Abstract
Do infants reared in poverty exhibit certain physiological traits that make them susceptible to the positive and negative features of their caregiving environment? Guided by theories of differential susceptibility and biological sensitivity to context, we evaluated whether high baseline respiratory sinus arrhythmia (RSA) operates as a susceptibility factor among infants reared in poverty (N = 73). Baseline RSA at 5 months, the quality of the attachment relationship at 17 months, and the interaction of these two factors were included in our models as predictors of problem behavior at 17 months. Consistent with theory, results showed no significant differences in problem behavior among infants with low baseline RSA; however, infants with high baseline RSA exhibited the lowest levels of problem behavior if reared in an environment that fostered security, and they exhibited the highest levels of problem behavior if reared in an environment that fostered disorganization. These results have important implications for the psychological health of infants living in poverty.
Keywords: poverty, infant development, environmental effects
Developmental psychologists have identified a discrete group of children who, despite similar biological or behavioral susceptibilities, either wither or bloom depending on the environments in which they are reared. The differential-susceptibility hypothesis (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007) and the related theory of biological sensitivity to context (Boyce & Ellis, 2005) contend that within the right rearing environments, individuals with traits that make them susceptible to environmental influences will achieve levels of adaptation that regularly exceed those of their less susceptible, presumably hardier peers (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). However, if susceptible individuals are born into environments that afford constant diets of adversity, their susceptibilities will function principally as vulnerabilities that predispose them to many of the worst outcomes possible.
Because of the provocative nature of such theorizing, the search is on for specific susceptibilities as well as for specific environments that foster or fetter such traits. Recent tests of these theories using physiological indices of susceptibility have been limited mostly to older children, which limits researchers’ ability to identify these susceptibilities earlier in life when children are presumably most responsive to experience (Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2008). In the present study, we investigated infants’ physiological susceptibilities in the context of what is arguably the most important early human environment, the primary attachment relationship.
How a baby reacts and recovers physiologically to environmental conditions is prognostic of later adjustment (Rothbart, 2011). One physiological measure that has received a great deal of attention in the regulatory literature because of its association with attentional and emotional systems is respiratory sinus arrhythmia (RSA), a noninvasive measure of vagal (parasympathetic) influences on the heart (Porges, 2007). Previous work has shown that high baseline RSA buffers older children against their parents’ marital conflicts (El-Sheikh, Harger, & Whitson, 2001) and prenatal substance exposure (Sheinkopf et al., 2007). However, such associations are much less straightforward in infancy. On the one hand, baseline RSA appears to be a particularly sensitive marker of the degree to which infants are attuned to their environment for better and for worse (Beauchaine, 2001; Propper, 2012). Infants with higher baseline RSA tend to express more negative reactivity to arm restraint, cry more in response to complex stimuli, and exhibit more pain reactivity (Fox, Schmidt, & Henderson, 2000). On the other hand, infants with higher baseline RSA exhibit greater levels of sustained visual attention, express more interest and positivity toward strangers, and smile more while playing (Fox et al., 2000). Higher baseline RSA thus may be a type of biological susceptibility that makes infants sensitive to both the affordances and perturbations of their early rearing environments (Beauchaine, 2001).
Although a genetic basis for RSA has been reported (Kupper et al., 2005), baseline RSA appears to be remarkably sensitive to early caregiving experiences (Propper, 2012). We believe that emotion-regulatory processes within the primary attachment relationship shape infants’ physiological response to and recovery from stress (Calkins & Hill, 2007; Conradt & Ablow, 2010). In the study reported here, we examined the attachment relationship as Bowlby (1969/1982) and Ainsworth, Blehar, Waters, and Wall (1978) defined it, specifically, as an index of the qualities of the early caregiving environment rather than as a measure of individual differences.
We chose to examine secure and disorganized attachment environments because they are an ecologically valid indicator of the caregiving environment in which the infant is raised that takes into account both positive and negative rearing conditions, an essential step in examining processes of susceptibility (Belsky & Pluess, 2009). Caregiving environments that foster security and disorganization also represent caregiving extremes and may interact very differently with infants’ physiological susceptibilities (Solomon & George, 2011). In the one instance, increased engagement with the environment may be adaptive, enabling some young children to take advantage of positive rearing conditions. In the other instance, increased engagement may reflect vigilance that, in the short term, protects young children from the distressing aspects of their caregiving environments (Ellis et al., 2011). However, this increased vigilance can come at a cost. Chronic exposure to stress taxes the system in the form of bodily wear and tear and increased likelihood of disease (McEwen & Stellar, 1993) and psychopathology (McEwen, 2003). Thus, children who are more physiologically attuned to a negative or harsh caregiving environment may be unable to cope with the effects of their heightened sensitivity, which would lead to poor developmental outcomes.
