Abstract
Difficulties with thinking and problem solving are very common among breast cancer survivors. We tested a computerized cognitive training program for 41 breast cancer survivors. The training program was associated with significant improvements in thinking and problem-solving skills. Our findings demonstrate potential for our online, home-based cognitive training program to improve cognitive difficulties among breast cancer survivors.
Background
A majority of breast cancer (BC) survivors, particularly those treated with chemotherapy, experience long-term cognitive deficits that significantly reduce quality of life. Among the cognitive domains most commonly affected include executive functions (EF), such as working memory, cognitive flexibility, multitasking, planning, and attention. Previous studies in other populations have shown that cognitive training, a behavioral method for treating cognitive deficits, can result in significant improvements in a number of cognitive skills, including EF.
Materials and Methods
In this study, we conducted a randomized controlled trial to investigate the feasibility and preliminary effectiveness of a novel, online EF training program in long-term BC survivors. A total of 41 BC survivors (21 active, 20 wait list) completed the 48 session training program over 12 weeks. The participants were, on average, 6 years after therapy.
Results
Cognitive training led to significant improvements in cognitive flexibility, verbal fluency and processing speed, with marginally significant downstream improvements in verbal memory as assessed via standardized measures. Self-ratings of EF skills, including planning, organizing, and task monitoring, also were improved in the active group compared with the wait list group.
Conclusions
Our findings suggest that EF skills may be improved even in long-term survivors by using a computerized, home-based intervention program. These improvements may potentially include subjective EF skills, which suggest a transfer of the training program to real-world behaviors.
Keywords: Chemotherapy, Cognitive flexibility, Memory, Processing speed, Rehabilitation
Introduction
Results of studies suggest that approximately 17% to 75% of patients with breast cancer (BC) experience long-term cognitive deficits that significantly reduce quality of life.1 Women who have under-gone adjuvant chemotherapy are at the highest risk for such deficits.2,3 Patients who were treated with chemotherapy show altered brain structure and function compared with patients who were not treated with chemotherapy, which suggests a pattern of diffuse brain injury that underlies cognitive deficits.2,4 Cognitive impairments significantly extend disease-related disability, which affects quality of life by limiting activities of daily living, impeding the ability to perform in the workplace, and making it more difficult to follow treatment regimens.5,6
Cognitive impairment in BC patients appears to follow a course similar to traumatic brain injury in which most deficits occur within the first 6 months after adjuvant therapies followed by a 1- to 2-year recovery and/or stabilization period.7 However, a longitudinal study by Wefel et al8 indicated that a majority of women do not show significant improvement in cognitive function over time and that approximately 30% actually develop new onset of previously nonexistent cognitive difficulties. Many women show continued neurobiologic and cognitive deficits at 10 to 20 years follow-up.2,9–11 The most common cognitive deficits noted among BC survivors include executive functions (EF) such as working memory, cognitive flexibility, multitasking, planning, and attention.12–14 In addition, neuroimaging studies have consistently and repeatedly demonstrated altered structure and function of the prefrontal cortex, which is the specialized neural region that subserves EF.2,4,9,11
Cognitive training is a behavioral method of treatment for cognitive deficits that involves improving or restoring cognitive function.15 Cognitive training has resulted in significantly improved function in a number of conditions that, like BC, demonstrate a profile of subtle cognitive impairments, which include mild traumatic brain injury16 and mild cognitive impairment,17 among others. Cognitive training has been used to improve a variety of cognitive skills, including EF, and has been shown to increase brain function, connectivity, cortical thickness, and neurotransmitter function.18,19 Importantly, cognitive training also shows significant potential for preventing cognitive decline.20,21 Cognitive training programs involve repeated skills practice, hierarchical or adaptive difficulty level, and an engaging and rewarding environment.22 Currently, no practice standards have been established regarding the number of sessions or duration of cognitive training programs. Most successful studies have involved training programs that ranged from 4 to 12 weeks or more, characterized by multiple hours (10-60) of distributed training.18,23–25
The current study focused on improving EF, given that EF difficulties are among the most common deficits described in BC survivors, as noted above. EF refers to higher order processes, integrating many domains of cognitive function that are critical for adaptive responses to the changing demands of the environment.26 Therefore, EF deficits can cause pervasive problems for BC survivors. Individuals with EF impairments tend to show rigid thinking patterns, fail to understand alternate perspectives or ideas, struggle with multitasking, have difficulty changing ideas or behaviors, have trouble recognizing that there is more than one answer or approach to a certain task, and often do not recognize when a mistake has been made.27 EF deficits are associated with increased behavioral and psychiatric problems as well as decreased response to psychiatric treatment.28,29
Impairments in EF can have significant downstream effects on other cognitive domains, such as language, social cognition, and declarative memory.27,30 EF deficits can have debilitating effects on psychosocial functioning, educational achievement, and occupational success.26,31 In addition, in patients with BC, lower EF is the single best predictor of medication nonadherence.6 Therefore, we conducted a randomized, controlled trial of a novel EF cognitive training program. The focus on EF was judged to have the greatest potential for impacting overall cognitive functioning in BC. Studies in other populations have shown that EF training can improve non-EF skills as well, including fluid reasoning32 attention, language, and social skills.33 Importantly, EF skills training can facilitate patients’ return to work and improve occupational functioning.34 Previous studies have demonstrated the efficacy of memory and processing speed training among BC survivors,35,36 but, to date, improvement of higher order EF skills, such as cognitive flexibility or verbal fluency has not been examined. In addition, no studies have investigated the efficacy of a completely computerized, home-based cognitive intervention program in BC survivors. We hypothesized that a group of long-term BC survivors randomized to an active EF training program would demonstrate significantly increased scores on a primary outcome measure of EF compared with a wait list control group.
