Abstract
Studies exploring the underpinnings of age-related neurodegeneration suggest fronto-limbic alterations that are increasingly vulnerable in the presence of disease including late life depression. Less work has assessed the impact of this specific vulnerability on widespread brain circuitry. Seventy-nine older adults (healthy controls=45; late life depression=34) completed translational tasks shown in non-human primates to rely on fronto-limbic networks involving dorsolateral (Self-Ordered Pointing Task) or orbitofrontal (Object Alternation Task) cortices. A sub-sample of participants also completed diffusion tensor imaging for white matter tract quantification (uncinate and cingulum bundle; n=58) and whole brain tract-based spatial statistics (n=62). Despite task associations to specific white matter tracts across both groups, only healthy controls demonstrated significant correlations between widespread tract integrity and cognition. Thus, increasing Object Alternation Task errors were associated with decreasing fractional anisotropy in the uncinate in late life depression; however, only in healthy controls was the uncinate incorporated into a larger network of white matter vulnerability associating fractional anisotropy with Object Alternation Task errors using whole brain tract-based spatial statistics. It appears that the whole brain impact of specific fronto-limbic vulnerabilities in aging may be eclipsed in the presence of disease-specific neuropathology like that seen in late life depression.
Keywords: ageing, prefrontal vulnerability, white matter tracts, myelin integrity, late life depression
1.0 Introduction
Neuroimaging studies have long explored the grey and white matter underpinnings of age-related neurodegeneration. Much of this work suggests that i) age-related grey matter alterations are most prominent in prefrontal and hippocampal regions (Resnick, Lamar, & Driscoll, 2007); and ii) reduced white matter integrity is consistently observed in fronto-limbic tracts connecting the prefrontal cortex to more posterior regions of brain (Sullivan, Rohlfing, & Pfefferbaum, 2010). Alterations in these regions not only contribute to the cognitive (i.e., executive and memory) and affective profile of aging (Charlton et al., 2006; Lamar, Charlton, Morris, & Markus, 2010) but appear to become increasingly vulnerable when normal aging is compromised by disease. For example, when compared to the age-related neurodegeneration seen in healthy aging, late-life depression (LLD) is associated with similar albeit greater alterations in grey (Ballmaier et al., 2004; Chang et al., 2011) and white (O'Brien et al., 2006; Tupler et al., 2002) matter regions. This exacerbated vulnerability is associated not only with clinically significant depressive symptomatology but also cognitive alterations in executive functioning and memory. Few studies have explicitly examined this suggestion that age-related fronto-limbic alterations are exacerbated in the presence of LLD; fewer still have assessed the larger impact of these vulnerabilities on widespread brain circuitry. Using translational tasks shown in non-human primates to rely on specific prefrontal structures may uncover the impact of these brain vulnerabilities on a wider cerebral network in aging and LLD.
White matter integrity and tract-specific alterations can be examined using diffusion tensor imaging (DTI) which has corroborated and extended previous MRI findings showing vulnerabilities within frontal and subcortical regions in aging (Charlton, Schiavone, Barrick, Morris, & Markus, 2010) and depression (Shimony et al., 2009). Lower white matter integrity exists – as measured by fractional anisotropy (FA) – in dorsolateral prefrontal (DLPFC) and orbitofrontal cortices (OFC) in aging (Charlton et al., 2010; Sullivan et al., 2010) but even more so in LLD (Ballmaier et al., 2004; Taylor et al., 2004). In fact, pre- and post-treatment functional imaging studies of anti-depressants in LLD suggest that these white matter alterations may cause irreversible physiological damage in some but not all prefrontal systems assessed (Aizenstein et al., 2009; Ishizaki et al., 2008). Thus, while regional vulnerability within DLPFC improves with anti-depressants in LLD, OFC functional integrity does not. This suggests that the OFC, altered with aging, may be more permanently altered (both structurally and functionally) in the presence of LLD. Several recent reviews of the LLD literature also support this notion of greater vulnerability for and more permanent alterations to the OFC and its associated neural circuitry in this vulnerable population (Arnone, McIntosh, Ebmeier, Munafo, & Anderson, 2012; Bora, Harrison, Davey, Yucel, & Pantelis, 2012).
Numerous DTI studies have used region of interest and fiber tracking techniques to determine alterations in specific areas of white matter and their cognitive associates (see (Madden et al., 2012 for review). Prefrontal regions and their associated networks including the uncinate – with its direct connections to the OFC – and the cingulum bundle have shown significant associations to executive and motor functioning in aging (Zahr, Rohlfing, Pfefferbaum, & Sullivan, 2009) as well as mood regulation in aging and depression (Zhang et al., 2012). Despite strong evidence for damage within the DLPFC and OFC negatively impacting regional connectivity and cognition, the impact of these specific vulnerabilities on more widespread brain circuitry and cognition is less well understood. This may be due, in part to the fact that few studies have investigated brain-behavior associates using tract-based spatial statistics (TBSS) and fewer still have used tasks known to directly target these prefrontal regions in the aging brain.
