Abstract
Melanoma differentiation-associated protein 5 (MDA-5) is a novel autoantibody frequently characterized by interstitial lung disease and a distinct cutaneous phenotype with palmar papules, ulceration, and rash. Virtually all patients have underlying dermatomyositis, but many lack the characteristic clinical myopathy associated with it. In the setting of amyopathic disease, the absence of clinically available biomarkers or clear pathologic diagnosis can complicate effective prognostic and therapeutic intervention. Until recently the presence of MDA-5 antibody associated dermato-pulmonary syndrome was described only in Asian populations. We present 2 cases of MDA-5-associated dermato-pulmonary syndrome and provide a comprehensive review of available literature.
INTRODUCTION
Dermatomyositis (DM) is an autoimmune inflammatory myopathy that involves the muscle, skin, and lung to various extents in different patients. At one end of the spectrum, myopathy is prominent with weakness of skeletal muscle, and even muscles of swallowing and respiration. At the other end of the spectrum, there is prominent skin disease, but minimal or absent muscle disease (clinically amyopathic DM [CADM]). [4] Patients with either overt myopathy or clinically amyopathic disease can manifest interstitial lung disease (ILD) as part of their clinical phenotype. The ILD is also variable in its course and severity. [6]
Only 50%–70% of patients with DM and CADM have identifiable myositis specific antibodies, [12] leaving a significant proportion of patients with DM who are apparently seronegative. In the setting of clinically amyopathic disease and/or nondiagnostic clinical and pathologic findings in the skin, a definitive diagnosis can be difficult to obtain, which can affect classification and institution of therapy. In 2005, Sato et al [27] identified a novel autoantibody recognizing a 140 kDa protein in patients with DM and CADM (initially termed “CADM-140”). The 140 kDa autoantigen was subsequently identified as melanoma differentiation-associated protein 5 (MDA-5). [28]
In the initial studies in Japanese cohorts, most patients had clinically amyopathic disease, and some patients had rapidly progressive ILD. [27,28] The cutaneous findings were not well described in these cohorts. Although prior reports had linked both unusual cutaneous findings of ulceration [19] and palmar papules [16] with ILD, it was Fiorentino et al [7] who suggested that the link between all of these clinical findings was autoimmunity to MDA-5. Thus these skin findings, which include skin ulceration and palmar papules, along with ILD and MDA-5 antibodies, comprise what we now term a “dermato-pulmonary syndrome.”
We present 2 severely ill patients with progressive ILD and cutaneous findings not diagnostic for classic DM, absent myositis, negative autoantibodies as determined using clinically available assays, and without diagnostic findings on skin and lung biopsy. Both patients had MDA-5 autoantibodies. We detail the characteristics of these 2 cases, and review the literature describing the typical presentation of the MDA-5 autoantibody-associated dermato-pulmonary syndrome.
CASE REPORTS
CASE 1
A 54-year-old white man developed an erythematous rash surrounding both eyes while vacationing in Florida. Within 2 weeks, he noted shortness of breath with exertion and painful fissuring of the distal aspects of his fingertips bilaterally. He was initially treated with a course of levofloxacin for presumed pneumonia. No improvement was seen, so a 12-day taper of prednisone starting with 30 mg daily was prescribed. He had no improvement with this intervention either, and his shortness of breath and rash persisted. Two months after initial symptom onset, a computed tomography (CT) scan (Figure 1A) of his chest showed subtle changes consistent with early ILD. Two weeks after the CT scan, the dyspnea progressed rapidly and he presented to the emergency department with acute hypoxemia. A second CT scan was performed (Figure 1B), which showed no pulmonary embolism but significant progression of bilateral interstitial and ground-glass opacities, consolidation in the bilateral lower lobes, and mediastinal lymphadenopathy (largest 2.5 × 1.4 cm).
Fig 1.
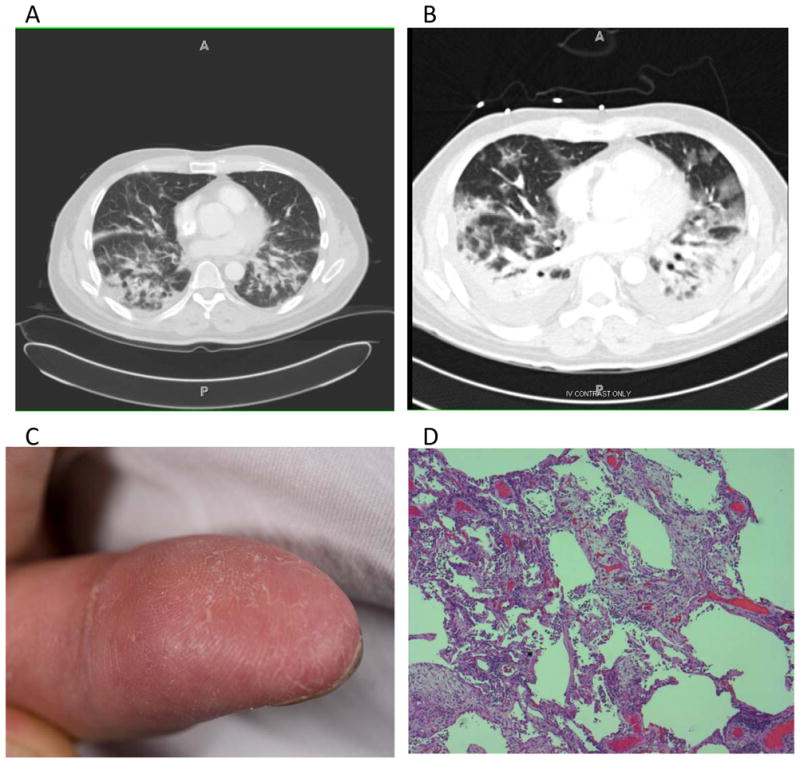
A) CT scan of chest with intravenous contrast 10 days before admission. The representative slice shows areas of patchy consolidation in the posterior portions of the lower lobes bilaterally. B) CT scan without intravenous contrast, taken on the day of admission, showing significant consolidation in the posterior aspects of the bilateral lower lobes, surrounded by patchy ground-glass infiltrates. C) Fingers of Case 1 showing dry, crackling skin at the fingertips, typical of mechanic’s hands. D) Hematoxylin and eosin stain of the left lingula showing areas of extensive interstitial fibroplasia and marked pneumocyte atypia and interstitial inflammation consistent with organizing diffuse alveolar damage with associated bronchopneumonia.
