Abstract
For this functional magnetic resonance imaging (fMRI) study, we assessed the impact of early social experiences on the social regulation of neural threat responding in a sample of 22 individuals that have been followed for over a decade. At 13 years old, a multidimensional measure of neighborhood quality was derived from parental reports. Three measures of neighborhood quality were used to estimate social capital—the level of trust, reciprocity, cooperation, and shared resources within a community. At 16 years old, an observational measure of maternal emotional support behavior was derived from a mother/child social interaction task. At 24 years old, participants were asked to visit our neuroimaging facility with an opposite-sex platonic friend. During their MRI visit, participants were subjected to the threat of electric shock while holding their friend’s hand, the hand of an anonymous opposite-sex experimenter, or no hand at all. Higher adolescent maternal support corresponded with less threat-related activation during friend handholding, but not during the stranger or alone conditions, in the bilateral orbitofrontal cortex, inferior frontal gyrus and left insula. Higher neighborhood social capital corresponded with less threat-related activation during friend hand-holding in the superior frontal gyrus, supplementary motor cortex, insula, putamen and thalamus; but low childhood capital corresponded with less threat-related activation during stranger handholding in the same regions. Exploratory analyses suggest this latter result is due to increased threat responsiveness during stranger handholding among low social capital individuals, even during safety cues. Overall, early maternal support behavior and high neighborhood quality may potentiate soothing by relational partners, and low neighborhood quality may decrease the overall regulatory impact of access to social resources in adulthood.
Childhood Maternal Support and Neighborhood Quality Moderate the Regulatory Impact of Social Relationships in Adulthood
Social proximity, peer bonding and soothing behaviors attenuate cardiovascular arousal (Grewen, Anderson, Girdler, & Light, 2003), facilitate non-anxious temperament (Weaver, et al., 2004), reduce glucocorticoid release (Wiedenmayer, Magarinos, McEwen, & Barr, 2003), and even extend life (Rohrbaugh, Shoham, & Coyne, 2006). Conversely, divorce, social subordination, rejection and isolation are major health risks (Hawkley & Cacioppo, 2010; Holt-Lunstad, Smith, & Layton, 2012; House, Landis, & Umberson, 1988; Sbarra, Law, & Portley, 2011).
In a recent functional magnetic resonance imaging (fMRI) study, Coan and colleagues suggested that the myriad benefits of social relationships are largely attributable to the emotion-regulatory benefits they confer (Coan, Schaefer, & Davidson, 2006). In their initial study, 16 happily married women were confronted with the threat of mild electric shock while either holding their spouse’s hand (cf., Gallace & Spence, 2010), a stranger’s hand, or no hand at all— all while functional images of the brain were collected. The shock paradigm was designed to create a state of anticipatory anxiety analogous to the kind of “background” anxiety many people face in their daily lives. Coan et al observed that among women in the highest quality marriages, neural threat reactivity while holding a spouse’s hand was limited to portions of the ventromedial prefrontal cortex (vmPFC) and supplementary motor cortex, possibly reflected some low level automatic regulatory activity. With decrements in either the quality or type of the relationship, threat-related brain activity increased. Individuals in lower quality marriages showed additional threat-related activations in regions associated with internal focus and stress regulation, such as the right anterior insula, left superior frontal gyrus and hypothalamus, even during spouse hand-holding. When the hand-holder was an unfamiliar stranger, increased vigilance and effortful self-regulation processes reflected in the superior colliculus, putamen, precentral gyrus and dorsolateral prefrontal cortex were all active as well. Finally, when facing the threat of shock alone in the scanner, all of the preceding activations were observed in addition to physiological preparation for action via the ventral anterior cingulate cortex, posterior cingulate, postcentral gyrus and supramarginal gyrus. In sum, as the social context changed from the presence of high-quality relational partners, to lower-quality partners, to strangers, and finally to social isolation, the brain became progressively more responsive to signs of threat.
Coan (2010) has characterized this pattern of socially dependent, monotonically increasing levels of threat-related activity as reflecting changes in the number of perceived demands placed on the individual in the scanner, a number that increases as perceived access to dependable social resources decreases. This perspective is a corollary assertion of Social Baseline Theory (Beckes & Coan, 2011; Coan, 2008; Coan, 2010), which states that the normative or baseline assumption of the human brain is to be embedded within a social network characterized by familiarity, predictability, shared goals and joint attention (cf., Herrmann, Call, Hernandez-Lloreda, Hare, & Tomasello, 2007; Schilbach, et al., in press; Schilbach, et al., 2010). In this way, the dominant ecology or habitat of the human brain is likely to be any that is rich with other humans (cf., Berscheid, 2003). Indeed, unlike most other animals, there is no specific terrestrial environment to which humans are specifically or even primarily adapted. Humans are capable of taking their highly cooperative social networks with them wherever they go—even to the moon. Taking a behavioral ecology perspective, the human brain can be understood in part as a model of the environment to which it is adapted (Friston, 2010). Thus, the human brain is phylogenetically prepared to find itself in the presence of trusted and interdependent relational partners with whom it will engage in cooperative action (Rekers, Haun, & Tomasello, 2011; Smith, 2010). When this baseline condition is met, cause for alarm is relatively low, even when potential environmental threats present themselves. By contrast, violations of the social proximity expectation signal the need for increased threat-related vigilance and reactivity, because being alone is in fact relatively dangerous.
Social Baseline Theory and the Social Regulation of Threat Responding
The adult attachment research tradition places a strong emphasis on identification of attachment figures, individuals toward whom one is attached in ways that are analogous to the attachments we had to our childhood caregivers. From this perspective, attachment figures are believed to be qualitatively different from others with whom humans interact. Although attachment theorists disagree about the extent to which attachment figures are necessary for the provision of social support, it is also true that studies of interpersonal relationships within the attachment literature tend to be highly attentive to the degree to which a given relational partner may satisfy the putative criteria of “attachment figure” (Hazan & Zeifman, 1999). Indeed, even Coan et al. included in their original study only those relational partners with whom their participants were 1) married for longer than 2 years, 2) subjectively highly satisfied with, and 3) identified as the one individual they would first turn to in times of dire need. This was an attempt to ensure that each participant arrived at the laboratory with his or her putative attachment figure (Coan et al., 2006).
