Abstract
HIV immune activation plays an important role in the immunopathogenesis of the disease. The mechanisms driving this immune activation are partially defined and likely are the result of multiple factors. The introduction of combination antiretroviral therapy (cART) has improved the life expectancy of HIV infected individuals, however there is evidence that in the setting of “undetectable” HIV-RNA plasma levels, there is some level of persistent immune activation in these patients. A better understanding of the immune activation pathways should be of value in developing complementary therapies to restore the immune systems’ of patients with HIV infection. This review discuss the cytokine mediated pathways of immune activation of the CD4 and CD8 T cell pools during HIV infection.
Keywords: CD4 and CD8 T cell immune activation, T cell homeostasis, IL-7, Type-I IFN
1. Pathogenesis of HIV infection
HIV infection targets the immune system leading to a state of immunodeficiency in a setting of immune activation. The molecular mechanisms causing the pathogenesis of HIV infection are still incompletely understood and are probably a composite of multiple factors. The acute phase of HIV infection or SIV infected rhesus macaques (RM), is characterized by a substantial drop in peripheral CD4 T cell counts and a substantial depletion of memory CD4+CCR5+ T cells [1–5]. In the chronic phase, a continued decline of CD4 T cells associated with ongoing HIV replication leads to the development of AIDS. The depletion of CD4 T cells by HIV direct infection only partially explains the CD4 T cell pool depletion and a variety of bystander mechanisms have been described as contributing factors to CD4+ T cell death [6–9].
Early observations had shown that the selective depletion of the CD4 T cells was accompanied by an aberrant immune activation of all the components of the immune system in patients with HIV infection [10–12]. In the setting of an adaptive immune response against the virus, virtually all the cellular components of the immune system: B cells, NK cells, monocytes, macrophages and T cells (HIV and non-HIV-specific CD4 and CD8 T cells) show evidence of immune activation [10, 12–20]. Especially, in the T cell compartment, immune activation is evidenced by increased T cell proliferation [21–25] and increased expression of cell surface activation markers such as HLA-DR and CD38 [26–28]. In some studies this immune activation has been found to be a better correlate of clinical disease progression than CD4 T cell counts or HIV-RNA levels [13, 29], leading to the hypothesis that immune activation is a critical component in the pathogenesis of the disease. The studies of SIV infection in natural hosts, Sooty mengabeys (SM) and African green monkeys (AGM) have contributed to a better understanding of the role of immune activation in retroviral infections. In these animals, primary acute infection is associated with a modest and transient decline of peripheral blood CD4 T cells in association with a severe depletion of CD4 T cells in mucosal tissues such as gut-associated lymphatic tissues (GALT) and lung [30, 31]. The chronic phase of infection is characterized by low levels of immune activation despite high levels of viremia. In contrast in non-human primates who develop an AIDS-like illness following SIV infection (such as rhesus macaques (RM)) the chronic phase of infection is characterized by chronic immune activation similar to that observed in human HIV infection [32].
There is substantial evidence that immune activation plays an important role in the immunopathogenesis of HIV disease, however, what triggers this immune activation; or how the natural hosts are able to down-regulate this response in the chronic phase of the infection are still unresolved questions.
The introduction of combination antiretroviral therapy (cART) has improved the life expectancy of HIV infected individuals. An increasing body of data has clearly demonstrated that despite “undetectable” HIV-RNA plasma levels (generally <50 copies/ml) following initiation of therapy there remains evidence of persistent immune activation, albeit at a lower level. This persistent immune activation takes a variety of forms. Its clinical significance is strongly suggested by the increased risks of all-cause mortality associated with elevated levels of soluble markers of inflammation and coagulation such as, IL-6, sCD14 and D-dimer [33]. A better understanding of the pathways involved in immune activation pathways during HIV infection should be of value in the development of adjunct therapies that might lead to a more quiescent immune system.
2. T cell immune activation
It is likely that multiple forces are responsible for the disruption of the immune systems of patients with HIV infection (Figure 1). An unresolved paradox in patients with HIV infection is that while both CD4 and CD8 T cells are activated, one sees depletion of the CD4 T cell pool and expansion of the CD8 T cell pool. A direct cytopathic effect of HIV infection on CD4 T cells may explain part of this difference, however, the low number of cells actively infected at any given point makes this an unsatisfactory explanation of the great dichotomy seen between these two subsets [6–8].
Figure 1. Cytokines mediated pathways of immune activation in HIV infection.