Beyond the immediate caregiving environment, infants’ biobehavioral development is deeply embedded in broader psychosocial contexts with known effects on long-term outcomes (Shonkoff, Boyce, & McEwen, 2009). Poverty is one such environmental circumstance that can augment the effects of early attachment conditions (Belsky & Fearon, 2002). Care-givers living in poverty often experience levels of stress that compromise their efforts to provide children with warm and supportive parenting (Repetti, Taylor, & Seeman, 2002). It is unclear whether infants’ biological characteristics will operate as added forms of susceptibility (i.e., in a manner for better and for worse) against the pernicious backdrop of poverty. There may be conditions of poverty that both trump the most secure caregiving environments and have no affordances that can interact adaptively with heightened forms of susceptibility.
Previous research has found that infants with greater negative emotionality (Pluess & Belsky, 2009), infants with specific allelic variants (Bakermans-Kranenburg & van Ijzendoorn, 2006), and children who are more physiologically reactive (Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010) are more susceptible to their rearing environments. The central aim of the current study was to extend this search for specific forms of susceptibility that operate during infancy. It is of critical importance to determine whether (a) these traits are both present and detectible early in life, when they are presumed to be most dependent on early experience for their finished form (Boyce & Ellis, 2005), and (b) whether these traits are predictive of later problems in adaptation. We tested the hypothesis that, compared with infants with lower baseline levels of RSA, infants with higher baseline levels of RSA would display low levels of problem behavior in toddlerhood if raised in environments that foster security but display high levels of problem behavior if raised in environments that foster disorganization.
Method
Participants
Participants were drawn from a prospective longitudinal study that followed 105 women at risk for parenting problems and their infants across the perinatal period. Details on enrollment and exclusion criteria are described elsewhere (Conradt & Ablow, 2010). We examined data from 95 dyads that participated in a laboratory visit when the infants were 5 months old (M = 20.99 weeks, SD = 2.55; 53 female, 42 male) and from 86 of the dyads that returned when the toddlers were 17 months old (M = 17.6 months, SD = 1.76; 48 female, 38 male). At the prenatal assessment, mothers’ mean age was 24.11 years (SD = 4.77, range = 18–38). Mothers were primarily European American (81.0%), with 2.9% African American, 5.8% Hispanic, 3.8% American Indian, 1% Asian, and 5.7% identifying themselves as “another group.” Detailed information regarding the demographics of our sample is shown in Table 1. Our analyses were limited to the 73 infants of this sample who were classified as secure and disorganized.
Table 1.
Descriptive Data for Individual Risk Items and the Cumulative Risk Score
| Risk-score item | M | SD | Range | Risk present (%) | Criterion for risk being considered present |
|---|---|---|---|---|---|
| Prematurity | — | — | — | 4.3 | Less than 37 weeks gestational age |
| Perinatal medical complications | — | — | — | 20 | Any medical complication (excluding jaundice) |
| Marital status | — | — | — | 26.7 | Single mother status at 5 or 17 months |
| Maternal education | — | — | — | 20 | Less than a college education at 17 months |
| Household income | — | — | — | 62.5 | Less than $20,000 at 5 or 17 months |
| Infant negative emotionality (Garstein & Rothbart, 2003) | 0.07 | 2.37 | −6.18–8.21 | 13.7 | Score ≥ 1 SD above the mean: (activity level + distress to limitations + fear) − (smiling + soothability) |
| Caregiver depression (Radloff, 1977) | |||||
| 5 months | 10.40 | 8.77 | 0–37 | 38.8 | CES-D score above 16 (clinical cutoff) at 5 or 17 months |
| 17 months | 12.09 | 9.38 | 0–44 | 38.8 | — |
| Family resources (Dunst & Leet, 1987) | 136.24 | 17.22 | 86–174 | 15.1 | Score ≤ 1 SD below the mean |
| Chaos (Matheny, Wachs, Ludwig, & Phillips, 1995) | 2.24 | 0.61 | 1–4 | 14.1 | Score ≥ 1 SD above the mean |
| Cumulative risk scorea | 2.06 | 1.53 | 0–6 | — | — |
Note: CES-D = Center for Epidemiologic Studies Depression Scale.
