Abstract
The lithium-pilocarpine model mimics most features of human temporal lobe epilepsy. Following our prior studies of cerebral metabolic changes, here we explored the expression of transporters for glucose (GLUT1 and GLUT3) and monocarboxylates (MCT1 and MCT2) during and after status epilepticus (SE) induced by lithium-pilocarpine in PN10, PN21, and adult rats. In situ hybridization was used to study the expression of transporter mRNAs during the acute phase (1, 4, 12 and 24 h of SE), the latent phase, and the early and late chronic phases. During SE, GLUT1 expression was increased throughout the brain between 1 and 12 h of SE, more strongly in adult rats; GLUT3 increased only transiently, at 1 and 4 h of SE and mainly in PN10 rats; MCT1 was increased at all ages but 5-10-fold more in adult than immature rats; MCT2 expression increased mainly in adult rats. At all ages, MCT1 and MCT2 up-regulation was limited to the circuit of seizures while GLUT1 and GLUT3 changes were more widespread. During the latent and chronic phases, the expression of nutrient transporters was normal in PN10 rats. In PN21 rats, GLUT1 was up-regulated in all brain regions. In contrast, in adult rats GLUT1 expression was down-regulated in the piriform cortex, hilus and CA1 as a result of extensive neuronal death. The changes in nutrient transporter expression reported here further support previous findings in other experimental models demonstrating rapid transcriptional responses to marked changes in cerebral energetic/glucose demand.
Keywords: lithium-pilocarpine, GLUT1, GLUT3, MCT1, MCT2, in situ hybridization, immunohistochemistry, development, temporal lobe epilepsy
Introduction
The model of epilepsy induced in rats by lithium-pilocarpine (li-pilo), features most clinical and neuropathological aspects of human temporal lobe epilepsy (Turski et al. 1989; Cavalheiro 1995). In adult rats, li-pilo leads to an acute phase of status epilepticus (SE) followed by a latent seizure-free phase lasting 14-25 days. Neuronal loss occurs in hippocampus, dentate gyrus, piriform and entorhinal cortices, septum, thalamus, amygdala and neocortex (Turski et al. 1989; Cavalheiro 1995; Dubé et al. 2001), leads to reorganization and generation of a hyperexcitable circuit underlying the expression of spontaneous seizures in 100% of adult rats. When li-pilo SE is induced at postnatal day 21 (PN21), neuronal loss is less extensive and only about 60-70% of the rats develop spontaneous seizures after a latency of 65-75 days. In PN10 rats, SE does not lead to damage or epilepsy (Sankar et al. 1998; Dubé et al. 2001).
During li-pilo SE in adult rats, marked increases in local cerebral metabolic rates for glucose (LCMRglc) occur in regions prone to neuronal loss, while in other areas LCMRglcs increase moderately (Fernandes et al. 1999). At PN10 and PN21, the pattern of metabolic increases is similar, although the immature brain undergoes limited or no neuronal loss (Fernandes et al. 1999). Changes in LCMRglc during latent and chronic phases occur only in regions undergoing neuronal loss in PN21 and adult rats (Dubé et al., 2000a,b, 2001).
Glucose delivery to the brain is mediated by facilitative transporter proteins. GLUT1 is located at the blood-brain barrier (BBB) endothelium and in glia, ependyma, and choroid plexus (Maher et al. 1994; Kumagai et al. 1995; Vannnuci et al. 1997b). GLUT3 is found in neurons (Maher et al. 1994; Vannucci et al. 1997b; McEwen and Reagan 2004). Ketone bodies and lactate are transported to brain via monocarboxylic acid transporters, MCTs. MCT1 is expressed by BBB endothelial cells, ependymocytes, and astrocytes (Pierre et al. 2000; Vannucci and Simpson 2003; Pierre and Pellerin 2005). MCT2 is a major neuronal transporter (Pierre et al. 2000, 2002; Vannucci and Simpson 2003; Pierre and Pellerin 2005). GLUT1 and GLUT3 levels are low in the immature brain and increase concurrently with LCMRglcs (Vannucci 1994; Vannucci and Simpson 2003). MCT1 expression peaks during suckling and declines with maturation as glucose becomes the major fuel. MCT1 and MCT2 levels in non-vascular compartments do not decline with maturation (Vannucci and Simpson 2003). Since changes in cerebral metabolism to meet changing energetic demand depend on the availability of the metabolic fuel in blood and capacity to transport this fuel into cells, age-dependent nutrient transport capacity is important to consider.
GLUT1 expression was reported to increase during kainate SE in adult rats, in response to heightened glucose demand (Gronlund et al. 1996). In human surgical tissue, GLUT1 expression was reduced interictally in the temporal lobe (Cornford et al. 1998) together with decreased neuronal density and reduction in glucose utilization (Mathern et al. 1997). To our knowledge, no data on the consequences of seizures on MCT1 and MCT2 expression are available. Here, we studied seizure-induced changes in GLUT1, GLUT3, MCT1 and MCT2 mRNA expression in PN10, PN21, and adult rats: (1) during the acute phase of li-pilo SE; (2) at the end of the latent phase; and (3) during the early and late chronic phases. In situ hybridization allowed precise and quantitative location of transporter expression easy to compare to our data on LCMRglcs (Fernandes et al. 1999; Dubé et al. 2000a,b, 2001). At one time point during SE in adult rats, we verified whether the increased transporter mRNA expression translated into increased protein expression using immunohistochemistry.
