Abstract
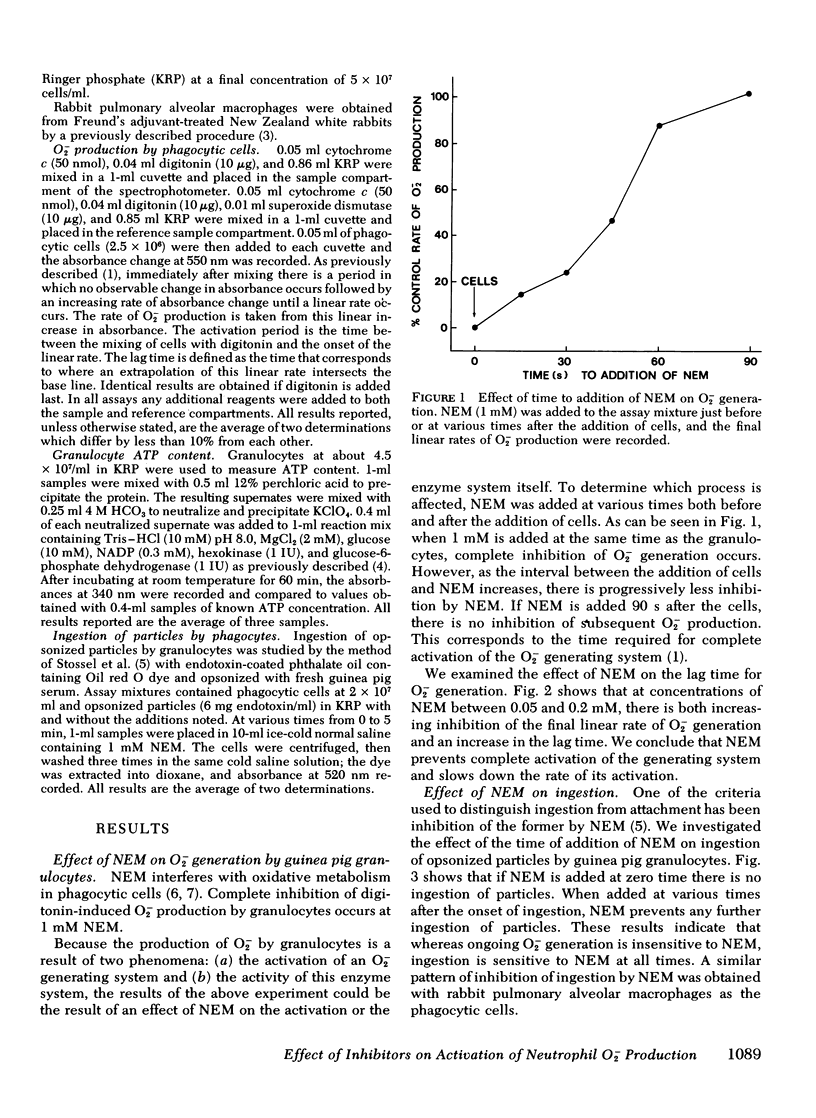
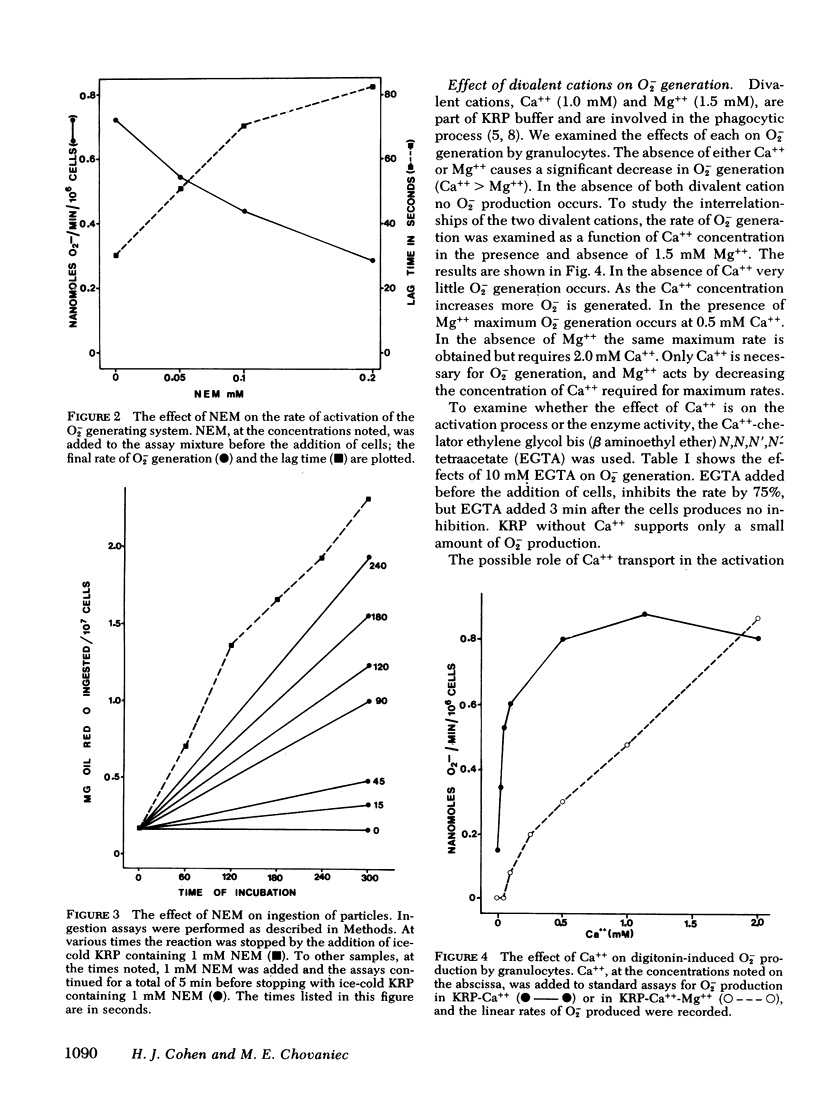
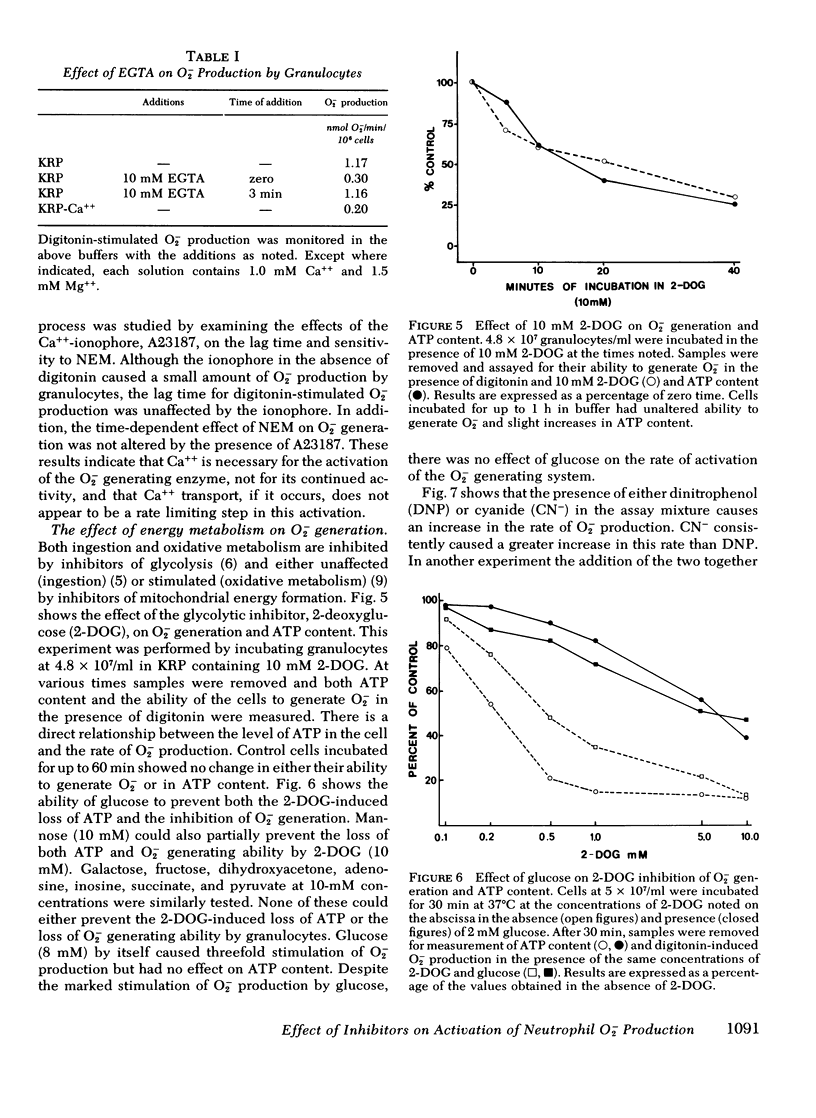
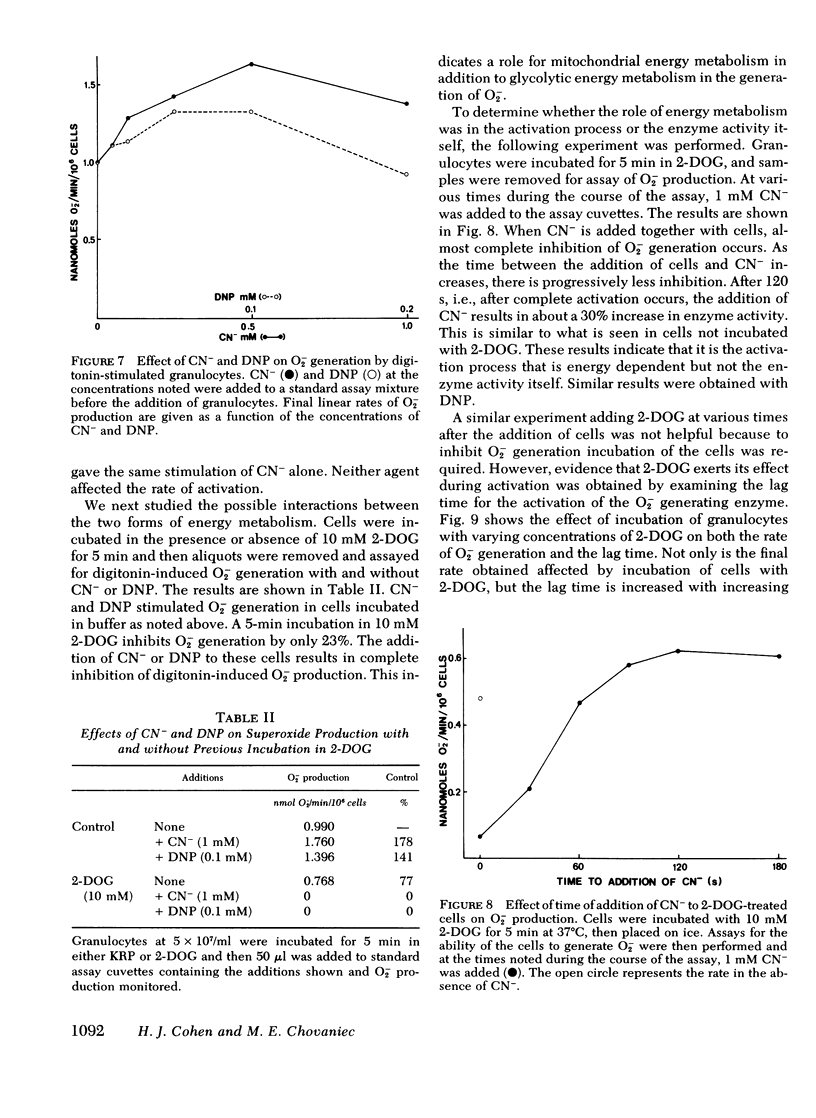
N-ethylmaleimide, divalent cations, ethylene glycol bis (β aminoethyl ether) N,N,N′,N′,-tetraacetate, 2-deoxyglucose, cyanide, and dinitrophenol were examined for their effect on the ability of guinea pig granulocytes to generate superoxide (O2−) when stimulated by digitonin. N-ethylmaleimide (1 mM) inhibits only when added before complete activation of the O2− generating system, and at lower concentrations (0.05-0.2 mM) slows the activation process. Ca++ is required for maximum O2− generation, and Mg++ decreases the amount of Ca++ required. Ethylene glycol bis (β aminoethyl ether) N,N,N′,N′,-tetraacetate (10 mM) inhibits only if added before complete activation. Incubation of cells in 2-DOG causes a time- and concentration-dependent inhibition of O2− generation. It also increases the time required for activation of this system. Cyanide and dinitrophenol increase the rate of O2− production. However, when these compounds are added