Each dyad received 1 point for each risk item for which the criterion was met.
There were no mean differences in baseline RSA and problem behavior among infants classified as avoidant and resistant and among those classified as secure and disorganized (all ps > .27). In addition, there were no significant demographic differences between dyads with complete physiological and attachment data and those with missing data or whose infants were classified as avoidant and resistant.
Procedure
Baseline RSA at 5 months
When infants were 5 months old, dyads watched a 2-min Baby Einstein video from the Baby Mozart Discovery Kit (The Baby Einstein Co., http://www.babyeinstein.com) while the infant sat on the mother’s lap. This assessment was used to examine infants’ RSA while in a relaxed state that did not involve contending with a stressful stimulus.
Infant physiological responses were collected with a 21-channel Bioamplifier (JCA-09; James Long Co., Caroga Lake, NY). Physiological channels were sampled continuously with low-pass filtering at 1000 Hz. High-pass filtering was recorded at 0.03 Hz. Epochs containing artifacts were edited manually for each channel. Editing the files included identifying outlier points relative to adjacent data and replacing them by determining the time between successive interbeat intervals (IBIs). Data files in which more than 2% to 3% of the data needed to be edited were not included in the analyses. The data were then scanned graphically using the Statistical Analysis System (Version 9.1; SAS Institute, Cary, NC), and outliers were removed. Outliers that were more than 3 standard deviations above or below the mean were removed and replaced with the mean of the episode. Infants with and without complete, usable physiological data did not differ in age or gender.
RSA was computed using respiration and IBI data according to Grossman’s peak-valley technique (Grossman, Karemaker, & Wieling, 1991). The difference between the minimum IBI during inspiration and the maximum IBI during expiration, in seconds, was used to calculate RSA. The difference was computed twice for each respiration cycle; once for each inspiration and once for each expiration. Using this method, RSA was computed without being affected by arrhythmia due to baroreceptor, thermoregulation, and tonic shifts in heart rate.1
Attachment measures at 17 months
When toddlers were 17 months old, they completed the strange-situation procedure (Ainsworth et al., 1978). This procedure consists of a series of episodes in which the mother and her infant are separated and reunited. The procedure is designed to activate the infant’s attachment system (see Ainsworth et al., 1978, for a full description). This procedure was videotaped, and the tapes were coded by E. Carlson and L. A. Sroufe at the University of Minnesota. Eight percent of infants were classified as avoidant, 67% as secure, 5% as resistant, and 20% as disorganized.
Infant temperament at 5 months
Mothers reported their infant’s temperament using the Infant Behavior Questionnaire—Revised (IBQ-R; Garstein & Rothbart, 2003) so we could determine whether baseline RSA was associated with hypothesized indices of engagement with the environment. The IBQ-R consists of 191 items on 14 scales. The IBQ has demonstrated moderate interrater reliability between caregivers (rs = .30–.71) and good internal consistency (Cronbach’s αs = .77–.90).
Cumulative risk
We developed a cumulative risk score to characterize the range of postnatal risk factors to which infants were exposed. Cumulative risk models assume that combinations of risk factors are powerful predictors of developmental outcomes (Sameroff, Seifer, Barocas, Zax, & Greenspan, 1987; Sheinkopf et al., 2007). Infants and their caregivers were assessed with a wide range of measures of development, behavior, home environment, and caregiver characteristics chosen a priori based on developmental theory (Table 1).
Problem behavior at 17 months
The Brief Infant-Toddler Social and Emotional Assessment (BITSEA; Briggs-Gowan & Carter, 2002) is a 42-item measure designed to evaluate symptoms of social and emotional problems and competence in children 1 to 3 years old. The BITSEA has demonstrated acceptable test-retest reliability (αs = .85–.87; Briggs-Gowan, Carter, Irwin, Wachtel, & Cicchetti, 2004). We used children’s total problem scores on the BITSEA, completed by mothers when their child was approximately 17 months old, to measure individual differences in toddler levels of social and emotional problems.