Translational measures shown in non-human primate lesion studies to rely on dorsolateral and orbitofrontal cortices may assist in determining the impact of prefrontal vulnerabilities on whole brain connectivity. Further, key white matter tracts implicated in aging and depression like the uncinate and cingulum bundle may help focus investigations in this area of research. The Self-Ordered Pointing Task (SOPT; Petrides, 1995) assesses planning and self-monitoring through sequential selection of visually presented abstract stimuli. Non-human primate lesion studies (Petrides, 1995) and human neuroimaging studies (Petrides, Alivisatos, & Frey, 2002) implicate the mid-DLPFC as key to successful SOPT performance. Investigations of the Object Alternation task (OA; Mishkin, Vest, Waxler, & Enger Rosvold, 1969) of decision making detail the importance of ventromedial orbitofrontal regions for successful performance in both non-human primate lesion studies (Mishkin et al., 1969) and human neuroimaging work (Zald, Curtis, Chernitsky, & Pardo, 2005). The uncinate, a ventral association bundle, connects the OFC with the amygdala and hippocampus (Catani & Thiebaut de Schotten, 2008). The cingulum, a medial association bundle, connects medial prefrontal, temporal, parietal and occipital regions to the cingulate (Catani & Thiebaut de Schotten, 2008). Both white matter tracts are involved in aging and depression (Zahr et al., 2009; Zhang et al., 2012) and both can be accurately quantified (Zhang et al., 2012) to ensure SOPT and OA task relevance to aging and depression before conducting whole-brain TBSS.
Using TBSS, we explored cognitive associates to whole brain connectivity to determine the presence and extent of altered white matter networks in aging and depression. We hypothesize that white matter (dis-)connectivity encompassing anterior and posterior regions of the brain will negatively impact task performance in both groups but more so in LLD. Given previously documented evidence for greater vulnerability of and more permanent alterations to the OFC in LLD (Arnone et al., 2012), we further hypothesize that the OA task – reliant on OFC for successful completion (Mishkin et al., 1969; Zald et al., 2005) – will be particularly affected by white matter (dis-)connectivity in LLD.
2.0 Material and methods
2.1 Participants
Data were collected as part of a larger research program at the University of Illinois at Chicago (UIC) approved by the UIC Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Individuals age 50 and older were recruited via community outreach (i.e., newspaper, radio and television advertisements).
All participants underwent a preliminary telephone screen. Exclusion criteria consisted of current or past history of neurological disorder (i.e., dementia, stroke, seizure, etc.), a history of head injury or loss of consciousness, an Axis I disorder other than major depression (e.g., bipolar disorder), a present or past history of substance abuse or dependence, psychotropic medication use including anti-depressant medication and the presence of metallic implant(s) that would preclude MRI. Thus, all study participants (including those with depression) were free of anti-depressant medication in order to study depressed mood in an untreated state.
After passing the telephone screen, participants were scheduled for a more detailed evaluation including cognitive, i.e., Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1974) and affective, i.e., Structured Clinical Interview for DSM-IV (SCID; Spitzer, Williams, Gibbon, & First, 1992) screens for final inclusion/exclusion determination. Screening measures were administered by a trained research assistant and followed by the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) completed by a board certified (AK) or board eligible (OA) psychiatrist. All raters were blind to telephone screen information.
All subjects, regardless of group, had an MMSE score ≥ 24 and were native English speakers. HC inclusion criteria included an absence of symptoms of depression based on the SCID and a HDRS score ≤8. Final inclusion criteria for adults with depression included a diagnosis of major depressive disorder based on the SCID and a HDRS score ≥15 (mean=18.7; s.d.=2.5).
Participants received an assessment of vascular risk using the Framingham Heart Study’s Stroke Risk Profile (FSRP; Wolf, D'Agostino, Belanger, & Kannel, 1991). The FSRP predicts stroke risk based on age, systolic blood pressure, anti-hypertensive therapy, diabetes mellitus, current cigarette smoking, cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy. Laboratory testing documented levels of health related variables (i.e., hypercholesterolemia and glucose levels) and an electrocardiogram assessed for atrial fibrillation and left ventricular hypertrophy. History of stable (e.g., diabetes) or remitted medical illness was not an exclusionary factor.
The final sample (n=79) included 45 HC participants and 34 older adults with LLD. Of those, 17 (HC=9; LLD=8) had inadequate MRI data and were excluded from all DTI analyses leaving a DTI sample of 34 HC and 26 LLD participants (n=62).
2.2 Procedures
Qualified subjects were scheduled for a second visit during which they were administered a neuropsychological assessment by a trained research assistant blind to participant group that included standardized measures of intelligence in addition to the tasks outlined below. Participants returned for a third visit for neuroimaging data acquisition.
2.2.1 Cognitive Assessment
Participants completed executive function measures translated from the non-human to human primate research setting namely the SOPT (Petrides, 1995; Petrides et al., 2002) and OA (Mishkin et al., 1969; Zald et al., 2005) detailed below.
The SOPT assesses planning and self-monitoring through sequential selection of visually presented abstract stimuli. Non-human primate lesion studies (Petrides, 1995) and human neuroimaging studies (Petrides et al., 2002) implicate the importance of the mid-DLPFC to successful performance. The study version of the SOPT consists of sets of 6, then 9 then 12 pages of abstract designs with a corresponding set of 6, or 9 or 12 abstract designs per page, respectively. Each page contains all the same designs per set; however, no design is in the same location twice. Participants are instructed to select a different stimulus design from those selected on previous pages in the trial. A trial is complete when all pages in the set (i.e., 6,9,12) have been presented; a total of three trials per set were administered. The dependent variable is the number of items selected more than once summed across all trial blocks per set (SOPT6, SOPT9, SOPT12).