to cells whose O2− production is partially inhibited by incubation in 2-deoxyglucose, complete inhibition results. If cyanide or dinitrophenol is added after activation of 2-deoxyglucose-treated cells, no further inhibition occurs. On the basis of the above results, we conclude that the activation of the O2− generating system is N-ethylmaleimide sensitive, Ca++ dependent, and energy requiring, but that the activity of the enzyme system in the cell is not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azimi P. H., Bodenbender J. G., Hintz R. L., Kontras S. B. Chronic granulomatous disease in three female siblings. JAMA. 1968 Dec 23;206(13):2865–2870. [PubMed] [Google Scholar]
- Boxer L. A., Baehner R. L., Davis J. The effect of 2-deoxyglucose on guinea pig polymorphonuclear leukocyte phagocytosis. J Cell Physiol. 1977 Apr;91(1):89–102. doi: 10.1002/jcp.1040910110. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Curnutte J. T., Karnovsky M. L., Babior B. M. Manganese-dependent NADPH oxidation by granulocyte particles. The role of superoxide and the nonphysiological nature of the manganese requirement. J Clin Invest. 1976 Apr;57(4):1059–1067. doi: 10.1172/JCI108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Shirley P. S. Effect of cyanide on NADPH oxidation by granules from human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):445–454. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J., Shafer A. W., Glass E. A., Karnovsky M. L. Metabolic and morphological observations on the effect of surface-active agents of leukocytes. J Cell Biol. 1967 Mar;32(3):629–647. doi: 10.1083/jcb.32.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvarstein B. Oxygen consumption during the initial stage of human leucocyte phagocytosis of polystyrene latex particles. Scand J Clin Lab Invest. 1970 Jun;25(4):337–348. doi: 10.3109/00365517009046214. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S., Wilfert C., Johnston R. B., Jr, McCall C. E. Deficiency of NADPH oxidase activity in chronic granulomatous disease. J Pediatr. 1977 Feb;90(2):213–217. doi: 10.1016/s0022-3476(77)80632-x. [DOI] [PubMed] [Google Scholar]
- PRANKERD T. A., ALTMAN K. I. A study of the metabolism of phosphorus in mammalian red cells. Biochem J. 1954 Dec;58(4):622–633. doi: 10.1042/bj0580622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- STAHELIN H., SUTER E., KARNOVSKY M. L. Studies on the interaction between phagocytes and tubercle bacilli. I. Observations on the metabolism of guinea pig leucocytes and the influence of phagocytosis. J Exp Med. 1956 Jul 1;104(1):121–136. doi: 10.1084/jem.104.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Phillips J. R., Acevedo J., Axline S. G., Theodore J. Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J Clin Invest. 1977 Mar;59(3):443–448. doi: 10.1172/JCI108658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolc V. Effect of calcium and lanthanum on iodine accumulation and release from human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1975 Oct;18(4):213–220. [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst D. B., Holmes B., Good R. A. A newly defined X-linked trait in man with demonstration of the Lyon effect in carrier females. Lancet. 1967 Apr 8;1(7493):737–739. doi: 10.1016/s0140-6736(67)91360-8. [DOI] [PubMed] [Google Scholar]


