Abstract
Purpose.
Activation of SIRT1 deacetylase prevents retinal ganglion cell (RGC) loss in experimental optic neuritis, an inflammatory optic neuropathy. While mechanisms of this effect are not known, evidence suggests it involves reduction of oxidative stress. We hypothesized that SIRT1 reduces RGC loss due to oxidative stress in noninflammatory optic neuropathies, and examined effects following traumatic injury.
Methods.
Optic nerve crush injury was induced in wild-type C57BL/6 mice, mice overexpressing SIRT1, and mice with conditional deletion of SIRT1 in neurons. Wild-type mice were treated daily with vehicle or 250 mg/kg resveratrol, a naturally occurring polyphenol that activates SIRT1. RGC function was assessed by pupillometry and optokinetic responses (OKR), and RGC survival was measured. Superoxide levels were measured to assess oxidative stress.
Results.
Significant decreases in pupillary light responses, OKR and RGC survival occurred 1 week after optic nerve crush, with progressive worsening at 2 to 4 weeks. Resveratrol treatment and SIRT1 overexpression delayed RGC loss and loss of pupillary light responses following optic nerve crush, although no change in RGC loss occurred in neuronal SIRT1-deficient mice. A significant accumulation of superoxide was detected in wild-type optic nerves following crush, and was reduced in mice overexpressing SIRT1 or treated with resveratrol.
Conclusions.
SIRT1 delays RGC loss following traumatic injury. Effects are associated with reduced oxidative stress. Results suggest SIRT1-activating drugs may have a specific role in preventing traumatic optic nerve damage, and suggest a broader role for this strategy in treating a variety of optic neuropathies that may include a component of oxidative stress.
Keywords: traumatic optic neuropathy, retinal ganglion cells, neuroprotection, resveratrol, SIRT1, oxidative stress
Resveratrol prevents RGC loss and preserves function following traumatic injury. Effects are mediated by SIRT1 and involve reduction of oxidative stress, suggesting a potential role in treating other optic neuropathies.
Introduction
SIRT1 is an NAD+-dependent deacetylase involved in cell stress responses and cell survival.1 We previously showed three distinct SIRT1-activating compounds—including the naturally occurring polyphenol resveratrol2—attenuate retinal ganglion cell (RGC) loss induced by experimental optic neuritis,3–5 an inflammatory demyelinating disease of the optic nerve that often occurs in multiple sclerosis patients.6 The mechanism of this neuroprotection is not fully understood, but results show the mechanism does not involve suppression of inflammation,3–5 suggesting other pathways must be involved.
Oxidative stress, marked by accumulation of damaging reactive oxygen species (ROS), is a common mechanism of neuronal damage in numerous disease models,7,8 including experimental optic neuritis.9 In vitro studies of neuronal cells, including primary RGC cultures, demonstrate that resveratrol and other SIRT1-activating compounds can prevent cell loss induced by oxidative stress.10 Together, results suggest that resveratrol may act downstream of inflammation-induced neuronal injury, by specifically attenuating accumulation of ROS, to prevent RGC loss from experimental optic neuritis. Furthermore, this suggests that resveratrol may be capable of providing neuroprotective effects in other optic neuropathies by promoting activation of SIRT1.
Experimentally induced optic nerve crush injury is an established model of traumatic optic nerve injury that results in significant axonal degeneration and loss of RGCs, and has been recognized for more than a decade to involve at least some level of oxidative stress.11–13 In the current studies, the ability of resveratrol to prevent RGC loss and preserve RGC function is examined following optic nerve crush. The role of SIRT1 in promoting RGC survival is further assessed by optic nerve crush in mice that overexpress SIRT114 and mice with a conditional deletion of SIRT1 in neurons.15
Methods
Mice
For resveratrol treatment studies, 7- to 8-week-old C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). SIRT1-overexpressing mice (SIRT1 KI) containing SIRT1 cDNA knocked into the beta-actin locus14 (Jackson Laboratory stock #013080) in a C57BL/6J background, and conditional neuronal SIRT1-knockout mice (SIRT1 KO) containing floxed SIRT1 and Cre recombinase expression driven by the nestin promoter in a C57BL/6J background15 were provided by Leonard Guarente, PhD (Massachusetts Institute of Technology, Cambridge, MA). Mice were bred as heterozygotes and genotyped by PCR using published methods and primers,14,15 with wild-type littermates used as controls. SIRT1-KI homozygotes are embryonic lethal, thus heterozygotes were used for optic nerve crush experiments, whereas both homozygous and heterozygous neuronal SIRT1-KO mice were used. Treatment of animals housed at the University of the Pennsylvania conformed to Institutional Animal Care and Use Committee guidelines and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Optic Nerve Crush
The optic nerve crush procedure used was adapted from published methods.11–13 Mice were anesthetized with ketamine and xylazine, and eyes were further anesthetized with one drop of 0.5% proparacaine. The conjunctiva was lifted with fine forceps and cut with Vannas scissors to expose the underlying sclera. Blunt tip forceps were used to retract orbital fat and muscle, gently rotating the globe to expose the optic nerve. Injury was induced by crushing the optic nerve 1 to 2 mm behind the globe with the blunt tip of self-closing forceps for 10 seconds. Care was taken to avoid orbital blood vessels in order not to induce ocular ischemia. Mice were excluded from experiments if any vessels were compromised and more than minimal bleeding occurred. For each experiment, optic nerve crush was performed on one eye, with the contralateral eye serving as control. The procedure was performed by an investigator blinded to the mouse genotype.