Materials and Methods
Animals and preparation of brain sections
Adult Sprague Dawley rats (Janvier Breeding Center, Le Genest-St-Isle, France), one male and two females per cage, were housed in mating groups for 5 days. Rats were kept in quiet, uncrowded facilities (21-22°C) on 12-h light-dark cycle (7:00 am–7:00 pm, lights on) and given unlimited access to lab chow and water. After delivery, litters were reduced to 10 pups (day of birth was considered as day 0). All animal experimentation was performed in accordance with rules of the European Committee Council Directive of November 24, 1986 (86/609/EEC) and the French Department of Agriculture (License N°67-97). A total number of 134 rats (35 PN10, 43 PN21 and 56 adults) were used.
Lithium chloride (3 meq/kg) was injected 18 h before pilocarpine to PN10, PN21, and adult rats (60, 30 and 25 mg/kg, respectively). Rats received 1 mg/kg methylscopolamine 30 min before the convulsant to reduce peripheral consequences of pilocarpine. Control animals received methylscopolamine, lithium, and an equivalent volume of saline instead of pilocarpine. Starting five days after SE induction, PN21 and adult rats were observed by video recording (Sony) 10 h/day for spontaneous seizure occurrence. These rats were then randomized to the latent or chronic groups. PN10 rats undergoing SE do not develop spontaneous seizures (Dubé et al., 2001; Sankar et al., 1998) and were not video-recorded.
As detailed in Figure 1, transporter mRNA expression during the acute phase was studied at 1, 4, 12 and 24 h of SE. Then, adult and PN21 rats with no seizures were sacrificed by the end of the latent phase, i.e. at 14 and 60 days after SE, respectively (Cavalheiro 1995; Dubé et al. 2001), at time points similar to our measurements of LCMRgc (Dubé et al., 2000a,b). During the chronic phase, some rats were sacrificed after a few days of spontaneous seizures (early stage), i.e. at 21 (adults) or 75 days (PN21) and other rats after two months of seizures (late stage), i.e. at 80 (adults) and 135 days after SE (PN21). In rats that underwent SE at PN10, we studied only a late time point of 120 days after SE.
Figure 1.

Schematic representation of the timing of the experiments and measurements of nutrient transporter mRNA expression in the long term in PN10 rats and during the acute, latent, early and late chronic phase in PN21 and adult rats.
For the preparation of sections, rats (3-6 animals per group) were deeply anesthetized with an overdose of pentobarbital (1.8 mg/kg) and sacrificed by decapitation. Brains were taken out, frozen in methylbutane at -25°C and kept at -80°C. 20 μm coronal sections were taken at four preselected levels and thaw-mounted on polylysine-coated slides. Adjacent sections were taken for classical thionin staining. The study of transporters was performed in 26 regions of interest (Paxinos and Watson, 1986). Only the data concerning the structures involved in the seizure circuit are presented here.
In situ hybridization and immunohistochemistry
The synthesis of 35S-labeled riboprobes and in situ hybridization were performed as previously described (Bondy et al. 1992; Vannucci et al. 1998, Koehler-Stec et al. 1998). Hybridization of additional sections with sense probes for all four transporters was performed as a control and did not produce any specific signal (data not shown). The tracer level in autoradiographic sections was quantified with an image processing system (Biocom 500, Les Ulis, France) by an observer unaware of the animals' treatment.
After exposure, slides were dipped in Kodak NTB3 photographic emulsion and stored at 4°C for six weeks. Slides were developed with Kodak D-19 developer and counterstained with hematoxylin. Sections were examined under a microscope by epiluminescence to reveal the silver grains resulting from in situ hybridization as bright green (Vannucci et al. 1997a). MCT1 and MCT2 immunochemistry was performed on 6 li-saline and 6 li-pilo rats at 6 h of SE using rabbit antisera raised against either MCT1 or MCT2, as previously described (Pierre et al. 2000).
Statistical analysis
GLUT1, GLUT3, MCT1 and MCT2 mRNAs were determined in 26 structures of groups of 3-6 animals, controls and rats exposed to SE. Tracer concentrations in controls were compared to those in li-pilo groups by a two-way analysis of variance for independent groups (ANOVA) followed by a post-hoc Fisher's LSD test for multiple comparisons.
Results
Seizure expression and behavior
Behavioral and electroencephalographic alterations induced by li-pilo were time- and age-dependent. They occurred in a temporal sequence similar to the one described previously (Motte et al. 1998; Fernandes et al. 1999). The mean duration of the latent phase was 18 ± 4 days in adult rats and 68 ± 7 days at PN21. 70% of the rats subjected to SE at PN21 (12/17) became epileptic. None of the PN10 rats developed epilepsy.