The OA task assesses establishing and maintaining mental set based on feedback during a trial and error decision making task. Non-human primate lesion studies (Mishkin et al., 1969) and human neuroimaging studies (Zald et al., 2005) implicate the importance of ventromedial prefrontal regions to successful performance. OA in the present study was computerized and consisted of a maximum of 50 trials in which participants were presented with a red circle and a blue square on each trial. They were instructed to find the star hidden under either the red circle or the blue square. After an initial guess trial on the part of the participant, visual (i.e., the presence or absence of the star) and auditory (i.e., a victorious or defeatist noise) feedback provided clues as to the object of the game – alternate between the two objects and get the star every trial. OA concluded upon the completion of 10 consecutively correct trials or maxed out at 50 trials. The dependent variables were trials to completion (OAttc), number of perseverative errors relative to total trials to completion (total errors / OAttc; OAE/T) and average reaction time per trial (OARTperT).
Given that OA involves reaction times and there are documented differences in motor speed between individuals with and without depression (Kertzman et al., 2010), as a comparison task participants were assessed on a motor version of the Trail Making Test (TMTM). Participants were asked to follow a dotted line connecting open circles on a page and time to completion was measured in seconds.
Depression is often seen as a risk factor for Alzheimer’s disease (Ownby, Crocco, Acevedo, John, & Loewenstein, 2006). To ensure that our LLD sample was comparable to their non-depressed non-demented aging counterparts across learning and memory (LM) skills, participants were evaluated along this cognitive domain using several clinical measures. Raw scores from select variables of each measure were transformed into z-scores and coded so high scores reflected good performance across all variables; z-scores were then collated to produce a mean score for the LM domain. The LM domain consisted of the following variables: immediate and long delay free recall of the 16-item word list of the California Verbal Learning Test-II (CVLT; Delis, Kramer, Kaplan, & Ober, 2000), both immediate and delay free recall of prose passages and simple geometric shapes derived from the Wechsler Memory Scale-III (WMS-III; Wechsler, 1997) Logical Memory I and II as well as Visual Reproduction I and II, respectively. Cronbach’s alpha was computed to assess how well the variables measured a latent construct and considered good (α=.832), indicating that each variable measured the unidimensional latent construct of learning and memory.
2.2.2 Neuroimaging Protocol
All whole brain MRI data were acquired using Philips 3.0T Achieva scanner (Philips Medical Systems, Best, the Netherlands) with an 8-channel sensitivity encoding (SENSE) head-coil. Participants were positioned comfortably in the scanner and fitted with soft ear plugs; foam pads minimized head motion. Participants were instructed to remain still throughout the scan.
A T2-weighted FLAIR scan was acquired with turbo spin echo sequence (FOV=240mm2; 67 contiguous axial slices; TR/TI/TE=11,000/2,800/68ms; voxel size=0.83×0.83×2.2mm3). Diffusion-weighted images, aligned to the AC/PC line, were acquired using single-shot spin-echo echo planar imaging sequence (FOV=240mm2 ; TR/TE=6,994/71ms; flip angle=90° ; voxel size=0.83×0.83×2.2mm3). Sixty-seven contiguous slices were collected along 32 gradient directions (b=700s mm2) and in one scan with minimal diffusion-weighting (b0 image). SENSE parallel imaging technique (reduction factor = 2.5) reduced scanning time to approximately 4 minutes.
2.2.3 Image Processing
All MRI results were visually examined to ensure good quality. Data with artifacts from movement or other causes such as space-occupying or other focal lesions, including stroke and other gross neuroanatomical abnormalities were excluded. T2 FLAIR images were also inspected to rule out cases with serious white matter hyperintensities in regions of interest for fiber tracking. Thus, four HC participants were not included in fiber tracking quantification of the uncinate or cingulum bundle.
The degree of subcortical white matter disease was estimated through volumetric quantification of white matter hyperintensities from FLAIR images using the Jim image analysis package, Version 6.0, (Xinapse Systems Ltd., Northants, UK, www.xinapse.com). Thus, white matter hyperintensities were outlined using the contour ROI function. All areas of increased signal were included unless: i) the area was less than 5mm3, ii) the area was a narrow band (less than one pixel wide) along the ventricles, iii) hyperintensities were due to the presence of blood vessels.
Diffusion-weighted images (32 gradient directions) were realigned to the b0 image using the automatic image registration (AIR) algorithm with affine transformation to minimize eddy current distortions. Diffusion tensor elements were computed at each voxel with the signals from the 32 diffusion weighted images fitted to obtain the six elements of the diffusion tensor (Basser, Mattiello, & LeBihan, 1994) which were then diagonalized to determine three eigenvalues (λ1, λ2, λ3) and three eigenvectors (v1, v2, v3) using FMRIB Software library (FSL; http://www.fmrib.ac.uk/fsl). Eigenvalue images were used to compute FA, axial (λ║= λ1) and radial [λ┴=(λ2+λ3)/2)] diffusivity maps. FA quantifies the relative degree of anisotropy in a voxel, axial diffusivity is assumed to represent diffusion along the local fiber direction (and subsequently axonal integrity) while radial diffusivity is assumed to represent diffusivity perpendicular to the fiber direction (and subsequently myelin integrity) (Song et al., 2003).
2.2.3.i Uncinate and Cingulum bundle fiber tracking
Fiber tracking methods are described in detail elsewhere (Zhang et al., 2012). Briefly, fiber tracking was performed in DtiStudio software (Laboratory of Brain Anatomical MRI, JHMI, Baltimore) using fiber assignment by continuous tractography method. For each subject, deterministic tractography on the whole brain was performed by initiating tracts at each voxel and terminating these tracts when FA was less than 0.15 or turning angles between coincident principal eigenvectors (v1) were greater than 60°. A region of interest (ROI) was carefully delineated to obtain our specific tracts of interest.