By contrast, the broad social support tradition is fairly agnostic about the status of individuals from whom one receives support (Cohen & Wills, 1985; Gottlieb & Bergen, 2010). In this tradition, very little, perhaps no, specific emphasis is placed on the status of supportive others as “attachment figures”. Indeed, the important question has simply been whether or not a given participant predicts that their social world—broadly defined—will be supportive of their emotional needs. Evidence suggests that relatively objective measures of social support are more weakly related to outcome measures of health and well being than are subjective measures that emphasize a participant’s private point of view on the question (Cadzow & Servoss, 2009). In this way, an individual may appear to have relatively few friends, but still report that the number of friends they have is large, or in any case sufficient (Haber, Cohen, Lucas, & Baltes, 2007). Similarly, a given individual may report feeling lonely despite the appearance of having a large number of friends (Hawkley & Cacioppo, 2010).
In common with the general social support tradition, Social Baseline Theory can be contrasted with Attachment Theory in its relative disinterest in qualitatively distinct relationship types (Beckes & Coan, 2011). Although many relationship researchers are highly concerned with the degree to which a relational partner is an attachment figure (Berscheid, 2010; Hazan & Zeifman, 1999), Social Baseline Theory regards putative attachment figure status as relatively unimportant and even possibly chimerical, just as Coan (2008) has argued that the putative “attachment behavioral system” postulated by (Bowlby, 1969/1982) does not map neatly onto any “attachment behavioral circuit” in the brain. As an alternative, and in contrast with much of the general social support literature, Social Baseline Theory acknowledges that most or all relationships vary quantitatively in terms of certain key variables the brain uses to make guesses about a relational partner’s availability as a resource, namely: familiarity (the partner is known), reliability (the partner is trustworthy), and interdependence (the partner is needed). From this perspective, when a potential friend is more familiar, reliable and interdependent, he will also be regarded as more of a resource. On the one hand, this assertion demands a conceptual replication of the original hand-holding study utilizing supportive relational partners that, unlike Coan et al (2006), may not classically qualify as “attachment figures.” On the other hand, there is also a need to begin the process of identifying other key moderators of the supportive impact of hand-holding on the brain’s threat response.
Our first hypothesis was that we would conceptually replicate the findings of Coan et al using platonic friends as hand holders, although we did not expect the effects of friend handholding to be either as strong or as pervasive as the effect of romantic partner handholding, because on average, friends are neither as familiar nor interdependent as romantic partners. To test this, we simply asked participants in the present study to visit our laboratory with a platonic friend. A large literature suggests that friendships provide substantial levels of social support (Chu, Saucier, & Hafner, 2010; van der Horst & Coffe, 2011), even surpassing, at certain periods of development, the levels provided by family members (Bokhorst, Sumter, & Westenberg, 2010). Because social support should be sensitive to the degree of certainty ascribed to potential support providers, we first predicted that although we would replicate the original findings of Coan et al among platonic friends, our effects in this sample would be neither as strong nor as widespread as those observed among happily married romantic couples.
Next, we hypothesized that higher levels of maternal support during adolescence would correspond with increased regulatory effects of friend, but not stranger, handholding. Attachment theorists have long argued that predictions about future relationship functions are rooted in “internal working models”—abstracted and generalized representations of past relationship experiences (Main, Kaplan, & Cassidy, 1985). More recently, researchers have argued for a strong link between these internal working models and capacities for both self- and socially-mediated emotion regulation (Allen & Miga, 2010; Mikulincer & Shaver, 2008). Indeed, supportive behavior by the mother has been widely associated with better emotional adjustment in adulthood, even among adopted children (Stams, Juffer, & van IJzendoorn, 2002). Recently, maternal support behavior during early childhood has even been associated with greater hippocampal volume by school age (Luby, et al., 2012), and hippocampal volume has itself been associated with attenuated threat responding (Francis & Meaney, 1999; Kalisch, et al., 2005). Our longitudinal sample allowed us to test this second hypothesis by modeling associations between maternal support behaviors measured at age 13 as moderating the impact of handholding on threat responding in adulthood.
Finally, we hypothesized that social capital (Lochner, Kawachi, & Kennedy, 1999) would, by virtue of being a more abstract and generalized indicator of social resources, correspond with less threat-related activity during both friend and stranger handholding. Although universally agreed-upon definitions of social capital do not exist, the construct generally refers to the degree of trust and interdependence shared within a social community. Many theorists and researchers have observed that high social capital is associated with decreased risk of a number of social ills, though the empirical evidence has not always been conclusive (De Silva, McKenzie, Harpham, & Huttly, 2005). For example, high social capital has been associated with attenuated depression risk (Fujiwara & Kawachi, 2008; Kouvonen, et al., 2008), better emotional adjustment during the transition to adulthood (Pettit, Erath, Lansford, Dodge, & Bates, 2011), increased baseline feelings of safety (Dallago, et al., 2009), and lower stress-related sequelae attributable to other social ills, such as poverty (Evans & Kutcher, 2011) and crime (Buonanno, Montolio, & Vanin, 2009).
In testing these hypotheses, we emphasize the value of using a longitudinal sample—an approach that introduces a within-subject temporal ordering to the observations described below. Such temporal ordering does not resolve questions of causation underlying these associations, but it does limit them, in that it is not possible for adult handholding effects to cause adolescent maternal support or social capital. Thus, our attempts to predict receptivity to social support using the hand-holding paradigm can be considered prospective.