Review of his systems was unremarkable for fever or chills, arthralgia, or myalgia. Past medical history was significant only for well-controlled diabetes on metformin. He had never smoked and did not use alcohol or illicit drugs. His family history was unremarkable.
On presentation, he was afebrile, normotensive, breathing 26 times/min, and was saturating 95% on 100% non-rebreather mask. Pulse was 105 beats/min. Pulmonary exam revealed tachypnea and diffuse crackles bilaterally. Cardiac and abdominal exam were unremarkable. Skin exam showed evidence of periorbital erythema, fissuring of the distal fingers, and small papular lesions on the palmar surface of his hands (Figure 1C). No additional rash or edema was noted. No evidence of Raynaud or abnormal nailfold capillaries were seen on nailfold capillaroscopy. Comprehensive musculoskeletal exam showed no evidence of erythema, deformity, or synovitis in any joints. Muscle strength was 5/5 in proximal and distal muscle groups. Bulk and tone were preserved in all extremities.
Initial blood gas showed a pH of 7.16, pCO2 of 101, and pO2 of 77 on 100% non-rebreather mask. White blood cell count was 9730. Electrolyte panel was unremarkable. Erythrocyte sedimentation rate (ESR) was 60 mm/h. Thyroid-stimulating hormone (TSH), complement C4 and C3, creatine kinase (CK), aldolase, urine protein to creatinine ratio, human immunodeficiency virus (HIV) ELISA, and serum protein electrophoresis were all normal. Lactate dehydrogenase was moderately elevated at 542 IU/L (normal range, 118–273 IU/L), antinuclear antibody (ANA) was negative but showed a granular cytoplasmic immunofluorescence staining pattern at a titer of 1:320. Antineutrophil cytoplasmic antibody (ANCA), antitopoisomerase (Scl70) antibody, anti-Smith antibody (Sm), rheumatoid factor (RF), anticyclic citrullinated protein (CCP), and anti-ribonucleoprotein (RNP) antibody were also negative.
Given the patient’s respiratory distress, he was admitted directly to the medical intensive care unit and intubated. He was started on empiric ceftriaxone and azithromycin for community-acquired pneumonia. On hospital day 1, he underwent a video-assisted thorascopic surgery (VATS) biopsy of the lingula. Histology was consistent with organizing diffuse alveolar damage with areas of patchy bronchopneumonia (Figure 1D). No evidence of infection was identified from sputum, blood, or tissue culture. Biopsy of the periorbital rash showed only dilated blood vessels in the superficial dermis. Biopsies of the right deltoid and right quadriceps muscle showed severe type 2 muscle atrophy without inflammatory changes.
On hospital day 2, the patient was started on methylprednisolone 250 mg every 6 hours for 3 days and then continued on prednisone 75 mg daily for the duration of his hospital course. One week following admission, another chest CT was performed that showed further progression of infiltrates. A trial of intravenous immunoglobulin (IVIG) was initiated for 5 days. The patient’s hypoxemia progressed on mechanical ventilation using ARDS-NET protocol, [1] and a trial of high frequency oscillator ventilation failed. He was transitioned to comfort care and died shortly after ventilator support was withdrawn. Autopsy revealed bilateral diffuse alveolar damage and acute interstitial pneumonitis. There was also focal intraalveolar hemorrhage, edema, and acute inflammation in the left upper lobe. No pulmonary emboli were identified. Paratracheal lymphadenopathy was noted with the largest lymph node measuring 4.5 cm in greatest dimension, benign on histologic examination. No other significant pathologic findings were seen on autopsy.
Postmortem, immunoprecipitation was performed on the patient’s serum to evaluate the presence of MDA-5 antibody, given the constellation of clinical findings and the absence of another cause of rapidly progressive lung disease. Immunoprecipitations performed in our research laboratory using in vitro transcription/translated 35S-methionine-labeled MDA-5 confirmed the presence of MDA-5 antibodies in serum from this patient (Figure 2, Case 1). Cause of death was ultimately determined to be the result of rapidly progressive ILD, likely secondary to MDA-5-associated dermato-pulmonary syndrome.
Fig 2.
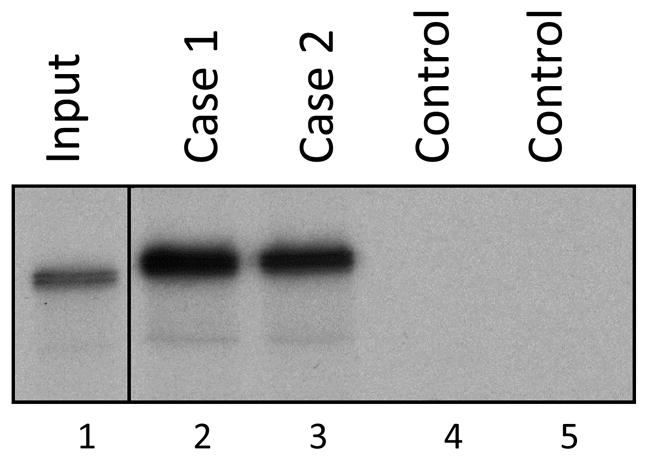
Confirmation of anti-MDA-5 antibodies in the sera of Cases 1 and 2 using an immunoprecipitation assay. 35S-methionine-labeled MDA-5, generated by in vitro transcription/translation, was immunoprecipitated using serum from Cases 1 and 2 (lanes 2 and 3) or 2 different normal controls (lanes 4 and 5). Input 35S-methionine-labeled MDA-5 (not subjected to immunoprecipitation) is shown in lane 1. These data demonstrate that Cases 1 and 2 have anti-MDA-5 antibodies.