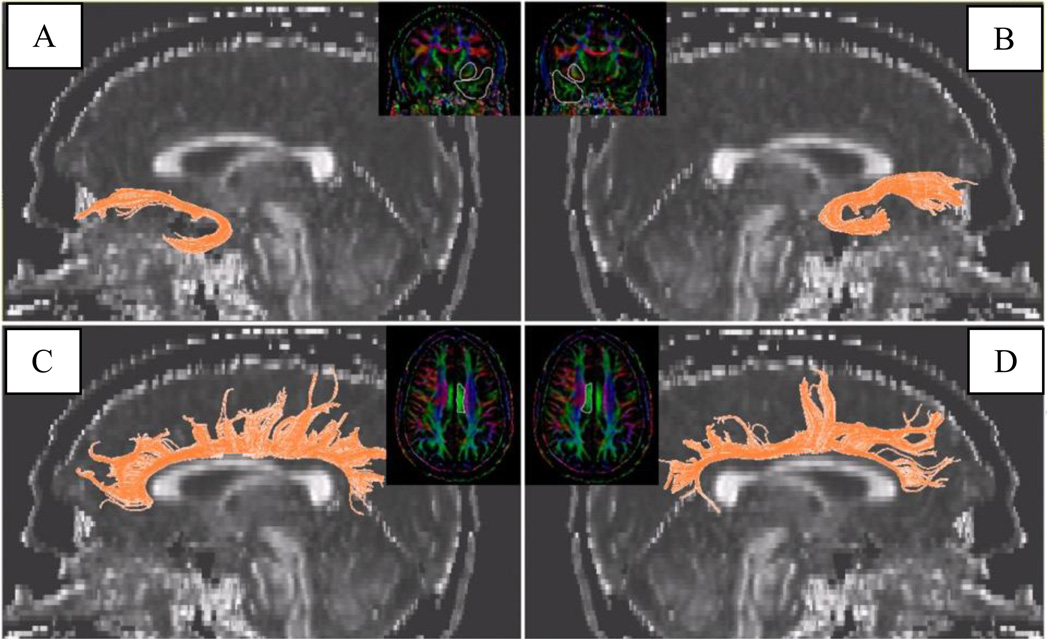
The extraction of the uncinate fasciculus followed the method described by Wakana (Wakana et al., 2007) such that the most posterior coronal slice in which the temporal lobe separated from the frontal lobe was identified and two ROI placed encompassing 1) the entire temporal lobe and 2) the entire frontal lobe. For the cingulum bundle, we used a single cigar-shaped ROI delineating the contour of the cingulum, as described by Catani and Thiebaut de Schotten (Catani & Thiebaut de Schotten, 2008). See Figure 1 for examples of extracted tracts. After tract extraction, the corresponding fiber data were loaded into an in-house program which calculated mean values for FA along the total length of each tract.
Figure 1.
Tract visualization of the (A) left uncinate fasciculus, (B) right uncinate fasciculus, (C) left cingulum bundle and (D) right cingulum bundle superimposed on a representative sagittal FA map from our research program at UIC. The inserts display the regions of interest drawn on the color map. Figure used with permission of the authors (Zhang et al., 2012).
2.23.ii Tract-based spatial statistics (TBSS)
Diffusion tensor images were transformed to standard space using TBSS software (Smith et al., 2006) and a 12-parameter affine transformation. This was followed by non-linear warping to normalize images to the Montreal Neurological Institute standard space (MNI152). Normalized images had isotropic 1 mm3 voxels and the skull and dura were removed (Brain extraction tool, BET; http://www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2002). These images were visually inspected to assure no gross errors had occurred during normalization (n=13 excluded). The normalized individual FA maps were averaged to produce a group averaged FA map and a group-wise (one pixel wide medial trajectory) skeleton map of white matter tracts. Axial and radial diffusivity maps were also transformed to the white matter skeleton by application of the parameters obtained from the FA maps.
2.3 Statistical Analyses
Group differences were assessed using individual analysis of variance (ANOVA) for continuous variables and chi-square testing for categorical variables. Task performance differences were assessed using separate ANOVAs controlling for any between-group demographic differences. We also controlled for sex and FSRP given that each of these variables is associated with depression (Alexopoulos et al., 1997; Barefoot, Mortensen, Helms, Avlund, & Schroll, 2001).
Two-tailed Pearson’s product moment correlations were performed between tract bundles extracted using DTIStudio and task variables by group collapsed across right and left hemisphere. Follow-up analyses were conducted where appropriate to investigate for possible hemispheric asymmetries in significant results. Sex was used as a covariate in all correlational analyses given known sex differences in brain morphometry where males have a greater percentage of white matter (Cosgrove, Mazure, & Staley, 2007) and differentially higher FA than females (Kanaan et al., 2012). Significant associations were also subjected to Fisher r-to-z transformation analyses to determine if tract bundle correlations with cognition were significantly different between groups. Only SOPT and OA variables reaching significance either through between-group analyses or partial correlations were used in TBSS analyses. Given the limited number of performance variables assessed as well as the exploratory nature of these analyses for ultimate use with TBSS, significance was set at p < .05.
Analysis of the relationship between FA, λ║ (axial) and λ┴. (radial) diffusivity 3D TBSS maps and our OA and SOPT variables of interest were performed using randomise software (http://www.fmrib.ox.ac.uk/fsl/). Statistical analyses were performed using linear correlation designs with OA and SOPT indices in turn as the dependent variable. All TBSS analyses were adjusted for sex given aforementioned differences in white matter integrity between males and females (Kanaan et al., 2012). The number of permutations in the analysis was set to 5000 and correction for multiple comparisons was achieved using threshold free cluster enhancement (TFCE; Smith & Nichols, 2009). As previously suggested (Smith & Nichols, 2009), cluster extent was set to 0.5 and peak height to 2.0; TFCE voxel clusters were deemed significant at p < 0.05.