Method
Participants
Twenty-five participants were recruited to bring an opposite gender friend to the scan. One pair was dropped due to a technical issue with the anatomical image, another was dropped upon the discovery that they were siblings as well as friends, and another was dropped for being a significant outlier on BOLD response, according to a Mahalabonis distance calculation. Thus, the final sample numbered 22 (11 female), the mean age of which was was 23.59 (SD = .959 ) and of the remaining friends was 23.14 (SD = 2.92). Within the scanned participants 14 identified as White and 8 identified as African American. The participants were recruited via mail and telephone from a larger group (n = 172) of participants involved in the ongoing Virginia Institute of Development in Adulthood (VIDA) study, a cohort of individuals that one of the authors (JPA) has been following and annually assessing for over a decade (Allen, Porter, & McFarland, 2006; Allen, Porter, McFarland, Marsh, & McElhaney, 2005; McElhaney, Antonishak, & Allen, 2008). Exclusion criteria included pregnancy and any issues with either the magnet or the scanning environment of the MRI, including severe claustrophobia or residual ferromagnetic items in the participant’s body. Only VIDA participants were scanned, while the friends they brought in provided the hand-holding. Informed consent was obtained from both members of each pair in accordance with the Internal Review Board of the University of Virginia and participants were paid $160 each for participation.
Materials
Supportive Behavior Task
At approximately age 16 or 17, during wave 4, participants were observed interacting with their mothers during a Supportive Behavior Task. During this task they ask for help with a “problem they were having that they could use some advice or support about.” These topics frequently involved issues with dating, problems with peers or siblings, raising money, or deciding about joining sports teams. The supportive behavior coding system was used to code the interactions (Allen et al. 2001). We focused on mother engagement as a general measure of responsiveness from the mother during the task. Specifically, mother engagement was comprised of levels of encouragement and advice offered by the mother, the level of emotional engagement by both the adolescent and mother, and the mother’s apparent success in understanding the adolescent’s problem. Two trained coders coded each interaction. Because inter-rater reliability was considered good, α = .69 (cf., Cicchetti & Sparrow, 1981), codes were then averaged across coders.
Social Capital
We derived a measure of social capital from a combination of neighborhood quality measures (see Buckner, 1992; Gonzales, Cauce, Friedman, & Mason, 1996) used in the early stages of the VIDA project (wave 1 of the study, when the participants were around 12 or 13 years of age). The measure captured three aspects of neighborhood social capital, including neighborhood connectedness (father’s α = .77; mother’s α = .76), crime and deterioration (father’s α = .73; mother’s α = .78), and neighborhood risk (father’s α = .91; mother’s α = .93) as reported by the participants’ mothers and fathers. The average zero-order correlation among these three scales was r = .74, high enough to take the average of the mothers’ and fathers’ assessments for one overall measure of neighborhood social capital. The connectedness subscale includes questions such as: “I believe my neighbors would help me in an emergency”; “The relationships I have with my neighbors mean a lot to me”. The crime and deterioration subscale includes: “In general people in my neighborhood do not watch out for each other”; “There are places in my neighborhood where you can buy or sell stolen property”. Finally, the risk subscale includes: “Theft is a problem in my neighborhood”; and “Violent crimes that involve weapons occur in my neighborhood”.
Procedure
Participants were phone screened for eligibility and compatibility with the scanner. Those who were determined to be eligible were informed they would be scheduled for an appointment for an MRI scan. In addition to the scan, participants completed a series of personality and relationship questionnaires and underwent a practice session with portions of the stimulus program to familiarize them with the scanning environment, the equipment, and the stimuli utilized in the study. Before scanning, two Ag-AgCl shock electrodes were applied to each participant’s ankle (left or right ankle was counterbalanced across participants) as well as to the partner’s ankle. Participants were then taken into the scanning chamber where high resolution anatomical scans followed the successful completion of the in-scanner practice session.
Participants viewed stimuli projected onto a screen at the back of the magnet’s bore through a mirror placed on the head coil, and responded to the stimuli accordingly using an MR-compatible button box. The study consisted of five experimental scanning blocks, during which the participant viewed ten threat cues with no shock, two with shock, and twelve safety cues in variable order, for a total of 24 cue trails per block. The first two scanning blocks made up the ‘Threat to Other’ portion of the study, where mild electric shock was delivered to the person the participant was holding hands with, either the friend or a stranger (these data are not reported here). The ‘Threat to Self’ task was composed of the final three scanning blocks, where mild electric shock was delivered to the participant while they were either holding hands with the friend, a stranger, or no one. Trials were varied within subject, and block order within each threat session was counterbalanced between subjects. The stranger was an anonymous member of the opposite gender, and participants did not meet their strangers until after the experiment. Participants’ right hands were employed for hand-holding, while their left hand held the button box they used to indicate ratings of their subjective arousal and valence immediately after each of the five scans. The threat cues consisted of a red “X” on a black background, and indicated a 17% chance of someone receiving an electric shock, depending on the current threat session (in Threat to Self, the participant was shocked, during Threat to Other either the stranger or the friend was shocked). Safety cues, a blue “O” on a black background, indicated no chance of shock. The shocks were generated by an isolated physiological stimulator (Coulbourn Instruments, Allentown PA), and lasted for 20-ms at 4mA. Two shocks were delivered in each block.
The trials were composed of a 1 second cue signifying threat or safety, followed by a 4 to 10 second period of anticipation during which participants were instructed to focus their attention on a fixation cross. The end of the anticipation period was signaled by a small dot. If the trial had been a threat trial, it was during the appearance of this dot that the electric shock was delivered. Participants were told to rest following the dot and until the next trial began, the duration of the rest period lasting between 4 and 10 seconds. Each block finished with participants rating their subjective feelings of unpleasantness (valence) and agitation (arousal) on the Self-Assessment Manikin (SAM) scales (Bradley & Lang, 1994). Participants indicated their level of valence and their level of arousal on a 9-point pictorial scale once per block via a button box placed in their left hand.
Image Acquisition and Data Analysis
Images were acquired using a Siemens 3.0 Tesla MAGNETOM Trio high-speed magnetic imaging device at UVA’s Fontaine Research Park, with a CP transmit/receive head coil with integrated mirror. Two hundred sixteen functional T2*-weighted Echo Planar images (EPIs) sensative to BOLD contrast were collected per block, in volumes of twenty-eight 3.5-mm transversal echo-planar slices (1-mm slice gap) covering the whole brain (1-mm slice gap, TR=2000ms, TE=40ms, flip angle=90°, FOV= 192 mm, matrix= 64 × 64, voxel size= 3 × 3 × 3.5mm). Prior to collection of functional images, one hundred seventy-six high-resolution T1-magnetization-prepared rapid-acquisition gradient echo images were acquired to determine the localization of function (1-mm slices, TR=1900 ms, TE=2.53ms, flip angle= 90°, FOV=250mm, voxel size= 1 × 1 × 1mm).