CASE 2
A 60-year-old healthy white woman began noticing increasing fatigue, followed 2 weeks later by acute onset of night sweats, chills, nonproductive cough, and dyspnea. She was treated with 2 courses of antibiotics over 3 weeks, with improvement in the dyspnea and chills, but continued fatigue. Two months later, she noticed skin changes including periorbital edema, and erythema on her forehead, nose, chin, elbows, and axillae, and she developed symmetric inflammatory arthritis involving her hands, wrists, shoulders, knees, and ankles. She was started on a steroid taper by her primary care physician and referred for further evaluation.
Complete blood count, electrolyte panel were unremarkable. ESR was 19 mm/h. TSH, CK, and aldolase were also unremarkable. Serologies, including ANA, ANCA, RF, antiphospholipid antibody, antihistidyl tRNA synthetase (Jo-1) antibody, cryofibrinogen, and cryoglobulins, were negative. Mammogram, Pap smear, and colonoscopy were normal. Steroids were reinitiated at this point with improvement in the arthralgia, but persistence of the rash.
Two months later, the patient was admitted to the hospital with worsening dyspnea and chest tightness. Chest CT showed multifocal areas of ground-glass infiltrates. A third course of antibiotics was prescribed with improvement in her dyspnea. A prednisone taper starting at 40 mg daily was prescribed given the persistent rash, arthralgia, myalgia, and fatigue. Only the arthralgia improved with prednisone, and pulmonary symptoms worsened. Repeat CT of the chest showed improved bilateral ground-glass infiltrates compared to the initial scan, but worsening peripheral, patchy airspace opacities. She also had developed new papules of the palms and painful fissures at the lateral aspects of the interphalangeal joints of the hands. Painful ulcers developed on her wrists, forearms, arms and buttocks. She continued to have active arthritis. She was started on methotrexate. Six months after symptom onset, she had lost 60 lb, and her exercise tolerance was limited to walking a few steps in her house.
She was hospitalized and transferred to our institution for progressive dyspnea. A third chest CT scan showed multilobar consolidation (Figure 3A). Pulmonary function tests demonstrated a restrictive pattern with severely impaired gas transfer (forced vital capacity [FVC] 2.34 L, 69% predicted; forced expiratory volume in the first second [FEV1] 1.87 L, 70% predicted; total lung capacity [TLC] 3.79 L, 69.6% predicted; diffusing capacity of lung for carbon monoxide [DLCO] 8.75, 41.3% predicted). On examination the patient was afebrile, normotensive, breathing 18 breaths/min, and was saturating 95% on room air. Her pulse was 98 beats/min. She had diffuse nonscarring alopecia, upper eyelid edema, livedoid rash on her chin and nasolabial folds, and V-neck erythema. Erythema was present over the dorsum of the second to fifth metacarpophalangeal joints bilaterally with an ulceration on the dorsum of the third left metacarpophalangeal joint (Figure 3B). She had exquisitely painful ulcers at the tips and lateral borders of the fingers, as well as a livedoid palmar rash. Palmar papules were found overlying the second metacarpophalangeal joint as well as thenar and hypothenar eminences. She had wrist and elbow ulcers and bilateral painful ulcers on her buttocks. She had no clinical evidence of weakness. An electromyogram was not performed.
Fig 3.
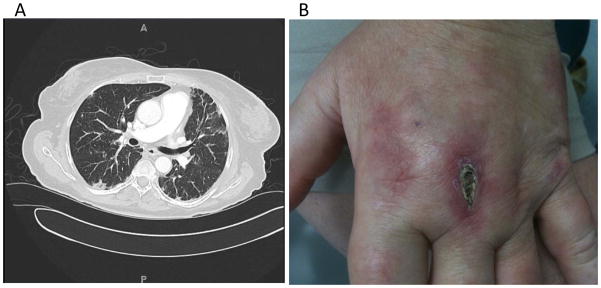
A) Thoracic CT images with intravenous contrast enhancement showing scattered peripheral interstitial thickening throughout both lungs and multiple subcentimeter ground-glass pulmonary nodules throughout all lobes of both lungs. B) Cutaneous ulceration of the dorsal third metacarpophalangeal joint of the left hand.
White blood cell count was 4270. Electrolyte panel was unremarkable. Sedimentation rate was elevated at 45 mm/h. Complement C3, complement C4, serum protein electrophoresis and urine protein to creatinine ratio were normal. Repeat serologies, including a myositis panel (Quest Diagnostics assay), were unremarkable with exception of ANA of 1:80 in speckled pattern and a positive anti-Ro antibody (ELISA assay). Ferritin was elevated at 713. Skin biopsies from multiple sites (chest, ulcers, palms, buttock ulcer) were nonspecific. A skin biopsy from the chest rash showed rare dying keratinocytes, diffuse pigment-laden macrophages, and a mild, superficial perivascular and interstitial infiltrate. Wedge biopsies of the lung (lingula, left superior lower lobe, left inferior lower lobe) showed interstitial fibrosis with honeycombing.