RGC Labeling and Quantification
RGCs were retrogradely labeled with 2.5 μL of 1.25% hydroxystilbamidine (Fluorogold; Molecular Probes, Eugene, OR) in sterile water injected in the superior colliculi 1 week prior to optic nerve crush as described in previous studies.16 To quantify RGC survival, eyes were fixed in 4% paraformaldehyde, retinas were removed and whole-mounted on glass slides; and RGCs were viewed by fluorescent microscopy, photographed in 12 standardized fields covering a total retinal area of 0.74 mm2, and counted by a blinded investigator as described previously.16
RGC Function
Optokinetic responses (OKR) were measured using visuomotor software and apparatus (OptoMotry; Cerebral Mechanics, Inc., Lethbride, AB, Canada) to estimate visual acuity, as in prior studies.17,18 OKR function is determined by the highest spatial frequency at which mice track a 100% contrast grating projected at varying spatial frequencies and data is reported as cyc/deg. Pupillary light responses were measured using a Neuroptics Pupillometer (San Clemente, CA) for rodents as described previously.19 A series of five 4.7 μW/cm2 light pulses, with a 100-ms duration of stimulus and a relaxation time of 9900 ms, was used to stimulate each eye, and the median percent constriction (change in pupil area following stimulus) of each pupil was used for statistical comparisons. This stimulus provided maximal detection of dysfunction as compared with controls in prior optic neuritis studies.19 Mice were dark-adapted overnight prior to pupillometry.
MitoSOX Staining
MitoSOX Red (Invitrogen, Grand Island, NY) mitochondrial superoxide indicator is a fluorogenic dye for selective detection of superoxide in the mitochondria of live cells. MitoSOX Red staining of optic nerves was performed as described previously.10 Briefly, optic nerves isolated at the time of killing were washed with PBS and incubated for 30 minutes at 37°C in 5 μM MitoSOX Red. Nerves were then washed, fixed with 4% paraformaldehyde, cryosectioned into 5 μM cross-sections, and viewed by fluorescent microscopy. One photograph centered within each optic nerve was taken at ×20 magnification by a blinded investigator. The optical density was calculated using Java-based image processing software (Image J; National Institutes of Health [NIH], Bethesda, MD) to quantify the level of staining.
Resveratrol Treatment
Resveratrol was purchased from Sigma-Aldrich (St. Louis, MO) and suspended in PBS. Mice were treated by oral gavage with 250 mg/kg resveratrol or PBS alone for controls, beginning on the day of optic nerve crush and repeated once daily until killed. The resveratrol dose used is the dose found previously to prevent RGC loss during experimental optic neuritis.5
Western Blot
Tissue was removed, placed in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% desoxycholic acid, 0.1% SDS, and 50 mM Tris, pH 8), and ultrasonicated on ice 5 × 5 seconds to obtain total protein extracts. Cell lysates were then centrifuged at 14,000g for 10 minutes at 4°C, and the protein concentration of the supernatant was determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA) according to manufacturer's instructions. Proteins were separated by electrophoresis on 10% polyacrylamide gels, 20 μg per lane, and transferred to nitrocellulose high-bound enhanced chemiluminescence membranes (GE Healthcare Biosciences, Pittsburgh, PA). The membrane was blocked with Odyssey Blocking Buffer (LI-COR Biosciences–Biotechnology, Lincoln, NE) for 1 hour at room temperature (RT) and probed overnight at 4°C with rabbit polyclonal antibodies against SIRT1 (1:1500; Abcam, Cambridge, MA). After washing with PBS, membranes were incubated for 1 hour at room temperature with infrared goat anti-rabbit IgG dye (IRDye 800CW; LI-COR Biosciences–Biotechnology) diluted 1:5000. Fluorescence was visualized using an infrared imaging system (Odyssey; LI-COR Biosciences–Biotechnology). Blotted membranes were stained for β-actin (Sigma-Aldrich) to normalize protein levels and the intensity of each band was determined using Java-based imaging software (NIH).