Using FMRIB Software Library (FSL) software (Version 5.98; www.fmrib.ox.ac.uk/fsl, Jenkinson, Bannister, Brady, & Smith, 2002), we preprocessed and analyzed the collected data. Motion was corrected for using an intra-modal correction algorithm tool known as using FMRIB’s Linear Image Registration Tool (MCFLIRT), with slice scan-time correction and a high-pass filtering cutoff point of 100 seconds, which removed signals that were irrelevant to the stimuli. The images were also subjected to BET (Smith, 2002) brain extraction, which eliminated unwanted, non-brain material voxels in the fMRI data. The images then underwent a spatial smoothing with a 5-mm full width at half minimum Gaussian kernel, and a grand-mean scaling, and were registered to the Montreal Neurological Institute standard space by FLIRT. Threat trials where participants actually received shocks were excluded from analysis due to the increased likelihood of movement artifacts.
Functional Regions of Interest (ROIs)
To determine the normative neural threat response of participants, a contrast of activation to threat cues and activation to safety cues (threat minus safe) during the alone (threat-to-self) condition was required. First level analysis of the functional data began with the determination of functional ROIs using FEAT and time-series statistical analysis by FILM. Third level analysis was performed by FLAME (FMRIB’s Local Analysis of Mixed Effects) state 1. Multisubject ROIs were identified via cluster-wise tests using the fsl standard Z-threshold of 2.3 and cluster p threshold of .05. We anticipated that this procedure would reveal activations in various neural regions that previous studies have indicated are associated with neural response to threat, negative affect, or anticipation of pain. Table 1 lists all the ROIs, means, and standard deviations within each condition. These ROIs were consequently used in further comparisons the threat-to-other conditions and in comparison of these conditions to the threat-to-self conditions.
Table 1.
Statistical Regions of Interest, Coordinates, Maxima, and Cluster Size, and Significant Effects of Handholding, Mother Engagement, Social Capital, and Gender
| x | y | z | Cluster Size (mm3) |
Effects | |
|---|---|---|---|---|---|
|
Frontal and Cingulate Regions
| |||||
| Left OFC | −33.4 | 23.9 | −7.17 | 669 | b |
| Right OFC | 37.4 | 24.9 | −5.6 | 839 | b |
| Left DLPFC | −33.6 | 47.0 | 13.6 | 542 | |
| Right DLPFC | 36.3 | 44.2 | 17.8 | 1552 | |
| Left SMC | −5.9 | 1.2 | 55.6 | 352 | a |
| Right SMC | 6.0 | 4.1 | 55.1 | 229 | c |
| Left SFG | −2.2 | 22.5 | 49.7 | 157 | a |
| Right SFG | 6.6 | 25.3 | 48.0 | 643 | b, c |
| Right SMG | 52.5 | −43.4 | 38.3 | 1603 | |
| Left IFG | −49.8 | 10.3 | 0.41 | 287 | |
| Right IFG | 51.5 | 17.9 | 3.48 | 491 | b |
| Dorsal ACC | 1.3 | 20.8 | 32.1 | 1208 | a |
| Posterior Cingulate | 2.6 | −27.1 | 24.4 | 376 | |
|
Insular and Subcortical Regions
| |||||
| Left Putamen | −25.2 | 8.5 | −0.8 | 351 | a, c |
| Right Putamen | 28.0 | 18.0 | 0.0 | 266 | |
| Left Insula | −34.4 | 16.3 | −2.5 | 799 | b, c |
| Right Insula | 35.3 | 19.0 | −2.2 | 674 | |
| Right Caudate | 13.4 | 6.6 | 9.1 | 305 | |
| Left Thalamus | −5.3 | −10.1 | −3.4 | 28 | c |
| Right Thalamus | 8.7 | −4.3 | 5.4 | 272 | |
significant main effect of handholding;
significant handholding by maternal support interaction;
significant handholding by social capital interaction.
ROIs determined by Feat fMRI Analysis were then used to create structural masks using FSLView’s Harvard-Oxford Cortical and Subcortical atlases. The voxels falling into the location where the statistical ROI overlapped with the atlas-derived brain structure were masked. Parameter estimates were then extracted from each ROI in each condition (with the threat-safe contrast) for each subject using FEATQuery and converted to percent signal change (PSC) values. These estimates were then used in an analysis using the PASW (PASW Statistics ,v 18, www.spss.com) statistical package, version 18. Means and standard deviations for PSC in each ROI and condition are displayed in Table 1.
Results
We hypothesized that holding a friend’s hand while anticipating shock would decrease the neural response to threat, much as it does for happily married women holding their husband’s hand. However we expected this effect to be less widespread and more susceptible to individual differences. We hypothesized that holding a stranger’s hand might have a similar effect, albeit to an even lesser extent, and that the degree to which any individual is benefitted by holding a friend’s hand should be moderated by previous experiences with potential social resources, defined here as early maternal support behavior (familial resources) and neighborhood social capital (community resources). Unlike Coan et al (2006), we did not observe any significant effects of handholding, or indeed of either of our hypothesized moderators, on subjective reports of valence and arousal. To test for the effects of social resources and handholding on threat-related brain activity, we predicted threat-safe contrasts derived from our functional ROIs using linear mixed models (LMM) with handholding and gender as fixed effects, and maternal support behavior and neighborhood social capital as covariates (West, Welch, & Galecki, 2007). For each test within the model, a type 1 sum of squares was specified, allowing each variable (handholding, maternal support, and social capital) to predict unique variance. A summary of all effects is presented in table 1.