3.0 Results
3.1 Demographic and Cognitive Data
There were no significant between-group differences on sex, MMSE scores, years of education or predicted verbal IQ (all p-values≥.50; Table 1). HC evidenced a slightly higher stroke risk, although this did not reach significance (p=.09) and were slightly older than LLD, F[1,78]=5.0, p=.03. HC also demonstrated slowed gross motor speed compared to the LLD group (F[1,78]=4.0,p=.05). Despite this, there were no between-group differences in terms of T2 FLAIR white matter hyperintensity volumes (p=.68). All subsequent behavioral analyses controlled for sex, FSRP, age and TMTM.
Table 1.
Group Demographics
| Healthy Controls n=45 |
Late Life Depression n=34 |
|
|---|---|---|
| Age* | 66.3±6.7 | 62.5±8.2 |
| Sex (M:F) | 19:26 | 9:25 |
| Degree Years of Education | 15.3±3.0 | 15.1±3.1 |
| MMSE Score | 28.9±1.1 | 29.1±1.4 |
|
WTAR Predicted Verbal Scale IQ Score |
106.1±15.0 | 108.2±12.2 |
|
Stroke Risk Factor Prediction Score |
11.0±4.4 | 9.2±4.6 |
| Motor Trails (seconds) | 28.3±12.1 | 23.4±8.8 |
p<.05
NOTE: M:F=male to female ratio; MMSE=Mini-Mental State Examination; WTAR=Wechsler Test of Adult Reading; IQ=Intelligence Quotient.
The LLD group took more trials to complete the OA task than HC after controlling for age, TMTM, FSRP and sex (F[1,74]=3.97,p<.05). No other OA variables were significant. There were no significant between-group differences on any SOPT variables after controlling for the covariates noted above (all p-values≥.21). One participant had an MMSE of 24. Behavioral results did not change when this individual was excluded; this person did not contribute to MRI analyses. It should be noted that the LLD and HC groups were equivalent in terms of LM performance (p=.54) after controlling for age, TMTM, FSRP and sex both with and without the individual with the MMSE of 24.
3.2 DTI Data
There were no demographic or performance based differences between the smaller MRI sample (n=62) and the overall sample described above with the exception that age was no longer significant (p=.23) and stroke risk no longer trended toward significance (p=.17). Thus, only sex was used as the covariate for DTI analyses.
3.2.1 Uncinate and Cingulum Bundle Associations
3.2.1.i Healthy Controls
The SOPT9 significantly correlated with FA in the cingulum bundle when controlling for sex (p=.04). This was driven by FA on the right, r(29)=−.37,p=.04. No other correlations reached significance (Table 2). Results no longer reached significance when age was added as a covariate.
Table 2.
Two-tailed partial correlations controlling for sex between FA in specific white matter tracts and behavioral performance
| Healthy Controls n=32 |
Late Life Depressed n=26 |
|||
|---|---|---|---|---|
| Uncinate | Cingulum | Uncinate | Cingulum | |
| SOPT 6-item error total | −.20 (.26) | −.27 (.13) | −.28 (.17) | .09 (.65) |
| SOPT 9-item error total | −.09 (.63) | −.40 (.04)* | −.32 (.11) | .07 (.74)* |
| SOPT 12-item error total | .07 (.70) | −.08 (.67) | −17 (.40) | .11 (.59) |
| OA Trials to Completion | −.19 (.28) | −.02 (.88) | −.31 (.12) | −.37 (.06) |
| OA Errors per Trials | −.25 (.16) | −.18 (.32) | −.39 (.05) | −.30 (.14) |
| OA RT per Trial (ms) | −.13 (.48) | −.11 (.55) | −.04 (.84) | .02 (.90) |
Fisher r-to-z transformation revealed a marginal but non-significant difference between HC and LLD r-values (p=.07).
NOTE: SOPT=Self-Ordered Pointing Task; OA=Object Alternation; RT=reaction time; ms=milliseconds; numbers represent r-value (p-value) with bolded values highlighting significant results.
3.2.1.ii Late-Life Depression
Total errors relatively to total trials to completion (i.e., OAE/T) significantly correlated with FA in the uncinate when controlling for sex, (p=.05). This was driven by FA on the right, r(23)=−.43,p=.03. No other correlations reached significance although there was a trend such that trials to completion (i.e., OAttc) negatively associated with FA within the cingulum bundle (p=.06), driven by the right cingulum [r(23)=−.43,p=.04]. Results no longer reached significance when age was added as a covariate; however, the association between FA in the right uncinate and OAE/T trended toward significance (p=.06).
3.2.1.iii Fisher r-to-z transformation analyses
Only conducted for r-values that were significant, two-tailed analyses did not reveal significant differences between groups for the relationship of specific fiber bundles and cognitive performance on either SOPT or OA tasks.
