713–720 [DOI] [PubMed] [Google Scholar]
- 8. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009; 27: 2126–2135 [DOI] [PubMed] [Google Scholar]
- 9. Utermohlen O, Baschuk N, Abdullah Z, et al. Immunologic hurdles of therapeutic stem cell transplantation. Biol Chem. 2009; 390: 977–983 [DOI] [PubMed] [Google Scholar]
- 10. Dressel R. Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Semin Immunopathol. 2011; 33: 573–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011; 52: 1573–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu B, Zhu D, Hinton D, Humayun MS, Tai YC. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices. 2012; 14: 659–667 [DOI] [PubMed] [Google Scholar]
- 13. Hu Y, Liu L, Lu B, et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res. 2012; 48: 186–191 [DOI] [PubMed] [Google Scholar]
- 14. Grisanti S, Szurman P, Jordan J, Kociok N, Bartz-Schmidt KU, Heimann K. Xenotransplantation of retinal pigment epithelial cells into RCS rats. Jpn J Ophthalmol. 2002; 46: 36–44 [DOI] [PubMed] [Google Scholar]
- 15. da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007; 26: 598–635 [DOI] [PubMed] [Google Scholar]
- 16. Yu W, Wang X, Zhao C, Yang Z, Dai R, Dong F. Biocompatibility of subretinal parylene-based Ti/Pt microelectrode array in rabbit for further artificial vision studies. J Ocul Biol Dis Infor. 2009; 2: 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montezuma SR, Loewenstein J, Scholz C, Rizzo JF III. Biocompatibility of materials implanted into the subretinal space of Yucatan pigs. Invest Ophthalmol Vis Sci. 2006; 47: 3514–3522 [DOI] [PubMed] [Google Scholar]
- 18. Hussain AA, Starita C, Hodgetts A, Marshall J. Macromolecular diffusion characteristics of ageing human Bruch's membrane: implications for age-related macular degeneration (AMD). Exp Eye Res. 2010; 90: 703–710 [DOI] [PubMed] [Google Scholar]
- 19. Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002; 75: 115–125 [DOI] [PubMed] [Google Scholar]
- 20. Timmers AM, Zhang H, Squitieri A, Gonzalez-Pola C. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol Vis. 2001; 7: 131–137 [PubMed] [Google Scholar]
- 21. Engelhardt M, Tosha C, Lopes VS, et al. Functional and morphological analysis of the subretinal injection of retinal pigment epithelium cells. Vis Neurosci. 2012; 29: 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998; 39: 2121–2131 [PubMed] [Google Scholar]
- 23. Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retina with retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999; 40: 1557–1564 [PubMed] [Google Scholar]
- 24. Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006; 8: 189–199 [DOI] [PubMed] [Google Scholar]
- 25. Coffey PJ, Girman S, Wang SM, et al. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. 2002; 5: 53–56 [DOI] [PubMed] [Google Scholar]
- 26. Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007; 21: 1345–1357 [DOI] [PubMed] [Google Scholar]
- 27. Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009; 8: 3822–3830 [DOI] [PubMed] [Google Scholar]
- 28. Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem. 2010; 111: 769–781 [DOI] [PubMed] [Google Scholar]
- 29. Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006; 203: 2315–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008; 22: 152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anisimov SV, Morizane A, Correia AS. Risks and mechanisms of oncological disease following stem cell transplantation. Stem Cell Rev. 2010; 6: 411–424 [DOI] [PubMed] [Google Scholar]
- 32. Rolstad B. The athymic nude rat: an animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev. 2001; 184: 136–144 [DOI] [PubMed] [Google Scholar]
- 33. Aramant RB, Seiler MJ. Human embryonic retinal cell transplants in athymic immunodeficient rat hosts. Cell Transplant. 1994; 3: 461–474 [DOI] [PubMed] [Google Scholar]
- 34. Preynat-Seauve O, de Rham C, Tirefort D, Ferrari-Lacraz S, Krause KH, Villard J. Neural progenitors derived from human embryonic stem cells are targeted by allogeneic T and natural killer cells. J Cell Mol Med. 2009; 13: 3556–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dawson PJ, Kluskens LF, Colston J, Fieldsteel AH. Transplantation of human malignant tumors to the athymic rat. Cancer. 1982; 50: 1151–1154 [DOI] [PubMed] [Google Scholar]
- 36. Stragand JJ, Drewinko B, Henderson SD, et al. Growth characteristics of human colonic adenocarcinomas propagated in the Rowett athymic rat. Cancer Res. 1982; 42: 3111–3115 [PubMed] [Google Scholar]
- 37. Kjonniksen I, Nesland JM, Pihl A, Fodstad O. Nude rat model for studying metastasis of human tumor cells to bone and bone marrow. J Natl Cancer Inst. 1990; 82: 408–412 [DOI] [PubMed] [Google Scholar]
- 38. Kjonniksen I, Storeng R, Pihl A, McLemore TL, Fodstad O. A human tumor lung metastasis model in athymic nude rats. Cancer Res. 1989; 49: 5148–5152 [PubMed] [Google Scholar]