Main Effects of Hand-holding
LMMs revealed significant main effects of handholding in the dorsal Anterior Cingulate Cortex (ACC), F(2, 15) = 6.0, p = .01, left Supplementary Motor Cortex (SMC), F(2, 15) = 4.7, p = .03, left Putamen, F(2, 15) = 4.4, p = .03, and left Superior Frontal Gyrus (SFG), F(2, 15) = 4.2, p = .04. Subsequent pairwise comparisons suggest threat-related activity during friend hand holding was significantly lower than during the alone condition in the ACC, the left SFG, and the left SMC. Interestingly, threat-related PSC during stranger hand holding was lower than in the friend condition in the left Putamen. With the exception of the left Putamen, these results generally replicated those of Coan and colleagues (2006).
Interactions Between Mother Support (Familial Resources) and Hand-holding
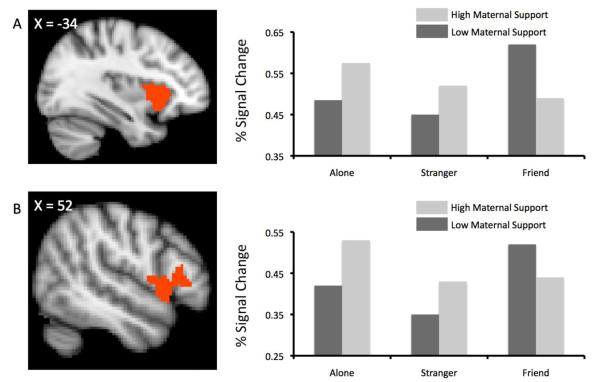
Several hypothesized interactions between mother support and handholding were observed, implicating the left Orbitofrontal Cortex (OFC), F(2, 15) = 5.9, p = .01, right OFC, F(2, 15) = 3.7, p = .05, right Inferior Frontal Gyrus (IFG), F(2, 15) = 4.1, p = .04, right SFG, F(2, 15) = 3.7, p = .05, and left Insula, F(2, 15) = 6.1, p = .01. In all regions, greater maternal support corresponded with less threat-related activation during friend hand holding relative to the alone and stranger conditions (see Figure 1). Overall, individuals with high maternal support tended to show the typical “handholding” effect, with a monotonic decrease in threat-related activity from the alone, to stranger, to friend handholding conditions (e.g., Figure 1-A). By contrast, low maternal support corresponded with a general increase in threat-related activity during friend handholding.
Figure 1.
Point estimates of percent signal change graphed as a function of handholding (alone, stranger, partner) by maternal support interaction effects. Point estimates were computed separately for individuals high (+1SD) and low (−1SD) in maternal support. Row A represents activity in the left insula. Row B represents activity in the right inferior frontal gyrus (IFG).
Interactions Between Social Capital (Community Resources) and Hand-holding
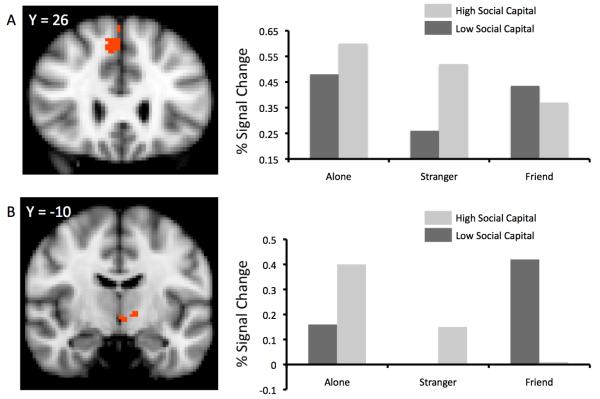
Handholding also interacted with social capital in several regions, including the right SFG, F(2, 15) = 4.1, p = .04, right SMC, F(2, 15) = 4.7, p = .03, left Insula, F(2, 15) = 6.1, p = .04, left Putamen, F(2, 15) = 6.3, p = .01, and left thalamus, F(2, 15) = 10.5, p = .001. In all of these regions, individuals with high neighborhood social capital also tended to show the typical “handholding” effect, with a monotonic decrease in threat-related activity from the alone, to stranger, to friend handholding conditions. By contrast, individuals with low neighborhood social capital showed evidence of either no difference between the alone and friend conditions, or relatively greater threat-related activity during friend handholding. Yet more interesting is that low social capital individuals appear to be least active during stranger handholding—an unexpected observation about which more will be discussed below.
Discussion
Our initial look into the role of platonic friends in the social regulation of neural threat responding revealed an easily interpretable replication of Coan et al. (2006). Specifically, threat-related neural activation was attenuated by friend, but not stranger, hand-holding in the ACC, left SMC, and left SMG. The relatively smaller number of implicated threat-related regions in comparison to Coan et al may reflect the use here of platonic friends instead of romantic partners, the more racially and socioeconomically diverse sample, or both. These main effects primarily implicated regions associated with alarm, self-monitoring and motivational aspects of motor planning in response to the threat—a pattern that occupies an intriguing “middle-ground” of threat-responsive regions between the partner and stranger effects reported by Coan et al (2006). This may simply reflect that friends are, on average, more trustworthy than strangers but less trustworthy than long term romantic partners.
Several interactions between mother support and hand-holding were observed, including effects in the left Orbitofrontal Cortex (OFC), right OFC, right Inferior Frontal Gyrus (IFG), and the left Insula, all highly integrative circuits, some (e.g., IFG) additionally associated with inhibitory control. Participants with more supportive mothers were less threat responsive while holding a friend’s hand. Although the nature of these date preclude causal conclusions, the pattern is consistent with the social baseline prediction that individuals with more positive social resource experiences will be more receptive to subsequent social regulation within close relationships. Another view of this, however, is that individuals with poor social resource experiences are less capable of depending on others, perhaps particularly those with whom they should be depending on most, such as familiar friends.