Statistics
Data are expressed as means ± SEM. Differences in RGC numbers, OKR responses, pupillary constriction, and MitoSOX staining were compared using one-way ANOVA followed by Tukey's multiple comparison test using commercial graphing software (GraphPad Prism 5.0; GraphPad Software, San Diego, CA). Differences were considered statistically significant at P < 0.05.
Results
Resveratrol Reduces RGC Loss Following Optic Nerve Crush
RGCs were retrogradely-labeled by injection of Fluorogold in the superior colliculi of adult (8–12-week-old) wild-type C57BL/6J mice. The left optic nerve was surgically crushed with blunt forceps 1 week later. Right eyes served as noncrushed controls. Mice were treated daily with 250 mg/kg resveratrol suspended in PBS, or with vehicle (PBS) only, by oral gavage beginning on the day of optic nerve crush and repeated until killed at 2 or 4 weeks. RGC numbers counted in retinal whole mounts from each eye demonstrate progressive loss of RGCs occurs from 2 to 4 weeks following optic nerve crush, with RGC numbers significantly decreased compared with control eyes at both time points (Fig. 1). RGC survival was significantly higher in eyes that underwent optic nerve crush in mice treated with resveratrol, as compared with optic nerve crush eyes from mice treated with vehicle alone, at both 2 and 4 weeks post–optic nerve crush (Fig. 1).
Figure 1.

Resveratrol reduces RGC loss after optic nerve crush. The number of Fluorogold-labeled RGCs were counted in 12 standardized fields of whole-mounted retinas from control eyes (total area of 0.74 mm2/retina), and from eyes that underwent optic nerve crush in mice treated daily by oral gavage with 250 mg/kg resveratrol or with vehicle alone. Two weeks post–optic nerve crush, significant RGC loss occurred in vehicle-treated mice as compared with control eyes (***P < 0.001), and resveratrol treatment lead to significantly higher RGC numbers (**P < 0.01). Four weeks post–optic nerve crush, RGC numbers were further reduced, with significant loss compared with controls in both vehicle- and resveratrol-treated mice (***P < 0.001), but RGC survival remained significantly higher in resveratrol-treated versus vehicle-treated optic nerve crush eyes (P < 0.05). Data represent mean ± SEM.
Retinal SIRT1 Expression Is Altered in SIRT1-KI and SIRT1-KO Mice
Despite expression driven by the β-actin locus, SIRT1 is not overexpressed in all tissues in the SIRT1-KI mice. Prior studies demonstrated variable levels of overexpression in adipose cells, brain and embryonic fibroblasts, but none in liver or muscle.14 Expression in the eye was not reported. We therefore examined SIRT1 expression in the retina by Western blot of protein extracts from wild-type and SIRT1-KI mice. SIRT1 expression is significantly higher in SIRT1-KI mouse retina (Figs. 2A, 2B).
Figure 2.
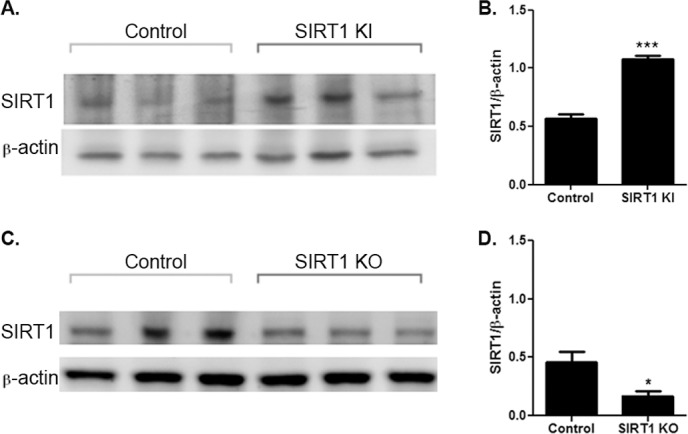
SIRT1-KI and SIRT1-KO mice have altered SIRT1 expression in retina. (A) Western blot of protein extracts from retinas of SIRT1-KI mice and wild-type littermates. Blots of three representative eyes are shown. (B) SIRT1 expression, normalized as a ratio to β–actin levels, is significantly increased in SIRT1-KI retina (***P < 0.001). (C) Western blot of protein extracts from retinas of homozygous SIRT1-KO mice and wild-type littermates. Blots of three representative eyes are shown. (D) SIRT1 expression, normalized as a ratio to β–actin levels, is significantly decreased in SIRT1-KO retina (*P < 0.05).