Effects of SE on the expression of nutrient transporters in PN10 rats
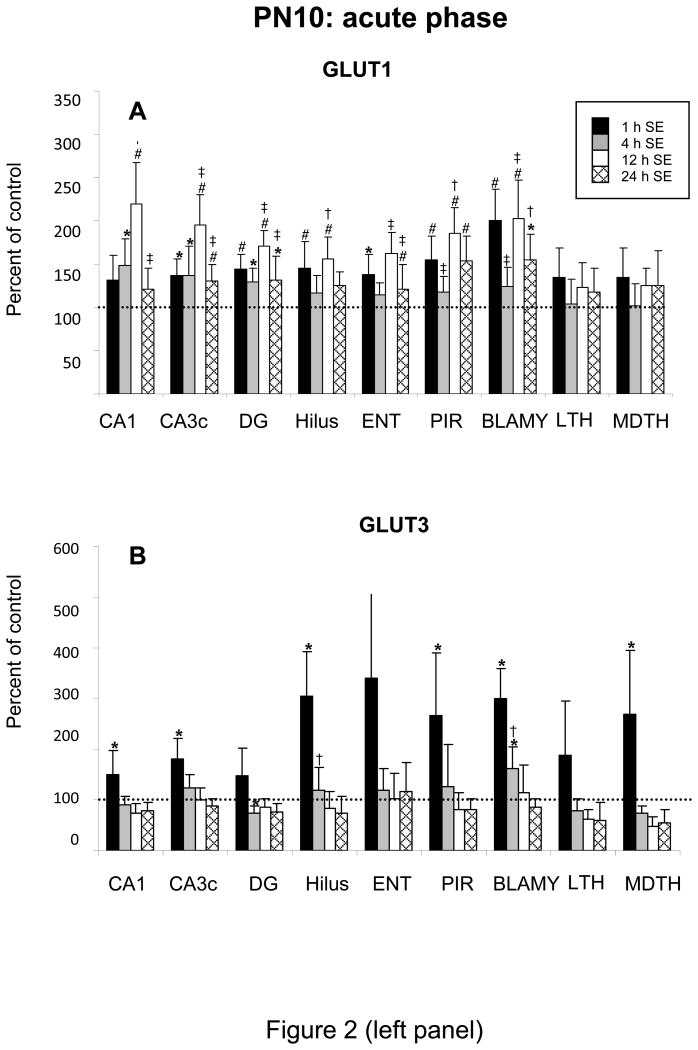
During SE in PN10 rats, GLUT1 mRNA expression increased in almost all brain regions and remained high during the 24 h of SE, except in thalamus (Figure 2A). Largest activations concerned hippocampus, amygdala, and piriform cortex. GLUT1 increased at 1 h of SE but most markedly between 4 and 24 h, reaching 18-119% over control levels. Changes in GLUT3 and MCT1 expression were temporally similar and increased markedly by 1 h in all regions. GLUT3 expression was mainly increased in hilus, entorhinal and piriform cortices, mediodorsal thalamus (49-239%). In most regions, GLUT3 mRNA levels had normalized by 4 h, except in amygdala. MCT1 expression increased by 78-179% at 1 h of SE in hippocampus, entorhinal and piriform cortex, remained high at 4 h in CA1, CA3, and piriform cortex and went back to control levels in the other regions. MCT1 mRNA increase lasted up to 12 h of SE in Ammon's horn (Figure 2B, C). The increase in MCT2 expression was moderate, occurring between 1 and ending by 12 h of SE. It was statistically significant (26-104%) in hilus, entorhinal and priform cortex (Figure 2D). Examples of increases of MCT2 mRNA expression in amygdala and entorhinal cortex of PN10 rats at 1 h of SE are given in Figure 3.
Figure 2.
Effects of lithium-pilocarpine SE on the expression of GLUT1 (A) and GLUT3 (B) MCT1 (C) and MCT2 (D) mRNAs in the brain of PN10 rats during the acute phase, i.e., at 1, 4, 12 and 24 h after SE onset.
Values are expressed as percent of control levels.
Abbreviations: CA1 and CA3c: pyramidal cell layers of Ammon's horn; DG: granular cell layer of the dentate gyrus; Hilus: hilus of the dentate gyrus; ENT: entorhinal cortex; PIR: piriform cortex; BLAMY: basolateral amygdala; LTH: lateral thalamus; MDTH: mediodorsal thalamus.
* p<0.05, # p<0.01, statistically significant differences from control
† p<0.05, ‡ p<0.01, statistically significant differences from the previous time
Figure 3.

MCT2 mRNA expression in basolateral amygdala and entorhinal cortex of control (upper row) and lithium-pilocarpine PN10 rats (lower row) at 1 h after SE onset. In both structures, the expression of the transporter was largely enhanced by the seizures.
At 4 months after SE in PN10 rats MCT levels were normal. GLUT1 and GLUT3 mRNA levels tended to be reduced in all regions, with only significant decreases in GLUT1 (44±16%, mean ± S.D.) and GLUT3 mRNA (52±15%) level in the CA3c (data not shown).
Effects of SE on the expression of transporters in PN21 rats
As in younger rats, GLUT1 mRNA levels underwent widespread increases (31-150%) in PN21 rats during SE, with maximal values between 4 and 24 h in most regions (Figure 4A). Largest increases were recorded at 24 h in Ammon's horn and amygdala. GLUT3 levels increased significantly by 72-138% over control levels during the initial 4 h of SE in piriform cortex, amygdala, and thalamus. They returned to control levels by 12 h. GLUT3 expression was unchanged in other structures during the acute phase (Figure 4B). Strong increases (117-340%) in MCT1 mRNA expression occurred in hippocampus, dentate gyrus, cortices, and amygdala. These increases lasted for 4-12 h and levels of MCT2 normalized by 24 h of SE (Figure 4C). In PN21 rats MCT2 mRNA expression was not affected by SE at any time, with the exception of a significant decrease at 12 and 24 h in mediodorsal thalamus (Figure 4D). Examples of increases of MCT2 mRNA expression in dentate gyrus and amygdala of PN21 rats at 1 h of SE are presented in Figure 5.
Figure 4.


Effects of lithium-pilocarpine SE on the expression of GLUT1 (A) and GLUT3 (B) MCT1 (C) and MCT2 (D) mRNAs in the brain of PN21 rats during the acute phase, i.e., at 1, 4, 12 and 24 h after SE onset.