She was treated with prednisone taper starting at 60 mg daily and azathioprine 75 mg daily, escalated to 150 mg daily. After 5 months on therapy her energy level improved and she returned to activities in her home. Pulmonary function tests showed preserved lung function (FVC 2.29 L, 69% predicted; FEV1 1.9 L, 71% predicted; TLC 3.55 L, 65% predicted; DLCO 10.75, 44% predicted). The lesions on her fingers and the ulcerative lesions of her wrists and elbows persisted despite therapy, and were complicated by wound infection necessitating surgical debridement and intravenous antibiotic therapy. No new skin lesions appeared.
The patient gave written informed consent for further research antibody testing given her constellation of symptoms and progressive skin and lung disease. She was found to have anti-MDA-5 antibody by immunoprecipitation assay (see Figure 2, Case 2).
METHODS
We reviewed the MEDLINE database (National Library of Medicine, Bethesda, MD) for publications between 1966 and July 6, 2011, using the following search terms: “amyopathic dermatomyositis,” “clinically amyopathic dermatomyositis,” “dermatomyositis,” “interstitial lung disease,” “MDA-5,” “melanoma differentiation-associated protein 5,” “anti-CADM-140,” and “anti-CADM-140 antibody.” We reviewed only articles written in English, and limited our search to humans. Additionally, articles were excluded if they did not contain discussion of clinical features of MDA-5 antibody, and/or only reviewed prior studies. One study was excluded for insufficient data. Abstract and full text reviews were performed independently by 3 authors (NFC, JP, A-MO). Data extraction was performed by the same 3 authors. Where possible, we attempted to identify publications that may have used the same patients in duplicate studies. Immunoprecipitations to confirm the presence of MDA-5 antibodies were performed as described [2] using 35S-methionine-labeled MDA-5 that was generated by coupled in vitro transcription/translation from full-length cDNA encoding human MDA-5 (Origene, Rockville, MD). One μL of serum was used in each immunoprecipitation, and the immunoprecipitates were visualized by fluorography after electrophoresis on 10% SDS-polyacrylamide gels.
RESULTS
We identified 11 studies that met our inclusion criteria (Table 1). Most studies were case series or cross-sectional analyses. One case report was included. No clinical trials or prospective analyses were identified. Two pairs of studies likely included data from similar patient cohorts. All studies but 1 evaluated Japanese or Korean patients; the other study involved American patients. In all studies but 1, the presence of MDA-5 antibodies was identified only in patients with DM or CADM. In the remaining study, a patient with systemic sclerosis was MDA-5 antibody positive. [15] Among MDA-5 antibody-positive patients, 8 studies included data regarding presence of myositis. [8,11,13,15,23,27,28,32] Accounting for studies that included overlapping patient cohorts, 80% of MDA-5-positive individuals were clinically amyopathic (CK <300 and clinically insignificant muscle weakness).
TABLE 1.
Demographic Characteristics for Both Anti-MDA-5 Antibody-Positive and -Negative Patients, Previous Reports
| Reference | Study Type | Population Demographic | Population Studied | Total Patients Studied | Total Patients With DM or CADM | Anti-MDA-5-Positive Patients | MDA-5-Negative Patients | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Patients With MDA-5 (M/F) | Age at Onset (yr) (mean ± SD) | % With CADM or DM (CADM Only) | Deaths (%) | % With ILD | % With ILD and A/SIP | % With ILD and A/SIP | ||||||
| 18 | Cross-sectional | Korean | DM and PM | 49 | 38 | 9 (4/5) | 45.8 ± 16 | 100 (NA) | 33 | 66 | 66 | NA |
| 10 | Case report | Japanese | MDA-5 | 2 | 2 | 2 (0/2) | 47.5 ± 24.7 | NA | 50 | 100 | 100 | NA |
| 7 | Case series | American | DM | 77 | 77 | 10 (3/7) | 51.5 ± 8.8 | NA | NA | 60 | 22 | 5 |
| 32 | Cross-sectional | Japanese | DM with ILD | 25 | 25 | 12 (4/8) | 53.5 ± 9.4 | 100 (50) | 58 | 100* | 42 | 8 |
| 28 | Cross-sectional | Japanese | DM, CTD, IPF, healthy controls | 294† | 67† | 27†‡ (NA) | NA | 100 (92) | NA | NA | NA | NA |
| 27 | Nested case-control | Japanese | DM, CTD, IPF, healthy controls | 255† | 42† | 8† (2/6) | 44.5 ± 12.7 | 100 (100) | NA | 88 | 50 | NA |
| 13 | Multicenter cross-sectional | Japanese | DM, CTD | 376† | 376† | 43† (9/34) | 53 ± NA | 100 (77) | 44 | 93 | NA | NA |
| 23 | Case series | Japanese | DM, CTD | 192 | 13 | 13 (3/10) | 57.3 ± 9.4 | 100 (84) | 46 | 92 | 54 | 4 |
| 11 | Case series | Japanese | DM | 65 | 65 | 14 (3/11) | 43.6 ± 14.6 | 100 (53) | 63 | 100* | 71 | NA |
| 15 | Case series | Japanese | DM, CTD, SSc with ILD | 135 | 82 | 21 (4/17) | 47 ± 15 | 95 (91) | 14 | 95 | 79 | NA |
| 8 | Cross-sectional | Japanese | DM | 30† | 30† | 8† (1/7) | 60.5 ± 10.9 | 100 (75) | 75 | 100 | 100 | 20 |
Abbreviations: A/SIP = acute or subacute interstitial pneumonitis, CTD = connective tissue disease, IPF = idiopathic pulmonary fibrosis, NA = not available; PM = polymyositis, SD = standard deviation, SSc = systemic sclerosis/scleroderma.
All patients had ILD as part of the inclusion criteria.
Indicates possible duplication of patients between studies.
Number of patients with positive result by immunoprecipitation.
It is notable that MDA-5 is a cytoplasmic protein, whose expression is interferon (IFN)-regulated. To our knowledge there are no systematic analyses of whether patients with MDA-5 autoantibodies also express other autoantibodies. The first patient described here was ANA negative, and the second patient had low ANA titer and a positive anti-Ro antibody.