Similarly, while neuronal SIRT1-KO mice have been shown to have to selective deletion of SIRT1 in brain, and specifically hypothalamus without loss of SIRT1 in the pituitary gland or nonneuronal peripheral tissues,15 expression in the eye was not reported. We therefore examined SIRT1 expression in the retina by Western blot of protein extracts from homozygous SIRT1-KO mice and wild-type littermates. SIRT1 expression is decreased in retinas from SIRT1-KO mice (Figs. 2C, 2D). As expected, SIRT1 expression is not completely eliminated in these neuron-specific knockout mice, as other retinal cell types continue to express SIRT1.
SIRT1-Mediated Mechanism of RGC Neuroprotection Following Optic Nerve Crush
Resveratrol demonstrates protective effects in a variety of tissues, both by direct intracellular effects, and by effects secondary to its ability to activate SIRT1.20–24 To examine whether RGC neuroprotection by resveratrol following optic nerve crush (Fig. 1) may be mediated by SIRT1, optic nerve crush was repeat in SIRT1-KI and SIRT1-KO mice and RGC survival was measured. One week post–optic nerve crush (Fig. 3A), significant RGC loss was observed in wild-type mice as compared with control eyes. In contrast, SIRT1-KI mouse eyes had significantly higher RGC numbers than wild-type mouse eyes 1 week following optic nerve crush. Interestingly, neither heterozygous nor homozygous neuronal SIRT1-KO mouse eyes showed any difference in RGC numbers compared with wild-type mouse eyes following optic nerve crush (Fig. 3A). Importantly, daily treatment with 250 mg/kg resveratrol failed to prevent RGC loss in homozygous SIRT1-KO mouse eyes that underwent optic nerve crush, confirming the importance of SIRT1 expression in neurons for resveratrol-mediated neuroprotection.
Figure 3.
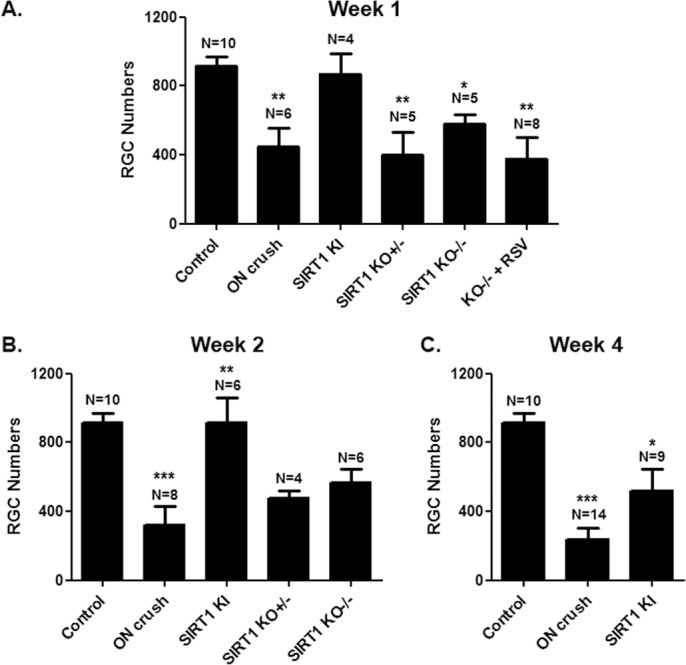
SIRT1 overexpression prevents RGC loss. (A) Mean ± SEM number of RGCs is significantly decreased 1 week post–optic nerve crush as compared with control eyes (**P < 0.01). No loss of RGCs compared with controls occurs 1 week post–optic nerve crush in SIRT1-KI mice, with RGCs numbers significantly higher than eyes from wild-type (**P < 0.01); heterozygous SIRT1-KO (**P < 0.01); or homozygous SIRT1-KO (*P < 0.05) mouse eyes that underwent optic nerve crush. There is no difference in RGC numbers between wild-type and SIRT1-KO optic nerve crush eyes. Daily treatment with 250 mg/kg resveratrol (RSV) failed to prevent RGC loss following optic nerve crush in homozygous SIRT1-KO mice, with RGC numbers decreased compared with controls (**P < 0.01). (B) Two weeks post–optic nerve crush, the number of RGCs is significantly decreased as compared with control eyes (***P < 0.001). No loss of RGCs compared with controls occurs post–optic nerve crush in SIRT1-KI mice, with RGC numbers significantly higher than eyes from wild-type, heterozygous SIRT1-KO, or homozygous SIRT1-KO (**P < 0.01) mouse eyes that underwent optic nerve crush. There is no difference in RGC numbers between wild-type and SIRT1-KO optic nerve crush eyes. (C) Four weeks post–optic nerve crush, RGC numbers are significantly decreased compared with control eyes (***P < 0.001) and with SIRT1-KI optic nerve crush eyes (*P < 0.05).