Results
Preliminary analyses
Relations between baseline RSA and temperament were examined to test the hypothesis that baseline RSA is reflective of both sensitivity to and engagement with environmental conditions. Baseline RSA was significantly and positively correlated with the following IBQ-R scales: Duration of Orienting, Perceptual Sensitivity, and Vocal Reactivity—these correlations provided support for our hypothesis (rs = .223–.229, ps < .05). To validate our measure of attachment security as an index of the type of environment in which the child was raised, we examined the relation between attachment security and cumulative risk. An independent-samples t test revealed that infants raised in an environment that fostered disorganization had risk indices that were significantly higher (M = 3.11, SD = 1.49) than the risk indices of infants raised in an environment that fostered security (M = 1.76, SD = 1.43), t(74) = −3.47, p < .001; this finding supports the theory that a disorganized attachment classification is reflective of an environment of greater instability during the early months of life than a secure attachment classification does.2 We then tested associations between baseline RSA and additional covariates. There were no significant associations between baseline RSA and gender (r = −.05, p = .63) or between baseline RSA and age (r = −.13, p = .20). Finally, the independence of baseline RSA and attachment classification was analyzed. A simple linear regression revealed that there were no significant differences in baseline RSA between infants raised in environments that fostered security and infants raised in environments that fostered disorganization, b = 0.10, p = .40.
Differential susceptibility analyses
Regression models were used to test the main effects of cumulative risk, infant baseline RSA, and attachment classification on problem-behavior scores at 17 months (assessed using the BITSEA). The regression models revealed no significant association between problem behavior and cumulative risk (b = 0.06, p = .58), baseline RSA (b = 0.06, p = .62), or attachment security (b = 0.10, p = .45).
Our next model tested the interaction between infant baseline RSA and attachment classification (secure vs. disorganized) to determine whether this interaction predicted problem behavior at 17 months. As Table 2 shows, cumulative risk, infant baseline RSA, and attachment classification (secure vs. disorganized) were entered in Step 1 of the linear regression model, and the interaction between infant baseline RSA (grand mean centered) and attachment classification was entered in Step 2. With all predictors entered simultaneously, neither cumulative risk, baseline RSA, nor attachment classification predicted variability in problem behavior. However, there was a significant interaction between infant baseline RSA and attachment classification, b = 0.36, p = .01, (ΔR2 = .09, p = .01).
Table 2.
Results of the Hierarchical Regression Predicting Problem Behavior at 17 Months
| Step and predictor | b | b SE | b 95% CI | β | t | p |
|---|---|---|---|---|---|---|
| Step 1: F(3, 69) = 0.30, p = .82, R2 = .01 | ||||||
| Cumulative risk | −0.13 | 0.69 | [−1.51, 1.28] | −0.03 | t(68) = −0.19 | .85 |
| Infant-attachment classification (secure vs. disorganized) | 1.84 | 2.45 | [−3.04, 6.73] | 0.10 | t(68) = 0.75 | .45 |
| Baseline RSA (natural-log-transformed) | 1.02 | 2.03 | [−3.04, 5.08] | 0.06 | t(68) = 0.50 | .62 |
| Step 2: F(4, 68) = 1.96, p = .11, R2 = .10 | ||||||
| Cumulative risk | −0.42 | 0.67 | [−11.43, 30.05] | −0.08 | t(67) = −0.62 | .54 |
| Infant-attachment classification (secure vs. disorganized) | 1.42 | 2.36 | [−3.28, 6.12] | 0.08 | t(67) = 0.60 | .55 |
| Baseline RSA (natural-log-transformed) | −1.94 | 2.25 | [−6.43, 2.56] | −0.12 | t(67) = −0.86 | .39 |
| Infant Attachment × Baseline RSA | 11.44 | 4.37 | [2.73, 20.16] | 0.36 | t(67) = 2.62 | .011 |
Note: CI = confidence interval; RSA = respiratory sinus arrhythmia.