There was a significant prevalence of ILD (60%–100%) among MDA-5 antibody-positive individuals, although criteria (radiographic changes or restriction on pulmonary function test) for defining ILD varied by study (see Table 1). A significant portion of patients (22%–100%) was classified as having acute or subacute ILD, characterized by evidence of progression of ILD within 1 or 3 months, respectively. This was significantly higher than MDA-negative patients (4%–20%). [8,23,32] One study evaluated differences in chest CT scan findings between MDA-5 antibody-positive and -negative patients with DM-associated ILD. [32] Patients with MDA-5 antibody tended to have regions of ground-glass attenuation in random distribution or focally in the lower lobes of the lung, whereas MDA-5 antibody-negative patients had predominately reticular changes in the lower lobes.
Significant skin findings in patients with MDA-5 antibodies included skin ulceration, arthritis, mechanic’s hands, palmar papules, heliotrope rash, Gottron papules, periungual erythema, periungual telangiectasias, and fever (Table 2). Evidence from a systematic study of patients referred to a dermatology clinic [7] indicates that the first 4 clinical findings are significantly more prevalent in MDA-5 antibody-positive individuals than in MDA-5 antibody-negative DM patients.
TABLE 2.
Skin Features and Biopsy Results Among Patients Who Were Anti-MDA-5 Positive by Immunoprecipitation
| Reference | Heliotrope Rash (%) | Gottron Papules (%) | Periungual Erythema (%) | Periungual Telanciectasias (%) | Skin Ulceration (%) | Arthritis (%) | Palmar Papules (%) | Mechanic’s Hands (%) | Fever (%) | Findings Compatible With DM on Skin Biopsy (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 100 | 100 | NA | NA | 50 | NA | NA | NA | NA | 0 |
| 7 | 70 | 70 | NA | 90 | 80 | 70 | 60 | 60 | NA | NA |
| 27 | 50 | 75 | NA | NA | NA | 50 | NA | NA | NA | 100 |
| 13 | 56 | 86 | 70 | NA | 30 | 42 | NA | NA | 74 | NA |
| 15 | 68 | 68 | NA | NA | NA | NA | NA | NA | NA | NA |
| 8 | 38 | 25 | 63 | NA | NA | NA | 63 | NA | NA | NA |
Abbreviations: See previous table.
Seven studies reported data on treatment, although most did not include specific dosing or mention treatment algorithms employed (Table 3). In all but 1 of the 7 studies, [15] prednisone or prednisolone was used. Other immunosuppressants included cyclophosphamide, cyclosporine, azathioprine, IVIG, and, in 1 study, methotrexate. Only 1 study reported individual treatment data. [13] In that study, all MDA-5 antibody-positive patients received prednisolone initially, but no specific preference for treatment could be identified beyond this.
TABLE 3.
Referenced Therapies Used in Patients With Anti-MDA-5 Antibodies*
| Reference | Therapy |
|---|---|
| 10 | Pulsed MP, MP, CsA, Tac, IVIG, AZA |
| 32 | MP, CsA, Cyc |
| 13 | Pulsed MP, MP, CsA, Cyc, IVIG |
| 11 | MP, Cyc, CI |
| 15 | Pulsed MP, CsA, Cyc, AZA, MTX |
| 8 | MP, immunosuppressants not otherwise defined |
Abbreviations: AZA = azathioprine, CI = calcineurin inhibitor not otherwise specified, CsA = cyclosporin A, Cyc = cyclophosphamide, IVIG = intravenous immunoglobulin, MP = methylprednisolone, MTX = methotrexate, Tac = tacrolimus.
Specific algorithms were not addressed in any study, and individual data were provided in only 1 study (reference 13).
Mortality data were provided in 8 studies. [8,10,11,13,15,18,23,32] Accounting for studies that included overlapping patient cohorts, overall mortality was 38% for MDA-5 antibody-positive patients, over follow-up periods ranging from 6 months to 14 years. Two studies reported mortality data at 6 months [8,23] that were much higher in MDA-5 antibody-positive patients compared with MDA-5 antibody-negative patients (57% [range, 46%–75%] vs. 9% [range, 8%–9%]). One study that followed patients an average of 588 days reported 7 of 12 patients with MDA-5 antibody died compared to none in the MDA-5 antibody-negative cohort. In all 8 studies, the majority of deaths in MDA-5 antibody-positive patients were attributable to progressive ILD.
DISCUSSION
DM encompasses a wide spectrum of disease, almost universally involving the skin, and frequently affecting muscle and lung. The disease phentoypes are complex, with the combination of tissues involved in different individuals varying considerably. [30] The disease associated with MDA-5 autoantibodies is characterized by striking ILD and a distinct cutaneous phenotype (especially ulcers and vasculopathy), but minimal to absent muscle inflammation and damage. This constellation of damage is suggestive of a common vascular target. The cutaneous and pulmonary involvement in the absence of myositis leads us to posit that this unusual dermato-pulmonary syndrome has distinct underlying causes and mechanisms. Although recent studies have suggested that muscle disease can occur in MDA-5 antibody-positive patients, it is unusual to have significant muscle weakness or elevation of muscle enzymes in this setting. [29,32]
Detection of commercially available autoantibodies is also rare in patients with anti-MDA-5. In the 2010 study by Gono et al, [11] only 21% of patients with MDA-5 antibodies had a positive ANA. However, the presence of cytoplasmic staining by immunofluorescence was noted in both of our patients and is characteristic in patients with MDA-5 antibody. [27] In a patient who presents with ILD, palmar papules, painful skin ulcers and absent myopathy, absent nuclear staining and presence of cytoplasmic immunofluorescence pattern should raise suspicion for MDA-5 antibodies.