Distinct pathways in the immune activation of the CD4 and CD8 T cell pools in HIV infection: role of the homeostatic forces (CD4 T cell counts/lymphopenia and IL-7) and inflammatory forces (HIV-replication and Type-I IFNs)
Increased activation of CD4 and CD8 T cells can be measured by proliferation, in vitro and in vivo, through examination of the expression of nuclear antigens such as Ki67, measurement of DNA content, or labeling with DNA precursors [21, 23, 34–37]. Studies of in vitro and in vivo labeling in patients with HIV infection have shown that proliferation of both the CD4 and CD8 T cell pools is directly related to the level of HIV viremia and significantly decreases after initiation of cART [21–23, 25, 38]. These earliers studies however, do not explain the selective depletion of CD4 T cells and expansion of CD8 T cells. One hypothesis suggested by this observation is that different pathways of activation are triggered in CD4 and CD8 T cell subsets with different end results. Among the possible pathways that have been studied are those associated with T cell homeostasis and those involved in the response to infection/inflammation (pathogen-induced factors).
2.1. CD4 T cell immune activation is driven by the homeostatic response to lymphopenia as well as HIV-induced inflammation
Homeostatic responses of the CD4 T cell pool are likely regulated to only allow a limited degree of expansion for each individual cell so as to preserve diversity of the repertoire [39, 40]. In lymphopenic conditions such as HIV-induced lymphopenia, post-bone marrow transplant and idiopathic CD4 T cell lymphopenia, there is a homeostatic response reflected by robust proliferation of T cells in response to increased levels of homoestatic cytokines such as IL-7 [41–46]. This process, triggered in response to alterations in the pool size, is designed to restore of steady-state levels of CD4 T cells. [44, 47].
We have recently tested the hypothesis that CD4 T cell activation in the setting of HIV infection is the net result of homeostatic forces derived from CD4 depletion coupled with the influence of an inflammatory environment that is generated and maintained by HIV-replication (Figure 1). By measuring ex-vivo proliferation in a large cohort of HIV infected individuals, we found that CD4 T cell proliferation was driven by the combination of CD4 depletion and HIV viral load. The strong associations between CD4 T cell proliferation and CD4 T cell depletion suggest that homeostatic forces represent an important factor in the CD4 T cell immune activation seen in patients with HIV infection [41, 48, 49]. The role of HIV viral load is discussed in the next section.
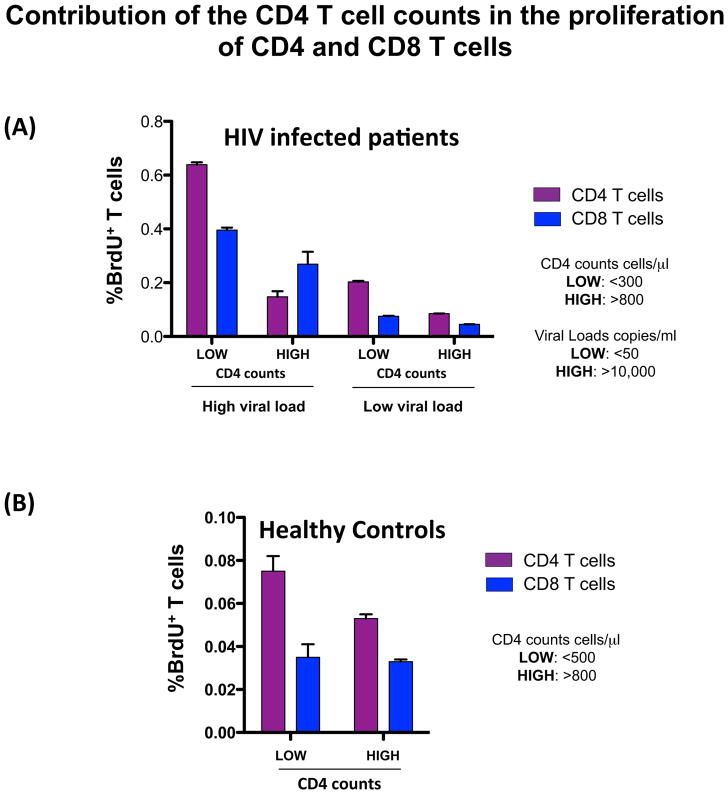
To better understand the contribution of homeostatic forces to the immune activation of the CD4 T cell pool, we analyzed the spontaneous ex vivo proliferation of CD4 T cells from healthy controls and HIV infected patients. Our data demonstrated that CD4 T cell proliferation was highly controlled by the CD4 counts and this regulatory mechanism was active during HIV infection (Figure 2). Interestingly, we also found that HIV-induced lymphopenia was the principal force driving proliferation of naïve CD4 T cells. In contrast, memory CD4 T cell proliferation was associated with both CD4 T cell depletion and HIV-RNA levels [50]. These same associations were also observed in studies utilizing the in vivo labeling of proliferating cells with BrdU [51]. These data are consistent with a recent report that, in humans, the maintenance of the naïve CD4 T cells pool is mainly mediated by expansion of peripheral naïve T cells, and also provides evidence of the important role of peripheral homeostasis in HIV infection [52, 53]. In addition, recent reports in the pathogenic model of SIV infection (RM) have suggested independent pathways of homeostasis in the naïve and memory T cell compartments in the context of chronic SIV infection [54].