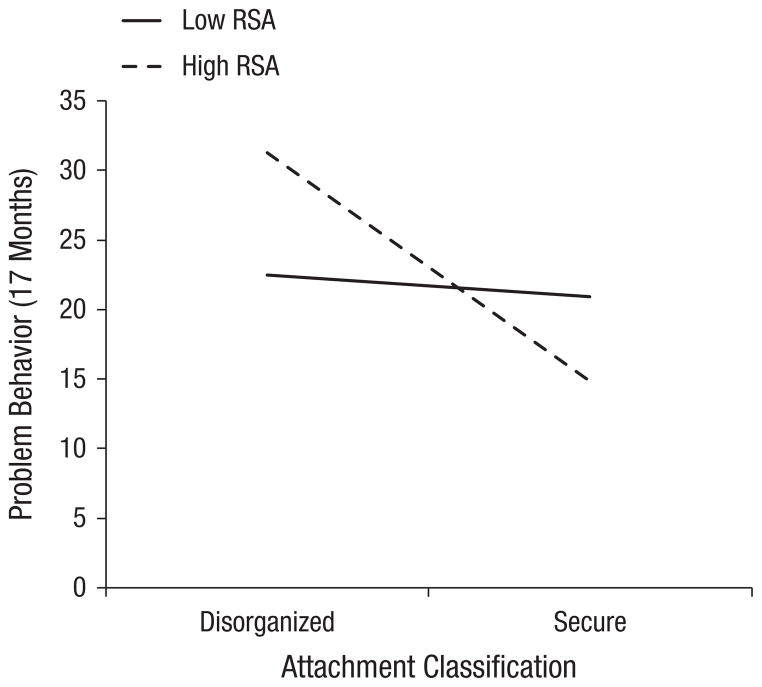
We used the online computational tools provided by Preacher, Curran, and Bauer (2006; http://www.quantpsy.org/interact/mlr2.htm) to clarify the nature of this interaction. The simple slopes of baseline RSA were calculated at 1 standard deviation above and below the mean. As Figure 1 shows, there was no significant difference in problem behavior between infants with low baseline RSA who were raised in environments that fostered either security (M = 20.89, SD = 2.93) or disorganization (M = 21.00, SD = 3.0), b = −4.40, p = .19. Of note, the group means for these infants were above the community norms presented by Briggs-Gowan and Carter (2002) for unselected community samples.
Fig. 1.
Problem behavior at 17 months as a function of attachment classification (secure vs. disorganized) and baseline level of respiratory sinus arrhythmia (RSA; low = 1 SD below the mean, high = 1 SD above the mean).
Among infants with high baseline RSA, there was a significant difference in problem behavior depending on attachment classification, b = 7.25, p = .02. Infants raised in an environment that promoted disorganization (M = 31.25, SD = 16.68) had significantly higher problem-behavior scores than infants who were raised in an environment that fostered security (M = 14.83, SD = 5.85). By contrast, infants with high RSA who were raised in an environment that fostered security had problem-behavior scores that were significantly lower as well as below the community norms of 19 for girls and 21 for boys (Briggs-Gowan & Carter, 2002) for unselected community samples. Additionally, an independent-samples t test confirmed that, among infants with a secure attachment relationship, infants with high RSA had significantly lower problem- behavior scores than did infants with low RSA, t(13) = 2.68, p = .019. Cohen’s d revealed a large effect size, d = 1.31 (95% CI = [0.25, 3.5]). Among infants raised in environments that promoted disorganization, those with high baseline RSA had the greatest problem-behavior scores relative to all others, including infants with low baseline RSA. Though this effect was large (Cohen’s d = 0.93, 95% CI = 0.46, 15.42]), it was not significant.
Discussion
The theory of differential susceptibility posits that some organisms are more susceptible than others to the positive and negative features of the environment; however, little is known about the early forms of differential susceptibility and whether these early markers of susceptibility are related to later problems in adaptation. This is the first study to identify a neuro-biological marker of susceptibility that already by 5 months of life is sensitive to experience. These data both support and extend the theories of differential susceptibility and biological sensitivity to context in several important respects.
First, we have identified a physiological marker of susceptibility that is operational at 5 months when the stress response is forming and potentially most malleable to both negative and positive environmental input (Ellis et al., 2011; Pluess & Belsky, 2011). Second, high baseline RSA has previously been conceptualized as a protective factor in environments of risk (e.g., marital conflict, prenatal substance exposure). When capitalizing on the full variability of environmental conditions—both positive and negative—we found instead that high baseline RSA may be better viewed as a susceptibility factor (Belsky & Pluess, 2009; Ellis et al., 2011). Third, we have extended the theory of differential susceptibility by investigating whether infants with susceptible traits can reap the benefits of a positive caregiving environment despite living in poverty. Although all infants in this study were reared in poverty, those with high baseline RSA raised in caregiving environments that fostered disorganization fared worse than comparably susceptible infants raised in caregiving environments of security and infants with low baseline RSA who were reared in environments of disorganization.