RGC survival was further assessed at 2 and 4 weeks post–optic nerve crush. Two weeks after crush, retinas from SIRT1-KI mice continue to demonstrate significant preservation of RGCs, with levels equivalent to control, non–optic nerve crush eyes, while SIRT1-KO mouse eyes again show RGC loss at levels equivalent to wild-type mouse eyes that underwent optic nerve crush (Fig. 3B). By 4 weeks post–optic nerve crush, RGC survival remains significantly higher in SIRT1-KI mice as compared with wild-type mouse eyes, although RGC numbers show a trend toward decrease compared with control eyes (Fig. 3C).
Resveratrol and SIRT1 Overexpression Reduce Loss of RGC Function After Optic Nerve Crush
OKR and pupillary light responses were assessed to determine whether RGCs that are preserved following optic nerve crush in mice treated with resveratrol, or mice overexpressing SIRT1, retain any function. OKR and pupillometry were performed at baseline (prior to optic nerve crush) and repeated at 1, 2, and 4 weeks post–optic nerve crush. Wild-type mice exhibit a progressive, significant reduction in pupillary constriction in response to light beginning 1 week post–optic nerve crush, as compared with control eyes not undergoing optic nerve crush (Fig. 4A). Eyes that underwent optic nerve crush in both SIRT1-KI mice, and wild-type mice treated daily with 250 mg/kg resveratrol, exhibited significantly better pupillary light responses at 2 and 4 weeks post–optic nerve crush compared with untreated mice (Fig. 4A), whereas pupillary light responses following optic nerve crush decreased in SIRT1-KO mouse eyes as severely as in wild-type mice. Similar to pupillary light responses, eyes of wild-type mice and SIRT1-KO mice had decreased OKR responses following optic nerve crush (Fig. 4B). However, SIRT1-KI mice and mice treated with resveratrol showed only a nonsignificant trend toward improved OKR responses compared with vehicle-treated wild-type optic nerve crush eyes (Fig. 4B).
Figure 4.

Resveratrol and SIRT1 overexpression attenuate loss of RGC function following optic nerve crush. (A) Pupillometry showed a trend toward decreased pupillary constriction 1 week post–optic nerve crush, with significant decreases compared with control eyes at 2 weeks (**P < 0.01) and 4 weeks (***P < 0.001) post–optic nerve crush. SIRT1 overexpression prevented this decrease in pupillary light responses at 2 (*P < 0.05) and 4 (**P < 0.01) weeks; and daily oral resveratrol treatment similarly prevented decreased pupillary responses at 2 (*P < 0.05) and 4 (*P < 0.05) weeks post–optic nerve crush. In SIRT1-KO mice, pupillary light responses decreased 1 and 2 weeks post–optic nerve crush similar to wild-type optic nerve crush eyes. (B) OKR demonstrates a trend toward decreased function 1 and 2 weeks post–optic nerve crush, with or without SIRT1 overexpression, neuronal SIRT1 deletion, or resveratrol treatment. By 4 weeks post–optic nerve crush, significantly decreased OKR responses were detected in vehicle- and resveratrol-treated mice (*P < 0.05). At all time points, SIRT KI and resveratrol-treated mice showed just a trend toward better OKR responses than vehicle-treated mice.