Figure 2. Contribution of the homeostatic forces (CD4 T cell counts) and HIV-RNA levels in the proliferation of CD4 and CD8 T cell pools.
HIV infected patients (A) and Healthy controls (B).
2.2. CD8 T cell immune activation is predominantly driven by HIV-replication and its associated inflammatory environment
The study to define the forces driving proliferation of CD8 T cells showed that levels of HIV-RNA were the main influence. In contrast to CD4 T cells, there was no evidence of homeostatic forces contributing to the proliferation of CD8 T cells [48]. This was in agreement with early studies demonstrating that proliferation of the T cells in HIV infected patients was a function of the level of viremia and was significantly reduced after treatment with cART [21, 23, 25, 55]. The studies of in vitro [50] and in vivo [51] proliferation of the CD8 T cell subsets, showed that the rates of proliferation of naïve CD8 T cells, unlike naïve CD4 T cells, only correlated with HIV-RNA levels, rather than both HIV-RNA levels and homeostatic forces. In addition, an analysis of the relative contributions of CD4 and/or CD8 T cell counts to the proliferation of the CD8 T cell pool in healthy controls (Figure 2 and [50]), revealed that CD8 T cell proliferation was not influenced by the size of either the CD4 or CD8 T cell pools. These results highlight intrinsic differences in the homeostatic regulation of the CD4 and CD8 T cell pool and suggest that the size of the CD8 T cell pool is not generally under tight homeostatic control [47, 50, 53]. In addition, these results showed evidence of the great capacity of the CD8 T cell pool to expand in response to inflammation/viral infection.
3. Cytokine and immune activation pathways
In the course of chronic HIV infection, homeostatic forces and HIV-induced inflammation differentially affect CD4 and CD8 T cell immune activation. These differences are reflected in the ways that these T cell pools respond to the inflammatory and homeostatic environments (Figure 1).
3.1. HIV-induced lymphopenia: the role of IL-7
It has been shown that levels of IL-7 in serum and tissue during HIV-induced lymphopenia are strongly correlated with the degree of CD4 T cell depletion [41]. IL-7 is a member of the common gamma-chain (γc) family of cytokines that includes IL-2, IL-15 and others. IL-7 is present in most tissues and produced by a variety of cells, including: fibroblastic reticular cells (FRC) in the T cell zone of lymphoid organs; thymic, liver and intestinal epithelial cells; fibroblasts; keratinocytes; and dendritic cells [56–58]. Studies have shown that IL-7 plays a crucial role in naïve and memory T cell homeostasis by regulating survival, proliferation and repertoire diversity [39, 43]. IL-7 signals through the IL-7 receptor (IL-7R), an heterodimer consisting of the common-gamma chain receptor (γc or CD132) and the IL-7 receptor alpha chain (IL-7Rα or CD127). Engagement of IL-7R by IL-7 activates Janus kinase-signal transducers and activators of transcription (JAK-STAT) (mainly JAK1, JAK3 and STAT5), phosphatidylinositol 3-kinase (PI3K) and Src family kinases signaling pathways [59, 60].
Consistent with the observation that homeostatic forces can drive proliferation of the CD4 T cell pool during HIV infection, we found increased mRNA expression of genes associated with γc cytokine signaling (such IL2RG, SOCS1 and STAT5) in naïve and memory CD4 T cells from patients with HIV-associated CD4 lymphopenia. In contrast, the CD8 T cell subsets showed decreased expression of these transcripts [50]. In addition, naïve CD4 and CD8 T cells were able to respond to in vitro stimulation with IL-7 as measured by STAT-5 phosphorylation. However, memory CD8 T cells showed an impaired response to IL-7 consistent with the reported decrease of CD127 on this subset [61, 62]. Memory CD4 T cells were able to phosphorylate STAT-5 in response to in vitro stimulation. These observations are again consistent with the hypothesis that CD4 and CD8 T cell subsets respond differently to homeostatic forces [50].