Most surprising to us was the detection of a biobehavioral susceptibility factor that operated in a “for better and for worse” manner, despite sample-wide levels of significant socioeconomic disadvantage. Although born into disadvantaged conditions, infants with high baseline RSA who were raised in contexts of security were buffered from the deleterious effects of the broader environmental context. This finding supports Propper’s (2012) hypothesis that family functioning and support can mediate the effect of poverty on developmental outcomes, and it speaks to the idea that poverty is not a uniform stressor, particularly for children raised by nurturing caregivers (Miller et al., 2011). For the developing infant who is particularly susceptible to environmental influences (i.e., one who has high baseline RSA), the best possible environment is one of support, whereby the infant learns to regulate using a sensitive, responsive teacher. Presumably, this physiological susceptibility enables these infants to be more attuned to—and affected by—a sensitive caregiver who aids the infant to self-regulate despite being raised with fewer economic resources. In fact, our results suggest that infants with high baseline RSA who were raised in environments that fostered security had the lowest levels of problem behavior, even falling below the community norms reported elsewhere (Briggs-Gowan & Carter, 2002).
In contrast, infants with high RSA who were raised in caregiving environments characterized as disorganized were faced with two obstacles: an insensitive caregiver and poverty. If high baseline RSA reflects greater engagement with the environment, then this group of infants were presumably more engaged with—and responsive to—negative parenting experiences. This engagement might have adaptive significance. Pluess and Belsky (2011) speculate that, in the context of exceptionally harmful environments, the neonate learns to allocate more attentional resources in the form of increased vigilance. Under such suboptimal rearing conditions, high baseline RSA may lead to the consolidation of coping strategies that portend problems later in development. In fact, Ellis and colleagues (2011) argue that infants reared in stressful environments are not vulnerable or resilient per se, but instead learn to adapt—for better or for worse—to this harmful environment through increased vigilance, which may come at a cost, as evidenced by increased problem behavior. These infants are most likely deprived of the conditions needed to acquire and consolidate effective self-regulatory capacities. Instead, they might rely more heavily on maladaptive physiological mechanisms to regulate, which could lead to the chronic overactivation of these physiological systems and subsequent significant mental- and physical-health problems (Shonkoff et al., 2009). By the time these infants become children, they will have to depend on maladaptive methods of coping, which could potentially lead to an increase in problem behavior (Fearon, Bakermans-Kraneburg, Van IJzendoorn, Lapsley, & Roisman, 2010). In the sample analyzed in the present study, infants raised in environments that fostered a disorganized attachment with high baseline RSA fared the worst, as shown by problem-behavior scores that were far above clinical risk indices.
There were no differences in problem behavior among infants with low baseline RSA from either secure or disorganized caregiving environments. These infants typically are not as responsive to environmental variations—positive or negative (Ellis et al., 2011; Pluess & Belsky, 2011). In the present study, however, both groups of infants with low baseline RSA had problem-behavior scores that were above the mean for community samples (Briggs-Gowan & Carter, 2002); this finding suggests that they were exhibiting significant levels of problem behavior. Our results sharpen the theory of differential susceptibility by raising the possibility that less-susceptible infants can still be affected by broader environmental factors. For infants with low baseline RSA, poverty may be a more powerful predictor of problem behavior than the immediate parenting context. In this group of infants, the increased stress of living in poverty combined with a physiological profile that made them less susceptible to environmental influences may have affected their coping abilities, which resulted in higher levels of problem behavior.
Although this study provides some answers about how risk may be mitigated in contexts of poverty and whether physiological differential-susceptibility processes are at work in infancy, it also raises further questions. First, the durability of the independence between baseline RSA and quality of the early caregiving environment as indexed by attachment is unclear. Although the two were independent in this sample, it is possible that, with canalization and development, the association could become stronger. This reasoning fits with the theory of differential susceptibility; people are born with biological susceptibilities, but these susceptibilities might develop with environmental input and experience. If true, this is precisely the type of plasticity that developmentalists and interventionists are seeking (Boyce, 2006). Another limitation of these data is that we only had maternal report of infant temperament. Thus, laboratory observations of negative emotionality may be related to infant baseline RSA, a possibility that should be explored in future studies.