Resveratrol and SIRT1 Overexpression Reduce Oxidative Stress
Resveratrol and other SIRT1-activating compounds reduce oxidative stress in neuronal cultures and primary RGCs in vitro.10 Because oxidative stress contributes to optic nerve degeneration following crush injury,11–13 we examined whether the mechanism of resveratrol and SIRT1 overexpression mediated RGC neuroprotection following optic nerve crush could involve prevention of ROS accumulation, a marker of oxidative stress. The presence of the ROS superoxide was detected by MitoSOX Red staining of optic nerves from control eyes, as well as vehicle-treated wild-type, resveratrol-treated wild-type, and SIRT-KI eyes 1 week post–optic nerve crush. MitoSOX staining was significantly higher in vehicle-treated optic nerve crush eyes (Fig. 5). Optic nerves from SIRT1-KI and resveratrol-treated mice showed a trend toward increased ROS staining compared with controls, but significantly less than vehicle-treated optic nerve crush mice.
Figure 5.
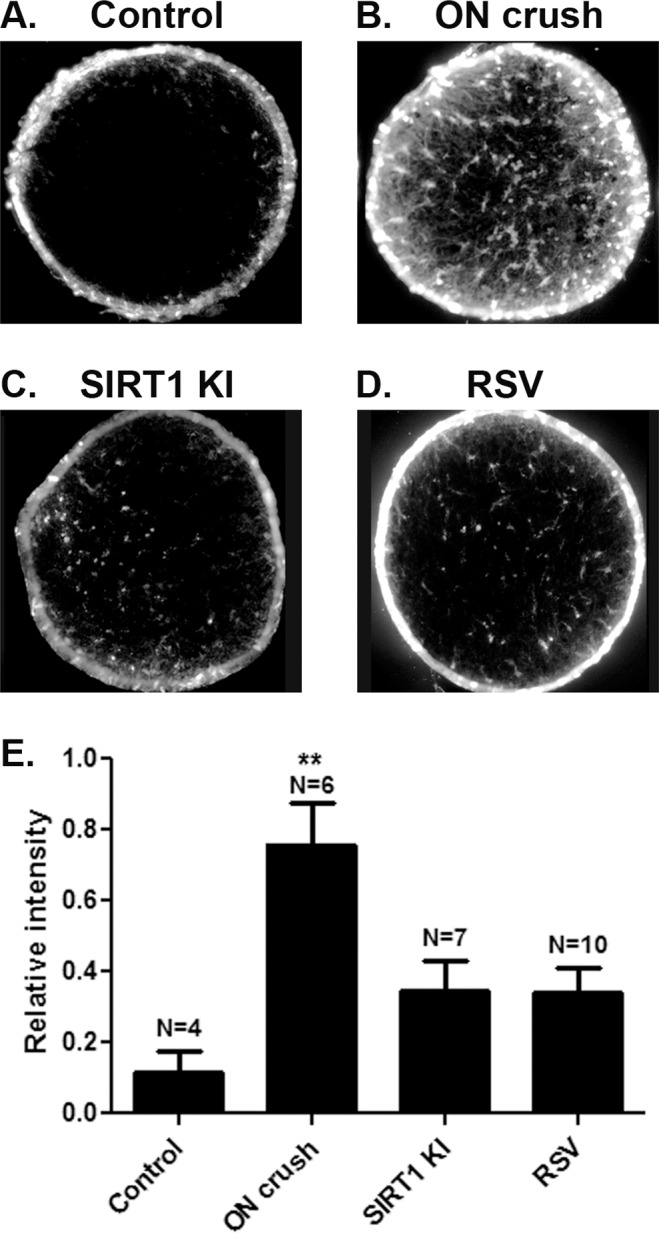
Resveratrol and SIRT1 overexpression prevent accumulation of superoxide in optic nerves. The ROS superoxide was detected by MitoSOX Red staining of optic nerve cross-sections. Representative sections from (A) control, (B) vehicle-treated optic nerve crush, (C) SIRT1-KI, and (D) resveratrol-treated mouse eyes demonstrate high levels of staining following optic nerve crush that is reduced by SIRT1 overexpression and resveratrol treatment. (E) Quantification of the intensity of staining demonstrates significantly higher ROS levels in vehicle-treated optic nerve crush eyes compared with control and with resveratrol-treated and SIRT1-KI optic nerve crush eyes (**P < 0.01). A trend toward increased ROS staining in resveratrol-treated and SIRT1-KI optic nerve crush eyes compared with controls was not significant.