It have been suggested that HIV-induced lymphopenia and the associated increased levels of IL-7 play a role in the up-regulation of the expression of the death receptor Fas on naïve T cells and an increased sensitivity to Fas-mediated apoptosis in T cells that express CD127 [63–66].
An important component of CD4 T cell homeostasis is the circulation of cells through lymphoid organs where they have the opportunity to encounter their cognate antigen and/or to receive survival signals from homeostatic cytokines [67]. Lymphoid organs of patients with chronic HIV infection and non-human primate modes of pathogenic SIV infection demonstrate increased fibrosis suggesting that these relationships may be altered in the setting of HIV infection [68–70]. Such alterations in lymphoid tissue mediated homeostasis including exposure to IL-7 and other survival signals may play a role in the depletion of CD4 and CD8 naïve [71] and memory [72] T cells. Of note is the fact that this process can be at least partially reversed by cART [73].
3.1.1. γc-cytokines and therapy
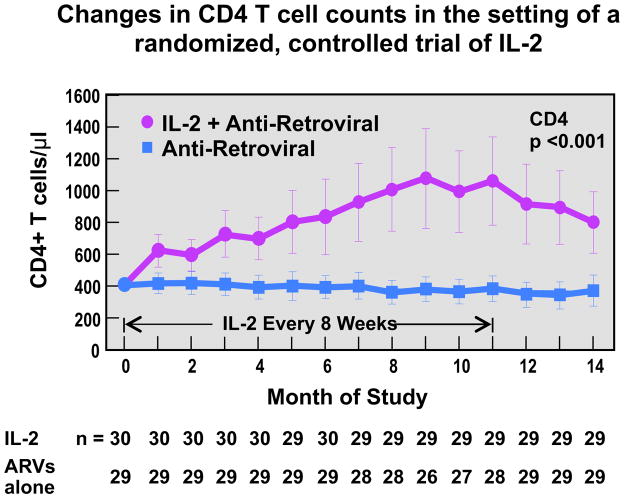
Given these data it is not surprising that γc using cytokines have been studied as possible therapeutic agents in patients with HIV infection in general, and patients with persistent lymphopenia in particular. Extensive studies with IL-2 have demonstrated that this cytokine is capable of increasing levels of naïve and central memory CD4 T cells with minimal effects on the CD8 T cell pool (Figure 3) [74, 75]. While capable of inducing “bursts” of HIV viremia in patients not on effective cART, IL-2 administration was not associated with increases in plasma levels of HIV. The cells induced by IL-2 exhibit increased expression of CD25 and FOXP3 and thus are similar to regulatory T cells [76]. Phase III trials of this cytokine, however, demonstrated that these increases were of no clinical benefit [77]. The reason for this paradox is still unclear but likely reflects the fact that any benefits derived from CD4 T cell expansion were countered by the well-known “cytokine-storm” side effects of IL-2. More recently IL-7 has entered the clinical arena and has been shown capable of inducing increases in numbers of not only CD4 T cells, but CD8 T cells as well [78, 79]. Whether or not theses increases will prove to be of clinical benefit is under study. As in the case of IL-2 the “blips” of viremia observed during IL-7 administration appear to be transient and similar to the viruses present prior to therapy [80]. IL-15 has been extensively studied in non-human primate SIV models of HIV infection. Studies of IL-15 in acute SIV infection have demonstrated increases in the levels of SIV [81] while studies in chronic SIV infection have shown little, to no effect [82, 83]. Of note is the fact that the route and duration of IL-15 administration may lead to drastically different effects on the immune system. In this regard, continuous I.V. infusion of low-dose IL-15 has been associated with a 100-fold increase in the levels of effector memory CD8 T cells [84].
Figure 3. Effect of IL-2 administration in CD4 T cell counts.
The error bars represent ±2 SE and approximate the 95 percent confidence intervals. Values at month 0 (base line) are the means of three values measured before the beginning of the study.