Values are expressed as percent of control levels.
Abbreviations: CA1 and CA3c: pyramidal cell layers of Ammon's horn; DG: granular cell layer of the dentate gyrus; Hilus: hilus of the dentate gyrus; ENT: entorhinal cortex; PIR: piriform cortex; BLAMY: basolateral amygdala; LTH: lateral thalamus; MDTH: mediodorsal thalamus.
* p<0.05, # p<0.01, statistically significant differences from control
Figure 5.

MCT1 mRNA expression in hilus and granular cell layer of the dentate gyrus, and the basolateral amygdala of control (upper row) and lithium-pilocarpine PN21 rats (lower row) at 4 h after SE onset. Note the large SE-induced increase in the expression of the transporter in the granular cell layer of the dentate gyrus compared to the quite moderate increase in the hilus (see data in Figure 8). In basolateral amygdala, the expression of MCT1 was also largely enhanced by the seizures.
During late phases, only GLUT1 mRNA expression increased over control levels by 36-92% in all regions and at all times (Figure 6A). GLUT3 mRNA levels tended to slightly increase in many regions during the late chronic phase and decreased at all times in piriform cortex, though not significantly. MCT1 levels increased by 18-67%, mainly in the late chronic phase in thalamus and hilus. MCT2 increased by 26-28%, only during the latent phase in CA1 and dentate gyrus (Figure 6B-D).
Figure 6.
Effects of lithium-pilocarpine SE on the expression of GLUT1 (A) and GLUT3 (B) MCT1 (C) and MCT2 (D) mRNAs in the brain of PN21 rats during the latent (60 days post SE) and during the early (75 days post SE) and late chronic phase (135 days post SE).
Values are expressed as percent of control levels.
Abbreviations: CA1 and CA3c: pyramidal cell layers of Ammon's horn; DG: granular cell layer of the dentate gyrus; Hilus: hilus of the dentate gyrus; ENT: entorhinal cortex; PIR: piriform cortex; BLAMY: basolateral amygdala; LTH: lateral thalamus; MDTH: mediodorsal thalamus.
* p<0.05, # p<0.01, statistically significant differences from control
Effects of SE and on the expression of transporters in adult rats
The temporal evolution of the influence of SE on transporter expression in adult rat brain is shown in Figure 7. In adult rats, GLUT1 mRNA expression was unchanged by 1 h SE and uniformly increased between 4 and 24 h up to 50-175% over control levels in hippocampus and dentate gyrus. In thalamus and amygdala, increases started at 1 h and lasted for the entire acute period. In entorhinal and piriform cortex, GLUT1 mRNA expression was enhanced at 1 and 4 h, normalized by 12 h and down-regulated by 24 h in piriform cortex (Figure 8A). GLUT3 mRNA expression was minimally affected by SE, with the exception of a 75-94% increase in amygdala between 1 and 12 h, and 62 and 47% decreases at 24 h in piriform and entorhinal cortices (Figure 8B). At 24 h, GLUT3 mRNA level was significantly up-regulated by 58% in dentate gyrus. A very strong activation in MCT1 mRNA occurred mainly in hippocampus, dentate gyrus, entorhinal cortex and amygdala (Figure 8C). It reached 600-1700%, was largest at 4 h and lasted up to 12 h of SE. In most other regions, the increase reached 150-600%. By 24 h, MCT1 mRNA levels were increased in most regions. MCT2 mRNA expression increased in hippocampus, piriform and entorhinal cortices, amygdala and thalamus (Figure 8D), starting at 1 h and lasting for 24 h, except in piriform cortex where MCT2 mRNA levels increased only at 1 h and in hilus where no change occurred. Increases were moderate in hippocampus and entorhinal cortex (39-110%) and marked in amygdala and thalamus, reaching 85-180% at all times.
Figure 7.

Temporal changes of the expression of the four different nutrient transporters studied in the adult rat brain in sections taken at the level of the dorsal hippocampus at 4 h, 24 h and 21 days after SE. Cryosections (20 μm) were analyzed for the expression of the transporters by in situ hybridization using a 35S-riboprobe.
Figure 8.


Effects of lithium-pilocarpine SE on the expression of GLUT1 (A) and GLUT3 (B) MCT1 (C) and MCT2 (D) mRNAs in the brain of adult rats during the acute phase, i.e., at 1, 4, 12 and 24 h after SE onset.
Values are expressed as percent of control levels.
Abbreviations: CA1 and CA3c: pyramidal cell layers of Ammon's horn; DG: granular cell layer of the dentate gyrus; Hilus: hilus of the dentate gyrus; ENT: entorhinal cortex; PIR: piriform cortex; BLAMY: basolateral amygdala; LTH: lateral thalamus; MDTH: mediodorsal thalamus.