Cutaneous Manifestations of MDA-5
The hallmark cutaneous manifestations of the DM spectrum include violaceous papules or plaques located on the dorsal aspect of the metacarpophalangeal or interphalangeal joints, called Gottron papules; erythematous or violaceous macules in the same distribution, called Gottron macules; and heliotrope rash, which is a periorbital violaceous erythema. These findings, which are present in over 70% of individuals with DM, [20] occur with similar prevalence in both MDA-5 antibody-positive and -negative patients. MDA-5 specific skin manifestations include 1) painful, erythematous papules, macules, or both on the palmar surfaces of the metacarpophalangeal and interphalangeal joints that can have a central, ivory coloration, ulceration, and sometimes manifest as 2 distinct papules on either side of the joint; 2) cutaneous ulceration, found in 80% of these patients compared to <20% of anti-MDA-5-negative DM patients. Ulcers can be found on the elbows and knees, lateral nailfolds, and within Gottron papules. Patients with MDA-5 antibodies also have a higher prevalence of tender gums and/or oral erosions compared with MDA-5 antibody-negative patients. [7,13] Our 2 patients both had palmar papules, and Case 2 had painful ulceration of the hands, wrists, elbows, and buttocks. In neither case could a clinical diagnosis of classic DM be made with certainty. Additionally, skin biopsies in both patients were nondiagnostic.
MDA-5-Associated Interstitial Lung Disease
The spectrum of ILD in patients with DM can range from the asymptomatic individual with incidental radiographic or pulmonary function abnormalities, to rapidly progressive and fatal disease. The reported prevalence of ILD among all patients with an idiopathic inflammatory myopathy varies widely. [5,21] In all studies of MDA-5-associated disease we identified, the lung was frequently involved and was often severely affected, highlighting the lung as a central focus in this syndrome. Studies indicate that ILD progresses more rapidly in those patients with MDA-5 antibodies, with approximately 40% of MDA-5 antibody-positive patients manifesting rapidly progressive ILD. In the largest group of MDA-5 antibody-positive patients reported to our knowledge to date, [13] 93% had ILD, using results from chest radiography, chest CT, and pulmonary function tests. Patients were classified as having rapidly progressive ILD if they had worsening dyspnea, hypoxemia, and progression of interstitial changes on radiography within 1 month of onset of symptoms. In general, MDA-5 antibody-positive patients who have coexistent muscle disease have a lower mortality than those without muscle disease. [11] Both of our patients initially described pulmonary symptoms close to the time of onset of skin manifestations, with pulmonary symptoms preceding skin disease in 1 patient, and following skin disease in the other.
We found only 1 study that looked at specific radiographic findings in patients with MDA-5 antibodies. [32] The findings of ground-glass attenuation in MDA-5 antibody-positive patients versus reticular patterns in MDA-5 antibody-negative patients remains very nonspecific and is unlikely to change management without further verification of underlying disease pathology by the clinician. Additionally, these data need to be validated in a large, blinded cohort before firm conclusions can be made. Pathologic data in patients with MDA-5 antibodies have not been well described to date either. In 1 of our patients, wedge biopsy revealed usual interstitial pneumonia pattern, while in the other a diffuse alveolar damage pattern was present. Findings of diffuse alveolar damage are uncommon in patients with CADM and DM, and in these cases, patients generally have poor response to therapy and dismal prognosis. [22,25]
MDA-5 Autoantibodies and Disease Pathogenesis
MDA-5 belongs to the family of retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), a family of RNA helicases that functions as cytoplasmic sensors of pathogen-associated molecular patterns within viral RNA. To date, 3 RLR members have been identified: RIG-I, MDA-5, and LGP2 (laboratory of genetics and physiology 2). While all are broadly expressed in most tissues, levels are typically low in resting cells but are greatly increased with IFN exposure and after virus infection. [17,34,35] The RLRs drive type 1 IFN production and antiviral gene expression, which mediates the intracellular immune response to control virus infection. Recent studies suggest that RLRs may also activate other inflammatory signaling pathways, including the inflammasome. [26] Different RLRs appear to detect infection preferentially with different viruses. Interestingly, MDA-5 detects several RNA viruses, including picornaviruses (like polio, coxsackie, and rhinovirus), flaviviruses (Dengue and West Nile), as well as vaccinia (DNA virus). The striking vascular character of the skin phenotype in MDA-5 antibody-positive patients and the rapidly progressive lung disease suggest that blood vessels are the primary target of the immune response, potentially initially targeted during virus infection, and driven later by type I IFN pathways. Defining the mechanisms underlying the initiation and propagation of the anti-MDA-5 immune response is a high priority. Elucidating the sites of MDA-5 expression in the target tissue (and especially blood vessels at these sites) will be important in this regard.
It has been noted that resistance to type 1 diabetes in humans is associated with polymorphisms that impair the function of the mda5 gene, suggesting that the relative strength of induction of type I IFNs may be important in establishing and propagating autoimmunity. [3,24] Since uncontrolled viral infection on the one hand (inadequate viral sensing by MDA-5) or heightened type I IFN-induced inflammation on the other (strong MDA-5 function) both have the capacity to render an individual susceptible to tissue damage, it will be of interest to define whether mda5 polymorphisms are present in these patients, which either augment or impair MDA-5 expression and function, and thereby regulate downstream type I IFN effects. If augmented MDA-5 signaling and type I IFN activity play a role in the amplified ILD and vascular phenotype seen in this dermato-pulmonary syndrome, this might have therapeutic implications for newly available agents that inhibit type I IFN signaling. Alternatively, if these pathways are decreased in MDA-5 antibody-positive patients, the possibility that increased virus-induced damage might play a role in initiating lung disease in these patients could prompt a different set of approaches.