3.2. The role of Type-I IFN and the HIV-induced inflammatory environment
Type-I IFNs are a group of cytokines that exhibit anti-viral and immunoregulatory properties in the setting of a viral infection [85]. In HIV infection, Type-I IFNs have also been associated with immunopathogenesis. In vitro, plasmacytoid dendritic cells from healthy controls can be induced by infectious or non-infectious HIV to secrete Type-I IFNs [86] that can lead to an increased expression of death receptors (DR5/TRAIL) on primary CD4 T cells [86, 87]. Type-I IFN-dependent increases of the enzyme 2,3-dyoxigenase (IDO) in plamacytoid dendritic cells have been detected in the lymphoid tissues of patients with HIV infection [88, 89]. IDO catalyzes the degradation of an essential amino acid (tryptophan) which is important for the metabolism of T cells [90].
Taken together, these observations have led to the hypothesis that chronic exposure to Type-I IFN can play a role in the pathogenesis of HIV infection. The role of the chronic exposure to Type-I IFNs and immunopathogenesis of HIV infection have also been noted in studies of non-pathogenic SIV infection (SM and AGM). The acute infection in these animals is very similar to that observed in pathogenic models of SIV infection (RM) in which a robust Type-I IFN response dominates this first phase of the infection. In the chronic phase, while SM and AGM are able to down-regulate the inflammatory response and interferon production, SIV infected RM are not and show a sustained Type-I IFN response and progression to AIDS [32]. This downregulation of Type-1 IFN response is seen in the non-pathogenic models despite high viral replication and is associated with decreases in immune activation and increases in transcriptional profiles of regulatory cytokines [91–93]. In humans with chronic HIV infection a strong transcriptional profile of genes associated with Type-I IFN signaling has been described [94–97]. To understand any differences in the effects of HIV infection and interferon exposure in the immune activation of CD4 and CD8 T cells we analyzed the transcriptional profile of genes associated with Type-I IFN signaling in naïve and memory CD4 and CD8 T cell subsets. Both, CD4 and CD8 T cells from viremic HIV infected individuals showed increased mRNA transcripts associated with Type-I IFN signaling. Interestingly, naïve and memory CD4 T cells demonstrated enhanced STAT1 phosphorylation in response to Type-I IFN in vitro. This was not observed in CD8 T cell subset [50]. These results highlight differences in the ability of CD4 and CD8 T cells from patients with HIV infection to respond to Type-I IFN. This enhanced responsiveness of CD4 T cells to Type-I IFN may have detrimental consequences on CD4 T cell homeostasis and survival.
Given this and the data on the impact of homeostatic cytokines in the setting of HIV infection, we can postulate a scenario whereby exposure to homeostatic cytokines creates a state within the CD4 (but not the CD8) T cell pool where chronic exposure to Type-I IFN leads to depletion of the pool. In contrast the CD8 pool has little response to homeostatic forces and undergoes expansion in the setting of an antigen-driven and inflammatory stimuli.
3.2.1 IFN-alpha and therapy
One seeming paradox in the clinical management of patients with HIV infection is the fact that this cytokine has both anti-neoplastic and anti-viral activities when given therapeutically (Figure 4). IFN-alpha is licensed for the treatment of AIDS-related Kaposi’s sarcoma. Of note is the fact that the clinical efficacy of IFN-alpha in this setting is directly correlated with the CD4 T cell count of the patient strongly suggesting that this anti-tumor effect is due to modulation of the immune system rather than due to a direct anti-proliferative effect of IFNs. Similarly, when given to patients with early stages of HIV infection, IFN-alpha has an anti-viral effect that is stronger than the anti-retroviral effect of the first licensed antiretroviral drug, zidovudine (AZT) [98]. Again supporting the hypothesis that IFN-alpha has potentially positive immunomodulatory effects in patients with early stages of HIV infection are the observations that the anti-retroviral effects of IFN-alpha are most pronounced in patients with the highest CD4 counts and that the loss of activity seen over time is not associated with the emergence of resistant strains of HIV. In other words, IFN-alpha is not acting as a classic antiviral in this setting. Also of note is the fact that responses of patients with hepatitis C to IFN-alpha are better in patients with lower levels of IFN-associated gene activation pre-therapy [99]. In addition, IFN-alpha may have a therapeutic effect in some patients with HIV infection, but only in those patients not exhibiting high levels of IFN-alpha in vivo at the time therapy is initiated.
Figure 4. Effects of IFN-alpha treatment.
(A) Effect of IFN-alpha administration on facial Kaposi’s Sarcoma (KS) lesions. (B) Model-based mean change in log HIV-RNA during IFN-alpha administration in HIV infected patients. Abbreviations: AZT, zidovudine.