* p<0.05, # p<0.01, statistically significant differences from control
† p<0.05, ‡ p<0.01, statistically significant differences from the previous time
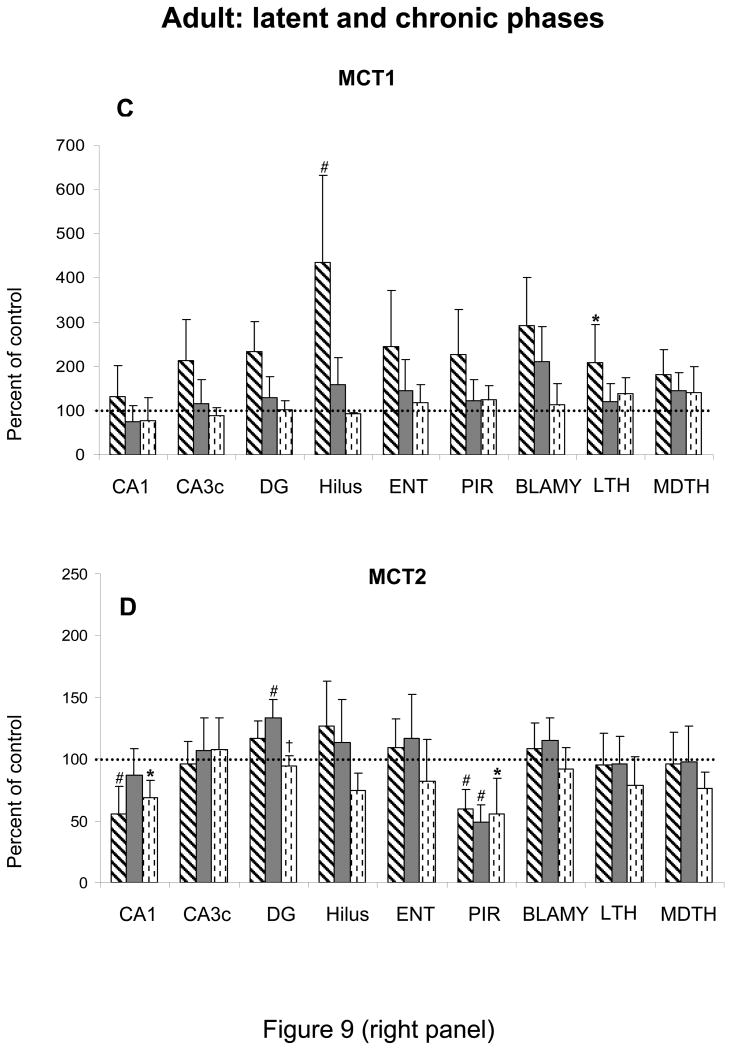
In adult rats, the mRNA expression of all transporters had normalized in most brain regions, except in hippocampus and piriform cortex. In the latter structure, the levels of all transporters, except MCT1 were strikingly decreased during all phases (Figure 9). In CA1, the expression of GLUT3 and MCT2 decreased during the latent and chronic phases. GLUT3 mRNA decreased also in hilus (Figure 9).
Figure 9.
Effects of lithium-pilocarpine SE on the expression of GLUT1 (A) and GLUT3 (B) MCT1 (C) and MCT2 (D) mRNAs in the brain of adult rats during the latent (14 days post SE) and during the early (21 days post SE) and late chronic phase (80 days post SE).
Values are expressed as percent of control levels.
Abbreviations: CA1 and CA3c: pyramidal cell layers of Ammon's horn; DG: granular cell layer of the dentate gyrus; Hilus: hilus of the dentate gyrus; ENT: entorhinal cortex; PIR: piriform cortex; BLAMY: basolateral amygdala; LTH: lateral thalamus; MDTH: mediodorsal thalamus.
* p<0.05, # p<0.01, statistically significant differences from control
† p<0.05, statistically significant difference from the previous time
Effects of lithium-pilocarpine SE on MCT protein expression
MCT protein changes were measured in adult rat brain sections at 6 h of SE (Figure 9). Compared to li-saline, SE enhanced the expression of MCT1 (Figure 10a-d) and MCT2 (Figure 10e-h) protein. Increases occurred hippocampus for MCT1 (Figure 10b vs a) and MCT2 (Figure 10f vs e) and in cortical neurons for MCT1 (Figure 10d vs c) and even more strikingly for MCT2 (Figure 10h vs g).
Figure 10.

MCT1 (a-d) and MCT2 (e-h) protein expression in hippocampus and cerebral cortex of control and lithium-pilocarpine treated adult rats at 6 h after SE onset. In both structures, protein expression of the two transporters was largely enhanced by seizures, mainly in neurons. Increased protein expression was prominent in CA1 pyramidal cell layer in hippocampus and scattered all over the different layers of the cortex. Peroxidase staining visualized with brightfield microscopy. Calibration bar: 50 μm.
Discussion
The present data highlight significant changes in expression of metabolic transporter mRNAs that are age-, region- and transporter-specific. Effects are prominent during the acute phase and more marked when the basal transporter expression is low, as is the case for GLUT3 in PN10 rats and MCT1 in adult rats.
Glucose transporters and hypermetabolism during SE: age-related response
Basal LCMRglcs are low and homogeneous in PN10 rats. They increase by 2-fold between PN10 and PN21 with regional differentiation, and by 1.3-fold between PN21 and adults (Nehlig et al., 1988). GLUT1 and GLUT3 expression is low at young ages and progressively increases to adult levels while it is the reverse for MCT expression, elevated in immature brain and progressively decreasing (Vannucci 1994; Vannucci and Simpson 2003). Li-pilo SE is also characterized by distinct age-related differences. In adults, it is followed by extensive neuronal loss (Turski et al. 1989; Cavalheiro 1995; Dubé et al. 2001). When SE is induced at PN21, neuronal loss is less extended; in PN10 rats there is no neuronal loss (Dubé et al. 2001).