Handholding also interacted with age 13 community social capital in several regions including the right SFG, right SMC, left Insula, left Putamen, and left thalamus, a set of regions that implicates many of the same or similar processes—integrative self monitoring and motor preparation—as the main handholding effects. The general pattern in each of these regions suggests on the one hand that greater social capital was associated with decreased activation in the friend condition, as predicted. On the other hand, it appears that lower social capital corresponds with decreased threat-related activity during stranger, but not friend, handholding. This last finding was not expected, and prompted a subsequent post hoc exploration of the social capital and handholding data. Specifically, an ANCOVA model predicting brain activity averaged across implicated ROIs (right SMC, right SFG, left putamen and left insula) using cue (safe versus threat) handholding (alone, stranger, partner), and social capital, yielded a significant 3-way interaction, F (2, 19) = 8.3, p = .003, partial η2 = .47.
Decomposition of this interaction revealed very different patterns in the association between social capital and BOLD activation—differences that depended upon both cue type and handholding condition. Specifically, although social capital was unrelated to safety cue BOLD activation per se during the alone (r = −.02) and friend handholding (r = .07) conditions, it was negatively (and unexpectedly) correlated with safety cue BOLD activity during stranger handholding (r = −.24). Meanwhile, social capital and threat-cue BOLD activation were positively correlated during both the alone (r = .25) and stranger (r = .31) conditions, but negatively correlated during the friend condition (r = −.16). It’s important to regard these correlations as descriptive, since they are all in the service of decomposing our three-way interaction, and since no single correlation is statistically significant. But although associations between social capital and BOLD activity during threat cues was more or less as expected (higher social capital predicts higher threat activation while alone and with a stranger, but not with a friend), it appears that lower social capital predicted higher BOLD activation even during safety cues—when holding a strangers hand. Thus participants from lower social capital backgrounds may find holding the stranger’s hand to be generally threatening in a way the high social capital participants do not. If true, this could give the appearance of reduced threat responding during stranger handholding among low social capital participants, even though the reality may be something like the opposite: low social capital participants find holding the hand of a stranger to be threatening in itself, regardless of other experimental conditions. Similar associations between low social capital and generalized stranger fear have been reported in the past (e.g., Dallago, Perkins, Santinello, Boyce, Molcho & Morgan, 2009). Although the data reported here are not conclusive, they do suggest directions for further research.
Possible Mechanisms
Social Entrainment
Social entrainment refers to a process by which physiological states become regulated through social contact (c.f., Sbarra & Hazan, 2008). Myron Hofer (e.g., 1994, 1995, 2006) described how entrainment processes occur in mother-pup rat dyads, observing that as a mother and pup interact throughout the pup’s early development, the mother’s physiological responses to the pup become contingent upon the pup’s physiological needs and, as the pup develops, the pup’s physiological needs become contingent on maternal behaviors. In this way, the behavior of one modulates—or regulates—the other. For example, the pup’s distress calls create a state of physiological arousal in the mother accompanied by maternal behaviors ranging from milk production to licking and grooming. The mother’s milk supply satisfies obvious metabolic needs, but the mother’s body warmth also regulates the pup’s cardiac activity, and her licking and grooming behaviors also regulate vigilance for, and responses to, potential threats in the environment (see also Weaver, et al., 2004). Hofer referred to these relationships as “hidden regulators,” because their later effects were not obvious until sufficient stress in the environment activated their regulatory activity. Such “hidden” regulation can be construed as a form of the social entrainment of physiological responding, and may play an important role in the social emotion regulation processes described in our current work. Drawing from these observations, one can certainly imagine maternal support behaviors becoming tightly linked to a child’s physiological stress response. It is possible that such entrainment is relatively generalizable to potentially supportive relationships later in life, which might explain why early maternal support behavior corresponded with decreased threat activity during friend, but not stranger hand holding. Less obvious from an entrainment perspective, however, are the links observed here between handholding and social capital.
Vigilance and Prediction
Across evolutionary time, and indeed during ontogeny, environmental dangers are ubiquitous, and humans are skilled at creating contingency plans in order to predict where, when and how they might occur. Such vigilance is costly and exhausting, however, so an equally important activity is to arrange one’s environment such that vigilance isn’t as necessary. Social baseline theory suggests that social relationships are resources that mitigate the need for costly vigilance throughout the lifetime (Beckes & Coan, 2011; Coan, 2008; Coan, 2010). However, social relationships are themselves contingent, for if our relational partner is not in fact engaging in some amount of vigilance on our behalf, then we place ourselves at increased risk by relaxing our own vigilance processing. The question is: how do we know whom to trust? According to social baseline theory, we rely on indicators of familiarity, predictability, shared goals and joint attention in deciding who among our possible relational partners is trustworthy. The human brain likely implements a kind of Bayesian inference, where “bets” are placed on the reliability of a social resource based on a prior probability distribution of past social experiences, and the deployment of personal resources are in turn based on this prediction (cf., Beckes, Simpson, & Erickson, 2010). A history of trustworthy and dependable relationships increases the presumed likelihood that new relationships will be similarly trustworthy, reliable, and so on. And given such a history, the risk of letting down one’s guard is sufficiently offset by the benefit of depending on one’s social resources. Both early maternal support behaviors and early social capital could be viewed as sources of useful information in this Bayesian process of deciding how dependable future relationships are likely be, and this could in turn determine the degree to which potential threats in the environment should be attended and responded to when in the presence of others.
Oxytocin and Endogenous Opioids
Among the more proximal potential mechanisms of the effects reported here are systems involving oxytocin and endogenous opioids. Oxytocin is vitally implicated in many forms of social behavior (Bales, van Westerhuyzen, Lewis-Reese, Grotte, Lanter, & Carter, 2007), including the inhibition of fear behavior (Taylor, 2006). For example, Kirsch and colleagues (Kirsch, Esslinger, et al., 2005) observed decreases in human amygdala sensitivity following administration of an intranasal oxytocin spray, suggesting oxytocin may attenuate threat vigilance. Endogenous opioid activity, particularly in the dorsal ACC (dACC), may inhibit threat detection (Nelson & Panksepp, 1998). Eisenberger and colleagues (Eisenberger et al., 2007) have suggested that positive social experience may desensitize the dACC through repeated exposure to endogenous opioids. The dACC is indeed dense with opioid receptors. And evidence suggests that opioid activity inhibits central and peripheral threat responding (Zubieta et al., 2003). Although neither of these possibilities has been ruled in as proximal mechanisms of the kinds of effects reported here, they remain strong candidates.