4. Inflammation and Biomarkers: IL-6, sCD14 and D-dimer
The introduction of cART has improved the life expectancy of HIV infected individuals. While antiretroviral therapies are capable of suppressing plasma levels of HIV to <50 copies/ml for extended periods of time, levels of viral replication return to baseline within weeks of stopping therapy and thus patients with HIV infection today face the likelihood of life-long therapy. An increasing body of data are clearly demonstrating that despite plasma levels of HIV that are “undetectable” there is evidence of persistent virus and persistent immune activation in patients receiving antiretroviral therapy [100, 101]. This persistent intracellular reservoir of HIV is felt to be present at several anatomical locations such as the peripheral lymphoid tissue, gastrointestinal tract and central nervous system [8, 102–105]. The persistent immune activation seen in patients with HIV infection despite HIV-RNA levels<50 copies/ml is likely associated with this reservoir and is clinically significant. Compared to control populations, patients with HIV infection have been shown to have increased levels of the inflammatory markers IL-6 and sCD14 and the fibrinogen breakdown product D-dimer. Patients with higher levels of these biomarkers are at an increased risk of all-cause mortality and significant hepatic, metabolic, renal and cardiovascular morbidity is observed in these patients despite HIV RNA levels <50 copies/ml [33, 106]. Levels of these biomarkers are directly correlated with levels of HIV RNA following discontinuation of cART reflecting the important relationships between viral load, inflammation, coagulation and end-organ damage in the setting of HIV infection.
In summary, the immune systems of patients with HIV infection are characterized by an immunodeficiency occurring in the setting of immune activation. The CD4 T cell pool declines while the CD8 T cell pool expands. Homeostatic cytokines such as IL-7 and pro-inflammatory cytokines such as IFN-alpha are elaborated and may play a significant role in some of the pathologic aspects of HIV infection. An interaction between IL-7 and IFN-alpha signaling may be responsible for the death of CD4 T cells. Both of these cytokines have been studied as potential therapeutic agents in the setting of HIV-1 infection. While IL-7 has been shown capable of expanding the CD4 and CD8 pools of T cells the clinical impact of these expansions is, as yet, unknown. IFN-alpha is a FDA-approved treatment for Kaposi’s sarcoma and has been shown to have anti-HIV properties but the precise mechanisms whereby these effects take place have not been determined. Further understanding the role of these and other cytokines in the setting of HIV-1 infection will not only expand our knowledge of the pathogenesis and treatment of HIV but also enhance our understanding of the role of the cytokines in health and disease.
Contributor Information
Cecile Le Saout, Email: catalfam@mail.nih.gov, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, Bldg 10 Room 11B07, Bethesda, MD 20892-1360, Phone: 301-443-8313, FAX: 301-402-4097.
H Clifford Lane, Email: CLANE@niaid.nih.gov, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, Rm. 4-1479, MSC 1460, Bethesda, MD 20892-1360, Office: 301-496-6572 I, Fax: 301-480-5560.
Marta Catalfamo, Email: catalfam@mail.nih.gov, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, Bldg 10 Room 11B07, Bethesda, MD 20892-1360, Phone: 301-496-5309, FAX: 301-402-4097.
References
- 1.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science (New York, NY. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 5.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 6.Brinchmann JE, Albert J, Vartdal F. Few infected CD4+ T cells but a high proportion of replication-competent provirus copies in asymptomatic human immunodeficiency virus type 1 infection. Journal of virology. 1991;65:2019–23. doi: 10.1128/jvi.65.4.2019-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science (New York, NY. 1996;274:985–9. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 8.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 9.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annual review of immunology. 1999;17:625–56. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 10.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. The New England journal of medicine. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 11.Lane HC, Fauci AS. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annual Review of Immunology. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- 12.Ascher MS, Sheppard HW. AIDS as immune system activation: a model for pathogenesis. Clinical and experimental immunology. 1988;73:165–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 14.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 16.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. 2004;173:2410–8. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 17.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nature reviews. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 18.Derdeyn CA, Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Current opinion in immunology. 2005;17:366–73. doi: 10.1016/j.coi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nature immunology. 2006;7:235–9. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 20.van Grevenynghe J, Cubas RA, Noto A, Dafonseca S, He Z, Peretz Y, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL- mediated apoptosis. The Journal of clinical investigation. 2011;121:3877–88. doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lempicki RA, Kovacs JA, Baseler MW, Adelsberger JW, Dewar RL, Natarajan V, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13778–83. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune JM, Hanley MB, Cesar D, Halvorsen R, Hoh R, Schmidt D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. The Journal of clinical investigation. 2000;105:R1–8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs JA, Lempicki RA, Sidorov IA, Adelsberger JW, Herpin B, Metcalf JA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. The Journal of experimental medicine. 2001;194:1731–41. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleury S, de Boer RJ, Rizzardi GP, Wolthers KC, Otto SA, Welbon CC, et al. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nature medicine. 1998;4:794–801. doi: 10.1038/nm0798-794. [DOI] [PubMed] [Google Scholar]