3.2.2 FA, λ∥ and λ┴ TBSS
3.2.2.i Healthy Controls
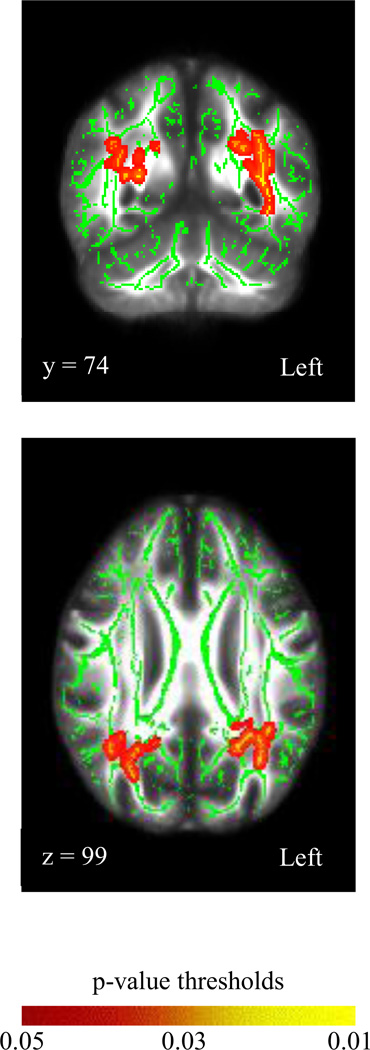
Only λ┴. was significantly associated with SOPT9 (corrected p-value of .03). Significant correlations were observed within the right internal capsule, the left posterior corona radiata and the left posterior thalamic radiation including the optic radiation (Figure 2). Specific tracts associated with these results included the inferior fronto-occipital fasciculi (IFOF; L>R), inferior longitudinal fasciculi (ILF; L>R) and the superior longitudinal fasciculi (SLF; bilaterally). Results did not change when age was added as a covariate.
Figure 2.
Visualization of the group averaged FA map and corresponding group-wise (one pixel wide medial trajectory) skeleton map of white matter tracts (z-coordinate=91) with tract-based spatial statistic results for radial diffusivity as it relates to errors on the Self-Ordered Pointing task 9-item trials in healthy controls. All results reflect 5000 permutations and a corrected p-value ≤.05.
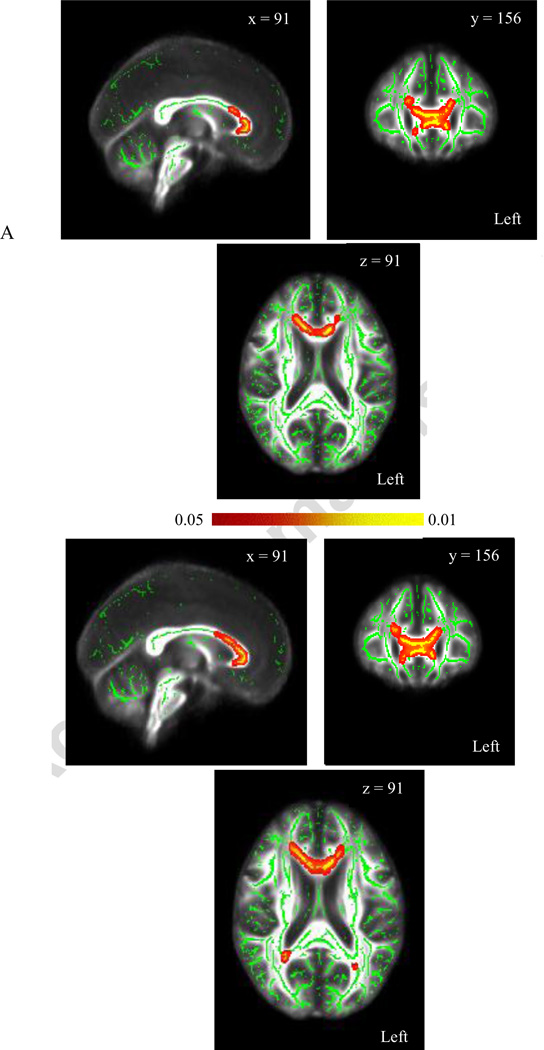
For the OA task, FA and λ┴ in the HC group correlated significantly with errors. As shown in Figure 3A, FA was negatively associated with errors relative to total trial completed (OAE/T) within the corpus callosum (genu) including the anterior thalamic radiation (ATR; bilaterally) and the anterior corona radiata bilaterally (corrected p-value=.02). Results also included uncinate and cingulum bundles (R=L) and the IFOF (R>L). Analyses for λ┴. (Figure 3B) revealed a positive association with OAE/T within the corpus callosum bilaterally (genu), right splenium, and the anterior corona radiata (corrected p-value of .01). This included the cingulum bundle and ATR bilaterally, the IFOF (R>L), right uncinate and left ILF. TBSS analysis for λ║ was not significant. Results did not change when age was added as a covariate.
Figure 3.
Visualization of the group averaged FA map and corresponding group-wise (one pixel wide medial trajectory) skeleton map of white matter tracts (z-coordinate=91) with tract-based spatial statistic results for FA (A) and radial diffusivity (B) as it relates to errors relative to total trials completed on the Object Alternation task in healthy controls. All results reflect 5000 permutations and a corrected p-value ≤.05.
3.2.2.ii Late Life Depression
In LLD, none of the TBSS analyses reached corrected significance levels. Results did not change when age was added as a covariate.
4.0 Discussion
We investigated the impact of regionally specific vulnerabilities on whole brain connectivity in aging and depression. Consistent with our hypothesis regarding the OA task, the LLD group took more trials to complete the OA task than their healthy aging counterparts. These behavioral differences were associated with specific white matter tract vulnerabilities in LLD (even after controlling for age) which only translated into a larger network of involvement for the HC group during whole brain analyses. Thus, isolated tracts involved with affective functioning, especially the uncinate (with its direct connections to the OFC), were associated with task performance in LLD but whole brain TBSS did not result in a significant or more global white matter pattern in this group.