One hypothesis for neuronal loss linked to severe seizures is based on the mismatch between cerebral metabolism and blood flow leading to hypoperfusion (Ingvar, 1986). During li-pilo SE in adult rats, early mismatch between LCMRglcs and blood flow occurs in regions undergoing neuronal loss. Surprisingly, in PN21 and PN10 rats, the distribution of this regional mismatch is similar but there is only little or no neuronal loss (Pereira de Vasconcelos et al., 2002). Thus, we explored whether seizure-induced neuronal loss could reflect substrate supply limitation during SE (Fernandes et al. 1999).
GLUT1 mRNA expression showed age-dependent widespread increases during SE. GLUT1 is expressed by the BBB endothelial cells and glial cells (Kumagai et al. 1995; Maher et al. 1994; Vannucci et al. 1997b) but increases in GLUT1 mRNA seem largely due to increased expression in cerebral microvessels upon activation (Vannucci et al. 1998). The widespread increases of GLUT1 mRNAs recorded here show the rapid transcriptional response to the widespread increases of LCMRglcs during severe seizures (Meldrum 1983; Ingvar et al. 1987; Fernandes et al. 1999). Largest increases were recorded in the structures of the seizure circuit. Hippocampus and amygdala underwent even further increases in GLUT1 mRNA expression at 24 h of SE in PN21 and adult rats, most likely due to high energetic demand (Fernandes et al. 1999). GLUT1 mRNA levels did not increase in thalamus of PN10 rats concurrently with the lack of increase in LCMRglcs during li-pilo SE in thalamus at that age (Fernandes et al. 1999). This further confirms the relation between metabolic needs and GLUT1 expression (Vannucci 1994).
The expression of GLUT3 mRNA was affected by seizure activity only transiently and inversely to age. GLUT3 increased mainly in cortex, amygdala, and thalamus of PN10 and PN21 rats, but only during the first 4 h of SE, reflecting also the transcriptional response to a rapid change in metabolic demand. The amplitude of GLUT3 up-regulation was largest in PN10, reflecting reduced density of the transporter at that age (Vannucci 1994) and capacity of the immature brain to adjust to increased metabolic demand at the neuronal level. In adult rats, GLUT3 expression was minimally affected by SE, with the exception of decreases at 24 h of SE in piriform and entorhinal cortices, most likely reflecting massive neuronal loss recorded at that time (Roch et al. 2002). This rapid decrease in GLUT3 mRNA density, if translated into insufficient transporter protein in neurons, could lead to insufficient metabolic supply to neurons during seizures. This deficit could lead to energy failure and subsequent neuronal necrosis resulting in cerebral dysfunction in the adult epileptic brain.
These data are in line with our earlier work showing early increases in GLUT1 and GLUT3 mRNA expression of PN10 and PN21 rats subjected to pentylenetetrazol (PTZ) SE. Increases were also widespread at 1 and 4 h of SE. As in li-pilo SE, the amplitude of the response was larger in PN10 than in PN21 rats. In contrast, most increases had disappeared by 24 h after PTZ, as opposed to li-pilo SE. This difference most likely reflects the short duration of PTZ SE (about 60-80 min) compared to that of li-pilo SE (8-12 h), hence leading to very long sustained metabolic demand in the latter model (Nehlig et al., 2006).
Monocarboxylate transporters and hypermetabolism during SE: age-related response
MCT1 and MCT2 mRNA up-regulation was limited to limbic regions outlining the seizure circuit, directly reflecting energetic demand linked to hyperactivity. Thus, MCT1 densities are adjusted to local glucose metabolism and transport and might represent a more sensitive marker than changes in GLUT1 densities (Maurer et al., 2004) which are increased in all brain regions. In limbic structures, MCT1 mRNA expression was up-regulated at 1 h of SE. MCT1 mRNA levels were increased to a similar extent as GLUT1 in PN10 and PN21 rats, but 5-10 times more than GLUT1 in adult rats. Thus, MCT1 levels depend also on metabolic rates. MCT1 up-regulation normalized rapidly in most regions and earlier in immature rats, reflecting the shorter duration, reduced severity and metabolic demand of SE in immature compared to adult rats. The more limited increase in MCT1 mRNA expression in immature rats might also be linked to the high level of this transporter during suckling (Cremer et al. 1976, 1979; Vannucci and Simpson, 2003) that would hence not be particularly inducible.
Marked up-regulation of MCT1 in adults likely reflects hypermetabolism during seizures that leads to massive accumulation of lactate in the brain. Its density rises proportionally with lactate concentration (Hertz and Dienel, 2004). During li-pilo SE in adult rats, cerebral lactate concentration reaches 11 mM compared to 2 mM in controls (Fernandes et al. 1999). The large increase in MCT1 expression could contribute to the release of lactate out of the brain. Neurons also increase the expression of their usual MCT transporter, MCT2, mostly in adult animals. However, MCT2 saturates rapidly when lactate concentration rises (Pierre and Pellerin 2005). In adult rats during SE, the expression of MCT2 increased to a more moderate extent than MCT1 while the reverse seemed to occur at the protein level.
Region-specific plasticity of transporter mRNA expression: correlation with neuronal damage in adult rats
Entorhinal and piriform cortices are involved early during SE (Roch et al., 2002) and undergo large increases in LCMRglcs in adult rats (Fernandes et al. 1999). In these structures, the up-regulation of GLUT1, MCT1 and MCT2 mRNAs was rather moderate and GLUT3 expression did not change. By 24 h of SE, all transporter systems had normalized and GLUT3 was down-regulated. This decrease most likely reflects the rapid cell disorganization and neuronal loss, which is fully established in basal cortices of adult rats by 24 h of SE (Roch et al. 2002). This rapid neuronal death may partly reflect the insufficient capacity of cerebral nutrient transporters to adapt to major hypermetabolism. As a result of extensive neuronal loss in these cortices, the expression of neuronal transporters, GLUT3 and MCT2, strikingly decreased during the latent and chronic phase. In the most affected structure, piriform cortex, GLUT1 also decreased in the long term.