- 25.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nature immunology. 2000;1:285–9. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 26.Ho HN, Hultin LE, Mitsuyasu RT, Matud JL, Hausner MA, Bockstoce D, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–9. [PubMed] [Google Scholar]
- 27.Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders RL, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clinical and experimental immunology. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam M, Peakman M, Davies ET, Pozniak A, McManus TJ, Vergani D. T cell activation and disease severity in HIV infection. Clinical and experimental immunology. 1993;93:337–43. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS (London, England) 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 30.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. Journal of immunology. 2007;179:3026–34. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. Journal of immunology. 2007;179:3035–46. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science (New York, NY. 2012;335:1188–93. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachsenberg N, Perelson AS, Yerly S, Schockmel GA, Leduc D, Hirschel B, et al. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. The Journal of experimental medicine. 1998;187:1295–303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ZQ, Notermans DW, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, et al. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1154–9. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 37.Sieg SF, Rodriguez B, Asaad R, Jiang W, Bazdar DA, Lederman MM. Peripheral S-phase T cells in HIV disease have a central memory phenotype and rarely have evidence of recent T cell receptor engagement. The Journal of infectious diseases. 2005;192:62–70. doi: 10.1086/430620. [DOI] [PubMed] [Google Scholar]
- 38.Mohri H, Perelson AS, Tung K, Ribeiro RM, Ramratnam B, Markowitz M, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. The Journal of experimental medicine. 2001;194:1277–87. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Asquith B, Borghans JA, Ganusov VV, Macallan DC. Lymphocyte kinetics in health and disease. Trends Immunol. 2009;30:182–9. doi: 10.1016/j.it.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nature medicine. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 42.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 43.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 44.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Seminars in immunology. 1997;9:339–46. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 45.Malaspina A, Moir S, Chaitt DG, Rehm CA, Kottilil S, Falloon J, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–8. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nature medicine. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 47.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–7. [PubMed] [Google Scholar]
- 48.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19851–6. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lane HC. Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Topics in HIV medicine: a publication of the International AIDS Society, USA. 2010;18:2–6. [PubMed] [Google Scholar]
- 50.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, et al. CD4 and CD8 T Cell Immune Activation during Chronic HIV Infection: Roles of Homeostasis, HIV, Type I IFN, and IL-7. J Immunol. 2011;186:2106–16. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasula S, Lempicki RA, Adelsberger JW, Huang CY, Roark J, Lee PI, et al. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood. 2011;118:262–70. doi: 10.1182/blood-2011-02-335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, Bregje de Boer A, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–97. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 54.Okoye AA, Rohankhedkar M, Abana C, Pattenn A, Reyes M, Pexton C, et al. Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection. The Journal of experimental medicine. 2012;209:641–51. doi: 10.1084/jem.20112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nature medicine. 2006;12:289–95. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 56.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nature reviews Immunology. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892– 904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 58.Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, et al. Visualization and identification of IL-7 producing cells in reporter mice. PloS one. 2009;4:e7637. doi: 10.1371/journal.pone.0007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109 (Suppl):S121–31. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 60.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nature reviews Immunology. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 61.Landires I, Bugault F, Lambotte O, de Truchis P, Slama L, Danckaert A, et al. HIV infection perturbs interleukin-7 signaling at the step of STAT5 nuclear relocalization. AIDS (London, England) 2011;25:1843–53. doi: 10.1097/QAD.0b013e32834a3678. [DOI] [PubMed] [Google Scholar]
- 62.Crawley AM, Angel JB. Expression of gamma-chain cytokine receptors on CD8(+) T cells in HIV infection with a focus on IL-7Ralpha (CD127) Immunology and cell biology. 2011 doi: 10.1038/icb.2011.66. [DOI] [PubMed] [Google Scholar]
- 63.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. Journal of immunology. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 64.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 signalling is sufficient to phenotypically and functionally prime human CD4 naive T cells. Immunology. 2005;114:322–35. doi: 10.1111/j.1365-2567.2004.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fluur C, De Milito A, Fry TJ, Vivar N, Eidsmo L, Atlas A, et al. Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection. Journal of immunology. 2007;178:5340–50. doi: 10.4049/jimmunol.178.8.5340. [DOI] [PubMed] [Google Scholar]