Response to Therapy
Effective therapeutic modalities to treat the MDA-5-associated dermato-pulmonary syndrome have not yet been identified, and no data exist comparing the effectiveness of 1 therapy over another. This is likely because this entity has only recently been described, and it is difficult to diagnose given the current lack of a widely available MDA-5 antibody assay. While no firm guidelines can be drawn from the literature regarding treatment of MDA-5 antibody-positive individuals, many experts continue to treat patients similarly to those who have DM and are MDA-5 antibody negative, usually beginning with corticosteroid therapy. Limited reports in the literature of patients with DM and rapidly progressive ILD suggest cyclophosphamide and cyclosporine early in the disease may be beneficial. [14,33] Cyclosporine has also been used to treat ulcerative lesions in a patient with CADM and intractable skin ulceration, [31] but these observations were made before the recognition of the MDA-5-associated entity. The poor outcomes associated with MDA-5 antibodies strongly suggest that the optimal therapy has not yet been defined. It is possible that rapid, aggressive institution of immunosuppression might avoid irreversible lung injury if diagnosis is prompt. On the other hand, standard approaches to DM and associated conditions may not be appropriate for this condition; instead, more specific therapy targeting type I IFNs, or potentially antiviral therapy if a pathogen is defined, may be more appropriate. In the current study, 1 patient died (Case 1) despite a trial of IVIG, pulse dose prednisone, and cyclophosphamide. The other patient (Case 2) received high-dose steroids and was maintained on prednisone and azathioprine since her diagnosis, but continued to have significant disease activity.
Outcomes
Data from previous studies show that the presence of MDA-5 antibody is associated with increased mortality in patients with DM and CADM compared to those who are MDA-5 negative. [8,23,32] Although many studies did not describe the cause of death, those that did suggested that the majority of individuals succumbed to ILD refractory to immunosuppressive therapy. The presence of MDA-5 antibodies clearly has prognostic value for those who present with this characteristic dermato-pulmonary syndrome. Although the numbers remain small, approximately 40% of MDA-5 antibody-positive patients died, mostly within the first year. This was very different from MDA-5 antibody-negative patients, where 1-year mortality was 0%, and overall mortality was <10%. In 2010, Gono et al [9] reported findings in individuals with CADM and ILD suggesting that increased ferritin levels correlated with faster progression of ILD. Presence of MDA-5 antibody was not measured in that study, but studies of individuals with MDA-5 antibodies support the observation that the higher ferritin levels might be associated with increased ILD progression. [10,11,32]
Conclusion
In the current report, we describe 2 patients with a progressive dermato-pulmonary syndrome that defied diagnosis using clinically available modalities. Unusual skin features included violaceous papules on the palmar surfaces of the metacarpophalangeal and interphalangeal joints, and painful ulcerative skin lesions. Inflammatory muscle disease was absent. Standard diagnostic studies, including autoantibodies, skin biopsy, and VATS lung biopsy, failed to reveal the underlying process. Antibodies to MDA-5 were present, strongly suggesting that autoimmunity to MDA-5 plays a role in generating this specific phenotype. In these settings, when extensive evaluation is otherwise nondiagnostic, clinical suspicion for MDA-5 antibodies should be high, and further testing should be aggressively pursued.
Acknowledgments
Financial support: This work was supported by National Institutes of Health grants AR44684 (to LC-R), P30 AR053503 (to AR), T32 AR048522 (to A-MO and JP), and T32 HL07534 (to NFC).
Abbreviations
- ANA
antinuclear antibody
- ANCA
antineutrophil cytoplasmic antibody
- CADM
clinically amyopathic dermatomyositis
- CK
creatine kinase
- CT
computed tomography
- DLCO
diffusing capacity of lung for carbon monoxide
- DM
dermatomyositis
- ESR
erythrocyte sedimentation rate
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- IFN
interferon
- ILD
interstitial lung disease
- IV
intravenous
- IVIG
intravenous immunoglobulin
- MDA-5
melanoma differentiation-associated protein 5
- RF
rheumatoid factor
- RIG-I
retinoic acid-inducible gene 1
- RLR
RIG-I-like receptor
- TSH
thyroid-stimulating hormone
- VATS
video-assisted thorascopic surgery
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Anonymous. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;18:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Casciola-Rosen LA, Pluta AF, Plotz PH, Cox AE, Morris S, Wigley FM, Petri M, Gelber AC, Rosen A. The DNA mismatch repair enzyme PMS1 is a myositis-specific autoantigen. Arthritis Rheum. 2001;44:389–396. doi: 10.1002/1529-0131(200102)44:2<389::AID-ANR58>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Downes K, Pekalski M, Angus KL, Hardy M, Nutland S, Smyth DJ, Walker NM, Wallace C, Todd JA. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS One. 2010;5:e12646. doi: 10.1371/journal.pone.0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Euwer RL, Sontheimer RD. Amyopathic dermatomyositis (dermatomyositis sine myositis). Presentation of six new cases and review of the literature. J Am Acad Dermatol. 1991;24:959–966. [PubMed] [Google Scholar]
- 5.Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;56:297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28:451–458. doi: 10.1055/s-2007-985666. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujikawa K, Kawakami A, Kaji K, Fujimoto M, Kawashiri S, Iwamoto N, Aramaki T, Ichinose K, Tamai M, Kamachi M, Nakamura H, Ida H, Origuchi T, Ishimoto H, Mukae H, Kuwana M, Kohno S, Takehara K, Sato S, Eguchi K. Association of distinct clinical subsets with myositis-specific autoantibodies towards anti-155/140-kDa polypeptides, anti-140-kDa polypeptides, and anti-aminoacyl tRNA synthetases in Japanese patients with dermatomyositis: a single-centre, cross-sectional study. Scand J Rheumatol. 2009;38:263–267. doi: 10.1080/03009740802687455. [DOI] [PubMed] [Google Scholar]