Conclusions
This study provides further evidence for the hypotheses produced by social baseline theory (Beckes & Coan, 2011; Coan, 2008; Coan, 2010). First, it suggests that social resources are broadly depended upon for degrees of social emotion regulation. Indeed, although the friends used as handholders in this study are unlikely to meet criteria for “attachment figure” status, they did nevertheless more strongly regulate threat responding than strangers. On the other hand, the number and type of threat-responsive regions subject to regulation by friend handholding was indeed limited in comparison to the effects of romantic partners reported by Coan et al (2006). This may reflect the nature of close romantic bonds—bonds that are inherently and strongly interdependent at a level that surpasses most platonic friendships.
Results presented here also suggest that past social experiences moderate the degree to which the brain responds to threats in the presence of potential social resources. Specifically, both maternal support and social capital corresponded with decreased threat responding during partner, but not stranger handholding. These observations also argue to an expanded understanding of social support, beyond orthodox attachment processes and more inclusive of the broader impact of social context during development. Interestingly, stranger handholding may have been somewhat threatening to individuals from low social capital backgrounds even during the presentation of safety cues. Although the evidence for this in the current dataset is post hoc and exploratory, it is reasonable to suggest that if this finding is ultimately reliable, it is because low social capital implies that strangers are not only unreliable as resources but also possibly dangerous.
Much remains to be learned about these social regulation processes and the factors that moderate them. The precise mechanisms of social emotion regulation remain poorly understood. Nevertheless, results reported here provide important clues. Moreover, although these data provide evidence of social emotion regulation among friends as opposed to close romantic partners, we have yet to directly compare the regulatory impact of different types of relationships (e.g., married, cohabiting, dating, friends) directly. Such comparisons may be useful for understanding how relationship types interact with relationship quality and past social experience to impact predict the regulatory effects of social contact and proximity. Answers to these and other questions will bring us closer to understanding precisely why and how social relationships impact our health and well being.
Research Highlights.
This paper focuses on early social moderators of the social regulation of neural threat responding by strangers and platonic friends.
Handholding by platonic friends caused significantly less neural threat responding than either handholding by strangers or lying in the MRI scanner alone.
Greater early adolescent maternal support behavior corresponded with less threat responding during friend, but not stranger handholding.
Low early adolescent social capital (neighborhoods characterized by high crime and poverty) corresponded with less regulation by friend handholding, and increased threat-related activity to stranger handholding even during the presentation of safety cues.
Figure 2.
Point estimates of percent signal change graphed as a function of handholding (alone, stranger, partner) by social capital score interaction effects. Point estimates were computed separately for individuals high (+1SD) and low (−1SD) in social capital. Row A represents activity in the right superior frontal gyrus. Row B represents activity in the left thalamus. Note that stranger condition activation among low social capital individuals, and partner condition activation among high social capital individuals are both at or near zero.
Acknowledgments
This research was supported by NIMH Award Number R01MH080725 to J.A.C. We thank Karen Hasselmo, Casey Brown, Alex Tatum, Lauren Everhart, Laura Long, Allison Mann, Rebecca Kelly, Jason Herzfeld, Mac Hodges, Kayla Lemons, Jamie Castle-Shifflet, Becky Martin, David Cohen, Mariah Sinden, Lauren Faulkner, Caroline Trower, and Matt Allen for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Results
- Allen JP, Hall FH, Insabella G, Land D, Marsh PA, Porter MR. The supportive behavior task coding system. University of Virginia; 2001. Unpublished manuscript. [Google Scholar]
- Allen JP, Miga EM. Attachment in adolescence: A move to the level of emotion regulation. Journal of Social and Personal Relationships. 2010;27:181–190. doi: 10.1177/0265407509360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Porter MR, McFarland FC. Leaders and followers in adolescent close friendships: Susceptibility to peer influence as a predictor of risky behavior, friendship instability, and depression. Development and Psychopathology. 2006;18:155–172. doi: 10.1017/S0954579406060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Porter MR, McFarland FC, Marsh P, McElhaney KB. The two faces of adolescents’ success with peers: Adolescent popularity, social adaptation, and deviant behavior. Child Development. 2005;76:747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Hormones and behavior. 2007;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes L, Coan JA. Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass. 2011;5:976–988. [Google Scholar]
- Beckes L, Simpson JA, Erickson A. Of Snakes and Succor: Learning secure attachment associations with novel faces via negative stimulus pairings. Psychological Science. 2010;21(5):721–728. doi: 10.1177/0956797610368061. [DOI] [PubMed] [Google Scholar]
- Berscheid E. The human’s greatest strength: Other humans. In: Staudinger UM, editor. A psychology of human strengths: Fundamental questions and future directions for a positive psychology. American Psychological Association; Washington D.C.: 2003. pp. 37–47. [Google Scholar]
- Berscheid E. Love in the fourth dimension. Annual Review of Psychology. 2010;61:1–25. doi: 10.1146/annurev.psych.093008.100318. [DOI] [PubMed] [Google Scholar]
- Bokhorst CL, Sumter SR, Westenberg PM. Social Support from Parents, Friends, Classmates, and Teachers in Children and Adolescents Aged 9 to 18 Years: Who Is Perceived as Most Supportive? Social development. 2010;19:417–426. [Google Scholar]
- Bowlby J. Attachment and loss. 2nd. ed Vol. 1. Basic Books; New York, NY: 1969/1982. [Google Scholar]
- Buonanno P, Montolio D, Vanin P. Does social capital reduce crime? The journal of law & economics. 2009;52(1):145–170. [Google Scholar]
- Cadzow RB, Servoss TJ. The association between perceived social support and health among patients at a free urban clinic. Journal of the National Medical Association. 2009;101:243–250. doi: 10.1016/s0027-9684(15)30852-x. [DOI] [PubMed] [Google Scholar]
- Chu PS, Saucier DA, Hafner E. Meta-analysis of the relationships between social support and well-being in children and adolescents. Journal of Social and Clinical Psychology. 2010;29(6):624–645. [Google Scholar]
- Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. American Journal of Mental Deficiency; American Journal of Mental Deficiency. 1981;86:127–137ß. [PubMed] [Google Scholar]
- Coan JA. Toward a neuroscience of attachment. In: Shaver J. C. a. P. R., editor. Handbook of attachment: Theory, research, and clinical applications. 2nd edition Guilford Press; New York: 2008. pp. 241–265. [Google Scholar]
- Coan JA. Adult attachment and the brain. Journal of Social and Personal Relationships. 2010;27:210–217. doi: 10.1177/0265407510368966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological bulletin. 1985;98:310. [PubMed] [Google Scholar]
- Dallago L, Perkins DD, Santinello M, Boyce W, Molcho M, Morgan A. Adolescent place attachment, social capital, and perceived safety: A comparison of 13 countries. American journal of community psychology. 2009;44(1):148–160. doi: 10.1007/s10464-009-9250-z. [DOI] [PubMed] [Google Scholar]
- De Silva MJ, McKenzie K, Harpham T, Huttly SRA. Social capital and mental illness: a systematic review. Journal of Epidemiology and Community Health. 2005;59(8):619–627. doi: 10.1136/jech.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kutcher R. Loosening the Link Between Childhood Poverty and Adolescent Smoking and Obesity The Protective Effects of Social Capital. Psychological Science. 2011;22(1):3–7. doi: 10.1177/0956797610390387. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9(1):128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nature Reviews Neuroscience. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Kawachi I. A prospective study of individual-level social capital and major depression in the United States. Journal of Epidemiology and Community Health. 2008;62(7):627–633. doi: 10.1136/jech.2007.064261. [DOI] [PubMed] [Google Scholar]
- Gallace A, Spence C. The science of interpersonal touch: An overview. Neuroscience & Biobehavioral Reviews. 2010;34:246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Gottlieb BH, Bergen AE. Social support concepts and measures. Journal of psychosomatic research. 2010;69(5):511–520. doi: 10.1016/j.jpsychores.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Anderson BJ, Girdler SS, Light KC. Warm partner contact is related to lower cardiovascular reactivity. Behavioral Medicine. 2003;29:123–130. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- Haber MG, Cohen JL, Lucas T, Baltes BB. The relationship between self-reported received and perceived social support: A meta-analytic review. American Journal of Community Psychology. 2007;39:133–144. doi: 10.1007/s10464-007-9100-9. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan C, Zeifman D. Pair bonds as attachments: Evaluating the evidence. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. Guilford Press; New York: 1999. pp. 336–354. [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Medicine. 2012;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Schubert M, Jacob W, Ke√üler MS, Hemauer R, Wigger A, et al. Anxiety and hippocampus volume in the rat. Neuropsychopharmacology. 2005;31(5):925–932. doi: 10.1038/sj.npp.1300910. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvonen A, Oksanen T, Vahtera J, Stafford M, Wilkinson R, Schneider J, et al. Low workplace social capital as a predictor of depression. American journal of epidemiology. 2008;167(10):1143–1151. doi: 10.1093/aje/kwn067. [DOI] [PubMed] [Google Scholar]
- Lochner K, Kawachi I, Kennedy BP. Social capital: a guide to its measurement. Health & Place. 1999;5:259–270. doi: 10.1016/s1353-8292(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main M, Kaplan N, Cassidy J. Security in infancy, childhood, and adulthood: A move to the level of representation. In: Bretherton I, Waters E, editors. Growing points in attachment theory and research (Serial No. 209). Monographs of the Society for Research in Child Development. Vol. 50. University of Chicago Press; Chicago, IL: 1985. pp. 66–104. [Google Scholar]
- McElhaney KB, Antonishak J, Allen JP. “They like me, they like me not”: Popularity and adolescents’ perceptions of acceptance predicting social functioning over time. Child Development. 2008;79:720–731. doi: 10.1111/j.1467-8624.2008.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Adult attachment and affect regulation. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. 2nd ed Guilford Press; New York, NY: 2008. pp. 503–531. [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience & Biobehavioral Reviews. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Pettit GS, Erath SA, Lansford JE, Dodge KA, Bates JE. Dimensions of social capital and life adjustment in the transition to early adulthood. International Journal of Behavioral Development. 2011;35(6):482–489. doi: 10.1177/0165025411422995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekers Y, Haun D, Tomasello M. Children, but Not Chimpanzees, Prefer to Collaborate. Current Biology. 2011;21:1756–1758. doi: 10.1016/j.cub.2011.08.066. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh MJ, Shoham V, Coyne JC. Effect of marital quality on eight-year survival of patients with heart failure. The American journal of cardiology. 2006;98:1069–1072. doi: 10.1016/j.amjcard.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Law RW, Portley RM. Divorce and Death. Perspectives on Psychological Science. 2011;6:454–474. doi: 10.1177/1745691611414724. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, et al. Toward a second-person neuroscience. Behavioral and Brain Sciences. doi: 10.1017/S0140525X12000660. (in press) [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, et al. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience. 2010;22(12):2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Smith EA. Communication and collective action: language and the evolution of human cooperation. Evolution and Human Behavior. 2010;31:231–245. [Google Scholar]
- Stams GJJM, Juffer F, van IJzendoorn MH. Maternal sensitivity, infant attachment, and temperament in early childhood predict adjustment in middle childhood: The case of adopted children and their biologically unrelated parents. Developmental psychology. 2002;38(5):806. doi: 10.1037//0012-1649.38.5.806. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and Befriend: Biobehavioral Bases of Affiliation Under Stress. Current Directions in Psychological Science. 2006;15:273–277. [Google Scholar]
- van der Horst M, Coffe H. How friendship network characteristics influence subjective well-being. Social Indicators Research. 2011:1–21. doi: 10.1007/s11205-011-9861-2. DOI10.1007/s11205-11011-19861-11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: a practical guide using statistical software. Chapman & Hall/CRC; Boca Raton, FL: 2007. [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Annals of the New York Academy of Sciences. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic {micro}-opioid neurotransmission. Archives of General Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]