- 66.Rethi B, Vivar N, Sammicheli S, Fluur C, Ruffin N, Atlas A, et al. Priming of T cells to Fas-mediated proliferative signals by interleukin-7. Blood. 2008;112:1195–204. doi: 10.1182/blood-2007-12-126698. [DOI] [PubMed] [Google Scholar]
- 67.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nature reviews Immunology. 2009;9:823–32. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 68.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. The Journal of clinical investigation. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Grevenynghe J, Halwani R, Chomont N, Ancuta P, Peretz Y, Tanel A, et al. Lymph node architecture collapse and consequent modulation of FOXO3a pathway on memory T- and B-cells during HIV infection. Seminars in immunology. 2008;20:196–203. doi: 10.1016/j.smim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. The Journal of clinical investigation. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Grevenynghe J, Procopio FA, He Z, Chomont N, Riou C, Zhang Y, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nature medicine. 2008;14:266–74. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 73.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT, Jr, Walker RE, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. The New England journal of medicine. 1996;335:1350–6. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 75.Kovacs JA, Baseler M, Dewar RJ, Vogel S, Davey RT, Jr, Falloon J, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. The New England journal of medicine. 1995;332:567–75. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 76.Sereti I, Imamichi H, Natarajan V, Imamichi T, Ramchandani MS, Badralmaa Y, et al. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. The Journal of clinical investigation. 2005;115:1839–47. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin- 2 therapy in patients with HIV infection. The New England journal of medicine. 2009;361:1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. The Journal of clinical investigation. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imamichi H, Degray G, Asmuth DM, Fischl MA, Landay AL, Lederman MM, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS (London, England) 2011;25:159–64. doi: 10.1097/QAD.0b013e328340a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. Journal of immunology. 2008;180:350–60. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. The Journal of clinical investigation. 2006;116:1514–24. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–9. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–8. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth factors. 2004;22:243–51. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- 86.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7000–5. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clinical immunology. 2007;123:121–8. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boasso A, Shearer GM. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr Drug Metab. 2007;8:217–23. doi: 10.2174/138920007780362527. [DOI] [PubMed] [Google Scholar]
- 90.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of clinical investigation. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. The Journal of clinical investigation. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nature medicine. 2008;14:1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 94.Sedaghat AR, German J, Teslovich TM, Cofrancesco J, Jr, Jie CC, Talbot CC, Jr, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. Journal of virology. 2008;82:1870–83. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. Journal of virology. 2007;81:3477–86. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. The Journal of infectious diseases. 2004;189:572–82. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 97.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. The Journal of clinical investigation. 2011;121:2391–400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tavel JA, Huang CY, Shen J, Metcalf JA, Dewar R, Shah A, et al. Interferon-alpha produces significant decreases in HIV load. J Interferon Cytokine Res. 2010;30:461–4. doi: 10.1089/jir.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lempicki RA, Polis MA, Yang J, McLaughlin M, Koratich C, Huang DW, et al. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV- coinfected persons. The Journal of infectious diseases. 2006;193:1172–7. doi: 10.1086/501365. [DOI] [PubMed] [Google Scholar]
- 100.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011 doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 101.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nature medicine. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 102.Clarke JR, Taylor IK, Fleming J, Nukuna A, Williamson JD, Mitchell DM. The epidemiology of HIV-1 infection of the lung in AIDS patients. AIDS (London, England) 1993;7:555–60. doi: 10.1097/00002030-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 103.Di Stefano M, Sabri F, Leitner T, Svennerholm B, Hagberg L, Norkrans G, et al. Reverse transcriptase sequence of paired isolates of cerebrospinal fluid and blood from patients infected with human immunodeficiency virus type 1 during zidovudine treatment. J Clin Microbiol. 1995;33:352–5. doi: 10.1128/jcm.33.2.352-355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van’t Wout AB, Ran LJ, Kuiken CL, Kootstra NA, Pals ST, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. Journal of virology. 1998;72:488–96. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chomont N, DaFonseca S, Vandergeeten C, Ancuta P, Sekaly RP. Maintenance of CD4+ T-cell memory and HIV persistence: keeping memory, keeping HIV. Curr Opin HIV AIDS. 2011;6:30–6. doi: 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
- 106.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, et al. Changes in Inflammatory and Coagulation Biomarkers: A Randomized Comparison of Immediate versus Deferred Antiretroviral Therapy in Patients With HIV Infection. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]