Despite null whole brain analyses in LLD, our findings are consistent with recent work reporting an anterior over posterior gradient of white matter involvement in whole brain TBSS seen in younger adults with depression (Zuo et al., 2012). Furthermore, some (Colloby et al., 2011; Kieseppa et al., 2010) but not all (Mettenburg, Benzinger, Shimony, Snyder, & Sheline, 2012; Sexton et al., 2012) TBSS studies in LLD lacked significant results after employing standard correction techniques like TFCE and/or family-wise error-correction. This may indicate that the robust correction applied in TBSS overwhelms the subtle changes observed in depression. This may also indicate that isolated white matter involvement may only affect abilities tapping such regions as the OFC when there is specific disease-related neuropathology such as that seen in LLD; LLD specific disease-related neuropathology that may be an exacerbation of overlapping age-related prefrontal vulnerabilities but also extends to a larger network of fronto-striatal involvement and neurochemical alterations (Sheline, 2003; Zhang et al., 2012).
Results of anterior white matter tract involvement in cognition within the LLD group are consistent with the larger literature advocating specific fronto-striatal involvement in depression (Sheline, 2003). Thus, lower FA in the uncinate (particularly the right uncinate) was associated with increases in OA errors relative to trials completed in the LLD group and, while statistically weakened, this pattern remained after controlling for age. The uncinate connects the OFC to subcortical structures including the amygdala and hippocampus and work from our laboratories has documented a significant association between the uncinate and depression severity in similar and younger age (Zhang et al., 2012) cohorts with depression. The current study extends these findings to include executive functions associated with decision making and reward processing in older adults. Previous work in a smaller sample found no between-group difference in grey matter structures including prefrontal and temporal regions between depressed and non-depressed older adults (Lamar, Charlton, Zhang, & Kumar, 2012). The current larger LLD sample lacked grey matter alterations in more specific regions such as the OFC, hippocampus and amygdala compared to HCs; they also lacked whole brain white matter alterations using TBSS (data not shown). Taken together, this may point toward the role of the uncinate as a harbinger of exacerbated prefrontal vulnerability in both structural and functional integrity in LLD when compared to their healthy aging counterparts. More work in a larger sample across the stages of risk for and development of depression in aging that incorporates grey and white matter quantification as it relates to cognitive and affective functioning is needed to confirm or refute this hypothesized role of the uncinate in the more widespread prefrontal involvement associated with LLD.
In contrast, whole brain analyses in the absence of depression revealed a more widespread network of age-related decline involving anterior and posterior connectivity that negatively impacted executive performance in healthy aging. Thus, the uncinate was also implicated in OA error production in HCs; however, unlike LLD, it was not significant in isolation but as part of a larger network of white matter vulnerability in healthy aging. Analysis of OA errors in HC also indicated that IFOF and ILF white matter integrity, more specifically, demyelination was significantly associated with performance. Damage to the ILF not only impairs the priming of medial temporal structures with visual input that may be relevant for accurate recall, it may also impact visual speed and dexterity (Voineskos et al., 2012). A decline in cognitive dexterity might lead to less flexible thinking and hinder one’s ability to determine OA rules for successful performance. Much like the cingulum (also associated with OA performance in the HC group), the IFOF facilitates attention to relevant stimuli; the IFOF also facilitates attention to unexpected stimuli (Corbetta, Kincade, & Shulman, 2002). Lower IFOF integrity may make processing unexpected outcomes and learning from these outcomes more difficult during OA. Meanwhile, the corona radiata contributes to perceptual and motor functioning (Catani & Thiebaut de Schotten, 2008) as well as executive control (Niogi, Mukherjee, Ghajar, & McCandliss, 2010). Taken together, this widespread network is associated with key cognitive constructs endangered by white matter damage and associated with errors on OA in HC.
Decreases in white matter integrity (specifically, myelin integrity) in the HC group also associated with declines in SOPT9 performance across multiple white matter tracts. Many aspects of this network including the cingulum, ILF, IFOF and SLF overlap structurally and encompass many of the functional skills necessary for SOPT performance including self-monitoring, working memory and planning. Structurally the ILF and IFOF contain white matter tracts that traverse across brain regions that the cingulum bundle also connects (Catani, Jones, Donato, & Ffytche, 2003). Functionally, the ILF has been shown to facilitate processing and recall of difficult to verbalize visual stimuli (Ross, 1980) by providing a direct pathway for short-latency priming between the visual cortex and medial temporal structures (Catani et al., 2003; Voineskos et al., 2012). In contrast, the executive processes of organization, planning and the facilitation of recall have been attributed to IFOF connections (Voineskos et al., 2012). The IFOF as well as the SLF facilitate attentional processes (Corbetta et al., 2002). Together, these tracts and their associated cognitive functions make up a whole brain network that fundamentally contributes to SOPT performance in healthy aging.
The SOPT is more reliant on DLPFC than orbitofrontal regions in human and non-human primate research (Mishkin et al., 1969; Petrides, 1995) but only the OFC has been shown to be vulnerable, as an isolated structure, to permanent functional damage in LLD (Aizenstein et al., 2009; Ishizaki et al., 2008). This may explain why the LLD group did not show significant behavioral or DTI-derived results for the more DLPFC reliant SOPT. Further, while posterior brain lesions in monkeys may prevent mid-dorsolateral prefrontal regions from contributing to successful SOPT performance (Petrides, 2000), those posterior regions are not implicated in depression. Instead lower FA in the uncinate, which would negatively impact set shifting and cognitive flexibility, was associated with more errors during the more OFC-oriented OA task in the presence of depression.