- 9.Gono T, Kawaguchi Y, Hara M, Masuda I, Katsumata Y, Shinozaki M, Ota Y, Ozeki E, Yamanaka H. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49:1354–1360. doi: 10.1093/rheumatology/keq073. [DOI] [PubMed] [Google Scholar]
- 10.Gono T, Kawaguchi Y, Ozeki E, Ota Y, Satoh T, Kuwana M, Hara M, Yamanaka H. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol. 2011;21:223–227. doi: 10.1007/s10165-010-0371-x. [DOI] [PubMed] [Google Scholar]
- 11.Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, Masuda I, Tochimoto A, Baba S, Okamoto Y, Ota Y, Yamanaka H. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49:1713–1719. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 12.Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 2009;48:607–612. doi: 10.1093/rheumatology/kep078. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, Komura K, Nakamura M, Kodera M, Suga N, Higashi A, Ogusu K, Tsutsui K, Furusaki A, Tanabe H, Sasaoka S, Muro Y, Yoshikawa M, Ishiguro N, Ayano M, Muroi E, Fujikawa K, Umeda Y, Kawase M, Mabuchi E, Asano Y, Sodemoto K, Seishima M, Yamada H, Sato S, Takehara K, Fujimoto M. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol. 2011;147:391–398. doi: 10.1001/archdermatol.2011.52. [DOI] [PubMed] [Google Scholar]
- 14.Hirakata M, Nagai S. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2000;12:501–508. doi: 10.1097/00002281-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 2010;49:1726–1733. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]
- 16.Kameda H, Nagasawa H, Ogawa H, Sekiguchi N, Takei H, Tokuhira M, Amano K, Takeuchi T. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719–1726. [PubMed] [Google Scholar]
- 17.Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, Fisher PB. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–1800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 18.Kang EH, Nakashima R, Mimori T, Kim J, Lee YJ, Lee EB, Song YW. Myositis autoantibodies in Korean patients with inflammatory myositis: anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord. 2010;11:223. doi: 10.1186/1471-2474-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawachi Y, Maruyama H, Furuta J, Fujisawa Y, Nakamura Y, Takahashi T, Otsuka F. Cutaneous deep necrosis with dermatomyositis: correlation with interstitial pneumonia. Eur J Dermatol. 2007;17:345–346. doi: 10.1684/ejd.2007.0220. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899–922. doi: 10.1016/s0190-9622(98)70263-4. [DOI] [PubMed] [Google Scholar]
- 21.Labirua A, Lundberg IE. Interstitial lung disease and idiopathic inflammatory myopathies: progress and pitfalls. Curr Opin Rheumatol. 2010;22:633–638. doi: 10.1097/BOR.0b013e32833f1970. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS, Chen TL, Tzen CY, Lin FJ, Peng MJ, Wu CL, Chen PJ. Idiopathic inflammatory myopathy with diffuse alveolar damage. Clin Rheumatol. 2002;21:391–396. doi: 10.1007/s100670200104. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, Kawabata D, Ohmura K, Usui T, Fujii T, Okawa K, Mimori T. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 2010;49:433–440. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 24.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parambil JG, Myers JL, Ryu JH. Diffuse alveolar damage: uncommon manifestation of pulmonary involvement in patients with connective tissue diseases. Chest. 2006;130:553–558. doi: 10.1378/chest.130.2.553. [DOI] [PubMed] [Google Scholar]
- 26.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 27.Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, Nishikawa T, Oddis CV, Ikeda Y. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, Kuwana M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–2200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 29.Sato S, Kuwana M. Clinically amyopathic dermatomyositis. Curr Opin Rheumatol. 2010;22:639–643. doi: 10.1097/BOR.0b013e32833f1987. [DOI] [PubMed] [Google Scholar]
- 30.Shamim EA, Rider LG, Pandey JP, O’Hanlon TP, Jara LJ, Samayoa EA, Burgos-Vargas R, Vazquez-Mellado J, Alcocer-Varela J, Salazar-Paramo M, Kutzbach AG, Malley JD, Targoff IN, Garcia-De la Torre I, Miller FW. Differences in idiopathic inflammatory myopathy phenotypes and genotypes between Mesoamerican Mestizos and North American Caucasians: ethnogeographic influences in the genetics and clinical expression of myositis. Arthritis Rheum. 2002;46:1885–1893. doi: 10.1002/art.10358. [DOI] [PubMed] [Google Scholar]
- 31.Shimojima Y, Ishii W, Kato T, Hoshi K, Matsuda M, Hashimoto T, Tanaka Y, Ikeda S. Intractable skin necrosis and interstitial pneumonia in amyopathic dermatomyositis, successfully treated with cyclosporin A. Intern Med. 2003;42:1253–1258. doi: 10.2169/internalmedicine.42.1253. [DOI] [PubMed] [Google Scholar]
- 32.Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Watanabe K, Aihara K, Oga T, Chin K, Nagai S, Mimori T, Mishima M. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med. 2011;105:1380–1387. doi: 10.1016/j.rmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki Y, Yamada H, Yamasaki M, Ohkubo M, Azuma K, Matsuoka S, Kurihara Y, Osada H, Satoh M, Ozaki S. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007;46:124–130. doi: 10.1093/rheumatology/kel112. [DOI] [PubMed] [Google Scholar]
- 34.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 35.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 36.Zahn S, Barchet W, Rehkamper C, Hornung T, Bieber T, Tuting T, Wenzel J. Enhanced skin expression of melanoma differentiation-associated gene 5 (MDA5) in dermatomyositis and related autoimmune diseases. J Am Acad Dermatol. 2011;64:988–989. doi: 10.1016/j.jaad.2010.08.004. [DOI] [PubMed] [Google Scholar]