Almost all thalamic nuclei underwent low to moderate increases of GLUT1 and MCT1 mRNA expression, and no change in GLUT3 during SE. The largest increase in MCT2 expression was observed in thalamus. MCT2, the major neuronal MCT of the rodent brain, becomes easily rate-limiting during activation (Hertz and Dienel 2004). The sustained up-regulation of MCT2 mRNA during SE may indicate the long-lasting activation during SE. The thalamus is highly sensitive to seizure activity, undergoing increases in BBB permeability (Leroy et al. 2003) concurrent with early lesions (Roch et al. 2002). This sensitivity may be linked to abundant thalamic interconnections to the critical role of thalamus in initiation and propagation of limbic seizures (Cassidy and Gale 1998; Bertram et al. 2001). In thalamus, neuronal loss does not translate into long-term changes in nutrient transporter levels.
In Ammon's horn and dentate gyrus, SE did not increase GLUT3 mRNA, moderately MCT2 and largely MCT1 expression. Hippocampal regions underwent the largest increases in GLUT1 mRNA levels. This up-regulation started at 4 h of SE and increased by 24 h. This delayed increase in GLUT1 expression most likely reflects delayed involvement of hippocampus in the seizure circuit and better transient structural preservation. In hippocampus, with the exception of hilus, neuronal damage is seen only at 6 days after SE (André et al. 2001; Dubé et al. 2001; Roch et al. 2002), possibly because of the rapid and strong up-regulation of MCT1 at 1 h of SE allowing the excess lactate to be efficiently released (Hertz and Dienel 2004). In the long term, neuronal loss in CA1 leads to the decrease of the neuronal transporters GLUT3 and MCT2. In hilus, which undergoes marked neuronal loss (about 70-80%, Dubé et al. 2001; Roch et al. 2002), only GLUT3 mRNA levels were reduced in the long-term which is concordant with decreases in LCMRglcs, 14 days and 2 months after SE in adult rats (Dubé et al. 2000a,b, 2001).
In conclusion, nutrient transporter expression was mostly affected during SE. The response of GLUT1 was uniform and more marked with increasing age. MCT1 increased strongly in adult brain and outlined the seizure circuit. The neuronal transporters GLUT3 and MCT2 were moderately or not affected in most limbic regions during SE. Changes in GLUT3 inversely related to age and this could reflect insufficient adaptation of this transporter in adult neurons, possibly leading to reduced metabolic supply during prolonged seizures. This failure could, together with other modifiers of injury such as heat shock proteins, receptors and transduction mechanisms, participate in neuronal necrosis leading to cerebral dysfunction in the epileptic brain.
Research Highlights.
Seizures lead to age-dependent overexpression of brain metabolic transporters
Up-regulation of glucose transporters is widespread
Up-regulation of monocarboxylate transporters is limited to the seizure circuit
In the long term, decreased expression reflects neuronal death
Acknowledgments
This study was supported by grants from the Institut National de la Santé et de la Recherche Médicale (U 398), the Fondation pour la Recherche Médicale, a UNESCO grant (CL), and NIH/HD30704 (SJV), NIH/NS41405 (IAS) grants. LP is supported by the Fonds National de la Recherche Suisse, grant N°. 31003A-125063. The excellent technical assistance of Estelle Koning, Arielle Ferrandon, Lisa Willing, and Marianne Klinger is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Lee W, Zhou J. Ontogeny and cellular distribution of brain glucose transporter gene expression. Mol Cell Neurosci. 1992;3:305–314. doi: 10.1016/1044-7431(92)90027-y. [DOI] [PubMed] [Google Scholar]
- Cassidy RM, Gale K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J Neurosci. 1998;18:9002–9009. doi: 10.1523/JNEUROSCI.18-21-09002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Cornford ME, Landaw EM, Delgado-Escueta AV. Interictal seizure resections show two configurations of endothelial Glut1 glucose transporter in the human blood-brain barrier. J Cereb Blood Flow Metab. 1998;18:26–42. doi: 10.1097/00004647-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Braun LD, Oldendorf WH. Changes during development in transport processes of the blood-brain barrier. Biochim Biophys Acta. 1976;448:633–637. doi: 10.1016/0005-2736(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem. 1979;33:439–445. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Dubé C, Boyet S, Marescaux C, Nehlig A. Progressive metabolic changes underlying the chronic reorganization of brain circuits during the silent phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2000a;162:146–157. doi: 10.1006/exnr.2000.7324. [DOI] [PubMed] [Google Scholar]
- Dubé C, Marescaux C, Nehlig A. A metabolic and neuropathological approach to the understanding of plastic changes that occur in the immature and adult rat brain during lithium-pilocarpine-induced epileptogenesis. Epilepsia. 2000b;41(Suppl 6):S36–S43. doi: 10.1111/j.1528-1157.2000.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Dubé C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001;167:227–241. doi: 10.1006/exnr.2000.7561. [DOI] [PubMed] [Google Scholar]
- Fernandes MJS, Boyet S, Marescaux C, Nehlig A. Correlation between hypermetabolism and neuronal damage during status epilepticus induced by lithium-pilocarpine in immature and adult rats. J Cereb Blood Flow Metab. 1999;19:195–209. doi: 10.1097/00004647-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Lindsberg PJ, Hallenbeck JM, Feuerstein GZ. Increased cerebral lactate output to cerebral venous blood after forebrain ischemia in rats. Stroke. 1990;21:614–617. doi: 10.1161/01.str.21.4.614. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22:272–281. [PubMed] [Google Scholar]