The results across both groups are not without limitations. For example, some investigators challenge the existence of the IFOF (Catani et al., 2003); thus, interpretations related to this white matter tract must be taken with caution. Even omitting this pathway, however, our results include other white matter tracts such as the ILF implicated in many of the same cognitive functions associated with the IFOF. Thus, the majority of our conclusions remain intact. Investigators also challenge the roll that axial and radial diffusivity play in the human brain (Madler, Drabycz, Kolind, Whittall, & MacKay, 2008). For example, although myelin may contribute to DTI-based radial diffusivity, factors other than demyelination affect this index making the interpretation of this metric cautionary.
While methods of analysis other than those used here and a larger sample may provide better spatial and/or microstructural resolution to detect significant results and should be investigated in future research, our study using TBSS suggests specific vulnerabilities in aging and depression exert their impact differently on a larger network level. Thus, while a widespread network of age-related alterations in anterior and posterior connectivity is associated with errors in performance for healthy older adults, the largest impact on cognition in LLD occurs in isolated tracts providing connections to and from anterior brain regions such as those between the OFC and the hippocampus-amygdala complex. Given that performance on tasks reliant on the OFC predict treatment remediation in LLD (Potter, Kittinger, Wagner, Steffens, & Krishnan, 2004) and that depression is a risk factor for dementia (Ownby et al., 2006), it may be all the more important to continue investigating structure-function correlates of shared vulnerabilities in aging and depression using translational tasks with enhanced neuroanatomical specificity to inform brain-behavior responses in normal and pathological aging.
Highlights.
Age-related fronto-limbic alterations are also seen in late life depression (LLD)
Little is known about whole-brain circuitry impact of this specific vulnerability
Participants completed translational tasks that rely on fronto-limbic networks
Only controls had associations between widespread tract integrity and cognition
Widespread impact of specific vulnerabilities in aging may be eclipsed in LLD
Acknowledgements
This work was supported by the National Institute on Aging [grant number K01 AG040192–01A1 to M.L.]; and the National Institute of Mental Health [grant numbers 7RO1 MH073989–04 to A.K., K23 MH011875 to O.A.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. 'Vascular depression' hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22(1):1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Mortensen EL, Helms MJ, Avlund K, Schroll M. A longitudinal study of gender differences in depressive symptoms from age 50 to 80. Psychol Aging. 2001;16(2):342–345. doi: 10.1037//0882-7974.16.2.342. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42(4):671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011;193(1):1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J Neurol Neurosurg Psychiatry. 2010;81(1):13–19. doi: 10.1136/jnnp.2008.167288. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, Firbank MJ, Thomas AJ, Vasudev A, Parry SW, O'Brien JT. White matter changes in late-life depression: a diffusion tensor imaging study. J Affect Disord. 2011;135(1–3):216–220. doi: 10.1016/j.jad.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio TX: 2000. [Google Scholar]
- Folstein MR, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1974;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki J, Yamamoto H, Takahashi T, Takeda M, Yano M, Mimura M. Changes in regional cerebral blood flow following antidepressant treatment in late-life depression. Int J Geriatr Psychiatry. 2008;23(8):805–811. doi: 10.1002/gps.1980. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Allin M, Picchioni M, Barker GJ, Daly E, Shergill SS, et al. Gender differences in white matter microstructure. PLoS ONE. 2012;7(6):e38272. doi: 10.1371/journal.pone.0038272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertzman S, Reznik I, Hornik-Lurie T, Weizman A, Kotler M, Amital D. Stroop performance in major depression: selective attention impairment or psychomotor slowness? J Affect Disord. 2010;122(1–2):167–173. doi: 10.1016/j.jad.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen VP, Luoma K, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120(1–3):240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Lamar M, Charlton R, Zhang A, Kumar A. Differential associations between types of verbal memory and prefrontal brain structure in healthy aging and late life depression. Neuropsychologia. 2012;50(8):1823–1829. doi: 10.1016/j.neuropsychologia.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18(7):634–642. doi: 10.1097/JGP.0b013e3181cabad1. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26:874–878. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI. Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage. 2012;60(4):2182–2190. doi: 10.1016/j.neuroimage.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Vest B, Waxler M, Enger Rosvold H. A re-examination of the effects of frontal lesions on object alternation. Neuropsychologia. 1969;7:357–363. [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, McCandliss BD. Individual Differences in Distinct Components of Attention are Linked to Anatomical Variations in Distinct White Matter Tracts. Front Neuroanat. 2010;4:2. doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JT, Firbank MJ, Krishnan MS, van Straaten EC, van der Flier WM, Petrovic K, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14(10):834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133(1):44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci U S A. 2002;99(8):5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–575. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- Ross ED. Sensory-specific and fractional disorders of recent memory in man. I. Isolated loss of visual recent memory. Archives of Neurology. 1980;37:193–200. doi: 10.1001/archneur.1980.00500530031001. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, et al. Magnetic Resonance Imaging in Late-Life Depression: Multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69(7):680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54(3):338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66(3):245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: History, Rationale, and Description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2010;31(3):464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161(7):1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53(2):665–676. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33(1):21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – III. 3rd ed. Upper Saddle River, NJ: Pearson Education; 1997. [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44(3):1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Curtis C, Chernitsky LA, Pardo JV. Frontal lobe activation during object alternation acquisition. Neuropsychology. 2005;19(1):97–105. doi: 10.1037/0894-4105.19.1.97. [DOI] [PubMed] [Google Scholar]
- Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J, et al. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37(4):959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo N, Fang J, Lv X, Zhou Y, Hong Y, Li T, et al. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS ONE. 2012;7(5):e37561. doi: 10.1371/journal.pone.0037561. [DOI] [PMC free article] [PubMed] [Google Scholar]