This study is the first to identify a biological marker of susceptibility to environmental influences in infancy, when the infant is learning through interactions with caregivers how to effectively respond and cope with environmental demands. High baseline RSA was predictive of later adaptation; it appeared to buffer certain infants from the effects of poverty if they were raised in contexts defined by supportive, sensitive caregiving. Among infants developing in the context of poverty, the ability to successfully adapt and regulate at biological and behavioral levels in response to a multitude of environmental pressures appears to differentiate children who develop more successfully from those who develop less successfully (Propper, 2012). We believe that early identification and prevention of risk is of extreme importance given the plasticity of the neurobiological stress response (Beauchaine et al., 2008). By identifying those children who are most vulnerable to developing problem behavior given biological and environmental risk factors early in life, it may be possible to halt the progression of psychological disorder.
Footnotes
One issue regarding the calculation of RSA is whether what one infers to be respiration actually is respiration (rather than chest-wall movements or nonrespiratory chest movements). We included tidal volume and respiration as controls in all analyses, but we report findings without these physiological control measures because including them did not change our results.
We included cumulative risk and the interaction between cumulative risk and baseline RSA as predictors in our regression models. Because the inclusion of these covariates did not substantially change our results, we do not report these findings.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Reprints and permission: sagepub.com/journalsPermissions.nav
References
- Ainsworth MD, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Fearon RMP. Infant-mother attachment security, contextual risk, and early development: A moderational analysis. Development and Psychopathology. 2002;14:293–310. doi: 10.1017/s0954579402002067. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 1. Attachment. 2. New York, NY: Basic Books; 1982. Original work published 1969. [Google Scholar]
- Boyce WT. Symphonic causation and the origins of childhood psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2. New York, NY: Wiley; 2006. pp. 797–817. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;13:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Brief Infant-Toddler Social and Emotional Assessment (BITSEA) manual (Version 2.0) New Haven, CT: Yale University; 2002. [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DC. The Brief Infant-Toddler Social and Emotional Assessment: Screening for social-emotional problems and delays in competence. Journal of Pediatric Psychology. 2004;29:133–155. doi: 10.1093/jpepsy/jsh017. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Hill A. Caregiver influences on emerging emotion regulation: Biological and environmental transactions in early development. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 229–248. [Google Scholar]
- Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior & Development. 2010;33:251–265. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Dunst CJ, Leet HE. Measuring the adequacy of resources in households with young children. Child: Care, Health and Development. 1987;13:111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, Van IJzendoorn MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: A meta-analytic study. Child Development. 2010;81:435–456. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Henderson HA. Developmental psychophysiology: Conceptual and methodological perspectives. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. 2. New York, NY: Cambridge University Press; 2000. pp. 665–686. [Google Scholar]
- Garstein MK, Rothbart MK. Studying infant temperament via the revised Infant Behavior Questionnaire. Infant Behavior & Development. 2003;26:64–86. [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Kupper N, Willemsen G, Posthuma D, DeBoer D, Boomsma DI, DeGeus EJC. A genetic analysis of ambulatory cardiorespiratory coupling. Psychophysiology. 2005;42:202–212. doi: 10.1111/j.1469-8986.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Matheny AP, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the Confusion, Hubbub, and Order Scale. Journal of Applied Developmental Psychology. 1995;16:429–444. [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Society of Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Annals of Internal Medicine. 1993;153:2093–2102. [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socio- emotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Differential susceptibility to rearing experience: The case of childcare. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50:396–404. doi: 10.1111/j.1469-7610.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Development and Psychopathology. 2011;23:29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Propper C. The early development of vagal tone: Effects of poverty and elevated contextual risk. In: Maholmes V, King RB, editors. The Oxford handbook of poverty and child development. New York, NY: Oxford University Press; 2012. pp. 103–123. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality in development. New York, NY: Guilford Press; 2011. [Google Scholar]
- Sameroff AJ, Seifer R, Barocas R, Zax M, Greenspan S. Intelligence quotient scores of 4-year-old children: Social-environmental risk factors. Pediatrics. 1987;79:343–350. [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, Das A. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Developmental Psychopathology. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Solomon J, George C. Disorganized attachment and caregiving. New York, NY: Guilford Press; 2011. [Google Scholar]