Discussion
Results demonstrate that oral resveratrol treatment reduces RGC loss and preserves pupillary light responses following optic nerve crush injury. Effects are similar to the neuroprotection of RGCs observed previously during experimental optic neuritis using the same dose of resveratrol,5 suggesting that the mechanism of resveratrol-mediated neuroprotection involves modulation of a common pathway leading to RGC loss as opposed to altering disease-specific pathology. Optic neuritis is an inflammatory optic neuropathy6 induced experimentally in autoimmune models of multiple sclerosis.16,18,25 Resveratrol was found previously to modulate some parameters of the immune response in these mice,26,27 mainly earlier during the disease onset, although other studies demonstrated no effect on the immune response during the time of active optic nerve inflammation,3–5 suggesting neuroprotective effects are likely independent of anti-inflammatory effects. Results in the current study, where RGC injury is induced by direct trauma, are more consistent with a nonimmunomodulatory role of resveratrol.
One likely mechanism of resveratrol-mediated neuroprotection involves activation of SIRT1.2 SIRT1 is a member of a conserved family of genes, the sirtuins, encoding NAD+-dependent deacetylases that promote increased lifespan through deacetylation of proteins involved in various cellular pathways, including stress responses, apoptosis, and axonal degeneration.28–30 Small molecule activators, including resveratrol, and inhibitors of sirtuins modulate SIRT1 activity by altering the Km for its substrates.31 The ability of sirtinol, a SIRT1 inhibitor, to block neuroprotective effects of resveratrol as well as the ability of other SIRT1-activating compounds to prevent RGC loss in experimental optic neuritis3–5 already suggested the role of SIRT1 in mediating effects of resveratrol. However, while the compounds used have a relative affinity for SIRT1, some activation or inhibition of other sirtuins can also occur. In the current studies, the ability of SIRT1 overexpression to similarly prevent RGC loss following optic nerve crush, and the inability of resveratrol to prevent RGC loss in SIRT1-KO mice, more definitively confirms that the mechanism relies specifically on SIRT1.
While SIRT1 shows a potent ability to reduce RGC loss following optic nerve crush, it is interesting to note that decreased SIRT1 expression in neuronal SIRT1-KO mice does not lead to increased RGC loss. In wild-type mice, not all RGCs die following crush injury, which led us to examine whether endogenous SIRT1 in RGCs may prevent some cells from dying. The similar levels of RGC loss seen in wild-type and SIRT1-KO mice after optic nerve crush suggest that is not the case; rather, endogenous resistance to apoptosis of a subset of RGCs occurs independent of SIRT1.
Both resveratrol treatment and SIRT1 overexpression reduce the levels of superoxide accumulating in optic nerves following crush injury. Studies showing protective effects by antioxidant mechanisms previously demonstrated a role of oxidative stress in RGC loss following axonal injury from optic nerve crush or axotomy,11,13 and ROS staining has shown prominent accumulation of ROS within the optic nerve itself, the site of injury during experimental optic neuritis as well.9 We therefore examined ROS levels specifically within the optic nerve. The ability to reduce oxidative stress suggests that resveratrol, or other therapies that increase SIRT1 activity, have the potential to prevent RGC loss in other optic neuropathies that involve oxidative stress. How increased SIRT1 expression in retina ultimately leads to reduced ROS in the optic nerve will require further examination, as specific intracellular signaling pathways in RGCs that influence ROS along their axons remain to be determined. Alternatively, SIRT1 signaling in other retinal cells, or glial cells along the optic nerve, may influence the mechanism of neuroprotection. Nonetheless, the potential of SIRT1 overexpression and SIRT1-activating compounds to modulate oxidative stress specifically in the optic nerve, at the site of axonal injury, suggests studies in other disease models where axonal injury may occur, such as glaucoma, may be warranted.
In addition to reducing RGC loss, resveratrol and SIRT1 overexpression significantly preserved at least some RGC function by maintaining pupillary light responses. OKR responses, however, only showed a trend toward improved outcomes. The reason for the different levels of preserved RGC function is not known. It is possible that this simply represents a difference in the sensitivity of the two functional tests if, for example, larger numbers of functioning RGCs are needed to drive OKR responses. Another intriguing possibility is that SIRT1 may provide relatively stronger neuroprotective effects selectively in melanopsin-expressing RGCs32 that can help drive light responses, and future studies of relative levels of SIRT1 expression and survival of melanopsin-expressing RGCs could help examine that possibility. Importantly, the preservation of some RGC function, which requires signaling to the brain, provides evidence that some RGC axonal protection is conveyed by SIRT1 as well.