- Gronlund KM, Gerhart DZ, Leino RL, McCall AL, Drewes LR. Chronic seizures increase glucose transporter abundance in rat brain. J Neuropathol Exp Neurol. 1996;55:832–840. doi: 10.1097/00005072-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Energy metabolism in the brain. Int Rev Neurobiol. 2002;51:1–102. doi: 10.1016/s0074-7742(02)51003-5. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res. 2004;79:11–18. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- Ingvar M. 1986. Cerebral blood flow and metabolic rate during seizures. Relationship to epileptic brain damage. Ann N Y Acad Sci. 1986;462:194–206. doi: 10.1111/j.1749-6632.1986.tb51254.x. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Folbergrova J, Siesjö BK. Metabolic alterations underlying the development of hypermetabolic necrosis in the substantia nigra in status epilepticus. J Cereb Blood Flow Metab. 1987;7:103–108. doi: 10.1038/jcbfm.1987.15. [DOI] [PubMed] [Google Scholar]
- Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. Am J Physiol Endocrinol Metab. 1998;275:E516–E524. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier Glut1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- Leroy C, Roch C, Koning E, Namer IJ, Nehlig A. In the lithium-pilocarpine model of epilepsy, brain lesions are not linked to changes in blood-brain barrier permeability: an autoradiograpic study in adult and developing rats. Exp Neurol. 2003;182:346–352. doi: 10.1016/s0014-4886(03)00122-5. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in the brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Armstrong DL. Hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 133–155. [Google Scholar]
- Maurer MH, Canis M, Kuschinsky W, Duelli R. Correlation between local monocarboxylate transporter 1 (MCT1) and glucose transporter 1 (GLUT1) densities in the adult rat brain. Neurosci Lett. 2004;355:105–108. doi: 10.1016/j.neulet.2003.10.056. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Metabolic factors during prolonged seizures and their relation to nerve cell death. In: Delgado-Escueta AV, Wasterlain CG, Treiman DM, Porter RJ, editors. Advances in Neurology, Vol 34: Status Epilepticus. New York: Raven Press; 1983. pp. 261–275. [PubMed] [Google Scholar]
- Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain: cDNA sequence of mouse Glut3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992;267:467–472. [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A, Boyet S. Quantitative autoradiographic measurement of local cerebral glucose utilization in freely moving rats during postnatal development. J Neurosci. 1988;8:2321–2333. doi: 10.1523/JNEUROSCI.08-07-02321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Rudolf G, Leroy C, Rigoulot MA, Simpson IA, Vannucci SJ. Pentylenetetrazol-induced status epilepticus up-regulates the expression of glucose transporter genes but not proteins in the immature rat brain. Brain Res. 2006;1082:32–42. doi: 10.1016/j.brainres.2006.01.078. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The Rat Brain in Stereotaxic Coordinates. 2nd. New York: Academic Press; 1986. [Google Scholar]
- Pellerin L, Pellegri G, Martin JL, Magistretti P. Expression of monocarboxylate transporters mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult tissue. Proc Natl Acad Sci USA. 1998;95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters MCT1 and MCT2 in the adult mouse brain revealed by double immunohistochemical labelling and confocal microscopy. Neuroscience. 2000;100:617–627. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizures. Electroencephal Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Roch C, Leroy C, Nehlig A, Namer IJ. Contribution of magnetic resonance imaging to the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43:325–335. doi: 10.1046/j.1528-1157.2002.11301.x. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Tseng MT, Miller JJ, Rigor BM. The glucose paradox in cerebral ischemia. New insights. Ann N Y Acad Sci. 1999;893:386–390. doi: 10.1111/j.1749-6632.1999.tb07862.x. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Tseng MT, Rigor BM. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001;895:268–272. doi: 10.1016/s0006-8993(01)02082-0. [DOI] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121:1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem. 1994;62:240–246. doi: 10.1046/j.1471-4159.1994.62010240.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Clark RR, Koehler-Stec E, Li K, Smith CB, Davies P, Maher F, Simpson IA. Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev Neurosci. 1998;20:369–379. doi: 10.1159/000017333. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Gibbs M, Simpson IA. Glucose utilization and glucose transporter proteins GLUT1 and GLUT3 in brains of diabetic (db/db) mice. Am J Physiol. 1997a;272:E267–E274. doi: 10.1152/ajpendo.1997.272.2.E267. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997b;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Reinhart R, Maher F, Bondy CA, Lee WH, Vannucci RC, Simpson IA. Alterations in GLUT1 and GLUT3 glucose transporter gene expression following unilateral hypoxia-ischemia in the immature rat brain. Dev Brain Res. 1998a;107:255–264. doi: 10.1016/s0165-3806(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Werner H, Raizada MK, Mudd LM, Foyt HL, Simpson IA, Roberts CT, Jr, Le Roith D. Regulation of rat brain/HepG2 glucose transporter gene expression by insulin and insulin-like growth factor-1 in primary cultures of neuronal and glial cells. Endocrinology. 1989;125:314–320. doi: 10.1210/endo-125-1-314. [DOI] [PubMed] [Google Scholar]