The current studies demonstrate that neuroprotective effects of resveratrol for RGCs are not limited to optic neuritis, and specifically occur following traumatic nerve injury. The mechanism involves SIRT1, and is associated with reduced axonal oxidative stress. Resveratrol represents a promising neuroprotective therapy for optic neuritis and traumatic optic neuropathies, and its common mechanism of action suggests that its potential effects should be examined in other optic neuropathies as well.
Acknowledgments
Supported by National Institutes of Health Grant EY019014; Research to Prevent Blindness; and the F. M. Kirby Foundation. The authors thank Leonard Guarente (Massachusetts Institute of Technology, Cambridge, MA) for providing SIRT1-KI and neuronal SIRT1-KO mice for breeding.
Disclosure: L. Zuo, None; R.S. Khan, None; V. Lee, None; K. Dine, None; W. Wu, None; K.S. Shindler, None
References
- 1. Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005; 26: 94–103 [DOI] [PubMed] [Google Scholar]
- 2. Pallàs M, Casadesús G, Smith MA. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009; 6: 70–81 [DOI] [PubMed] [Google Scholar]
- 3. Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007; 48: 3602–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010; 30: 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonseca-Kelly Z, Nassrallah M, Uribe J, et al. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012; 3: 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005; 139: 1101–1108 [DOI] [PubMed] [Google Scholar]
- 7. Ramalingam M, Kim SJ. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J Neural Transm. 2012; 119: 891–910 [DOI] [PubMed] [Google Scholar]
- 8. Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis. 2010; 20 (suppl 2); S453–S473 [DOI] [PubMed] [Google Scholar]
- 9. Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007; 48: 681–691 [DOI] [PubMed] [Google Scholar]
- 10. Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci. 2012; 6: 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci. 2000; 41: 4169–4174 [PubMed] [Google Scholar]
- 12. Al-Abdulla NA, Martin LJ. Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus. Am J Pathol. 1998; 153: 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castagne V, Clarke PG. Axotomy-induced retinal ganglion cell death in development: its time course and its diminution by antioxidants. Proc R Soc Lond B Biol Sci. 1996; 263: 1193–1197 [DOI] [PubMed] [Google Scholar]
- 14. Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007; 6: 759–767 [DOI] [PubMed] [Google Scholar]
- 15. Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009; 23: 2812–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shindler KS, Guan Y, Ventura E, Bennett J, Rostami A. Retinal ganglion cell loss induced by acute optic neuritis in a relapsing model of multiple sclerosis. Multiple Sclerosis. 2006; 12: 526–532 [DOI] [PubMed] [Google Scholar]
- 17. Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004; 45: 4611–4616 [DOI] [PubMed] [Google Scholar]
- 18. Quinn T, Dutt M, Shindler KS. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front Neurol. 2011; 2: 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shindler KS, Revere K, Dutt M, Ying GS, Chung DC. In vivo detection of experimental optic neuritis by pupillometry. Exp Eye Res. 2012; 100: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu LM, Lassaletta AD, Robich MP, Sellke FW. Resveratrol in the prevention and treatment of coronary artery disease. Curr Atheroscler Rep. 2011; 13: 439–446 [DOI] [PubMed] [Google Scholar]
- 21. Pallàs M, Casadesús G, Smith MA. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009; 6: 70–81 [DOI] [PubMed] [Google Scholar]
- 22. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006; 127: 1109–1122 [DOI] [PubMed] [Google Scholar]
- 23. De la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005; 49: 405–430 [DOI] [PubMed] [Google Scholar]
- 24. Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res. 2003; 523-524: 145–150 [DOI] [PubMed] [Google Scholar]
- 25. Meyer R, Weissert R, Diem R, et al. Acute neuronal apoptosis in a rat model of multiple sclerosis. J Neurosci. 2001; 21: 6214–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imler TJ Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(−) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009; 9: 134–143 [DOI] [PubMed] [Google Scholar]
- 27. Singh NP, Hegde VL, Hofseth LJ, et al. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007; 72: 1508–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000; 403: 795–800 [DOI] [PubMed] [Google Scholar]
- 29. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006; 444: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006; 8: E632–E643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010; 1804: 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002; 295: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]