Methods
Participants
We enrolled 41 women with a history of BC into this study. Inclusion criteria were (1) history of primary BC (stage I-IIIA at diagnosis), (2) a history of BC treatment, including surgery and adjuvant chemotherapy (participants were not excluded for radiation or hormonal therapies), (3) minimum age of 40 years to capture peak years of BC diagnosis, (4) at least 18 months after chemotherapy to allow for neural stabilization and recovery,4,7 (5) possessing access to an Internet-connected home computer, and (6) expression of interest in receiving the intervention.
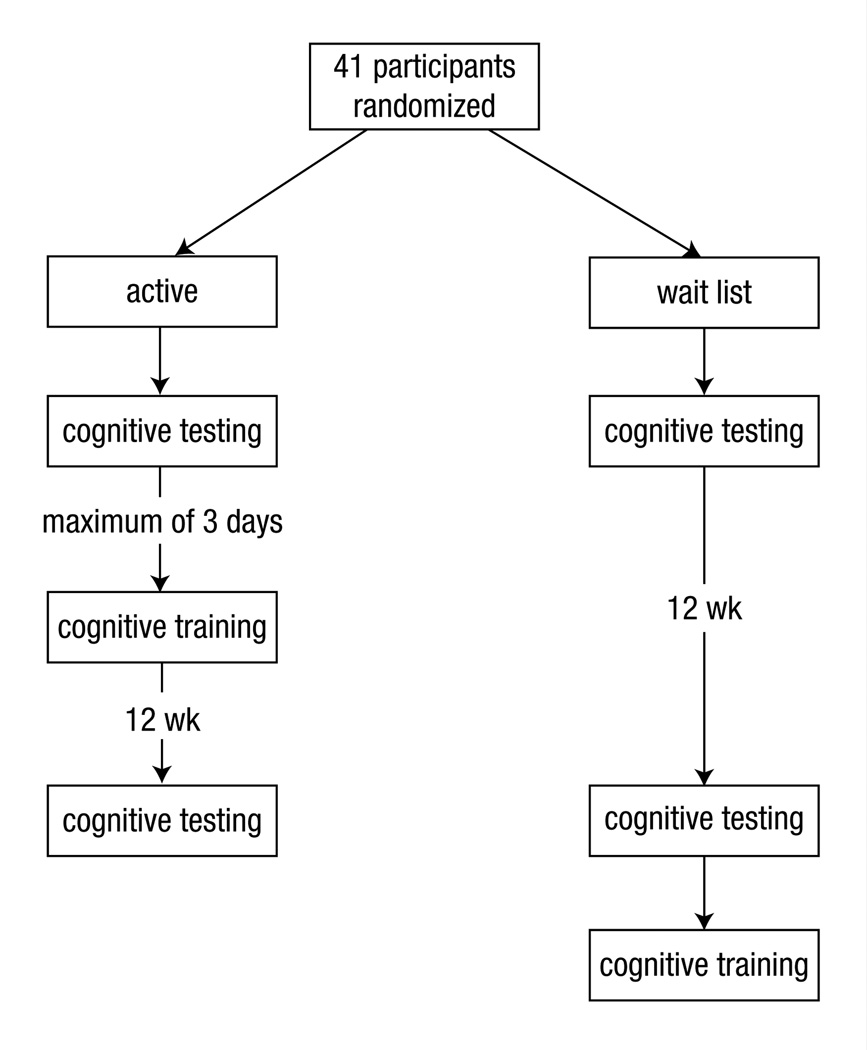
Exclusion criteria included (1) previous chemotherapy treatment; (2) major sensory deficit (eg, blindness); (3) color blindness, because some of the training tasks and cognitive tests relied on color; (4) neurologic or major medical conditions known to affect cognitive function; (5) history of inpatient psychiatric hospitalization; and (6) current psychostimulant or central nervous system depressant medication use (common antidepressants were not excluded). The participants were randomized by computerized coin toss software to an active treatment group or to a wait list group (Figure 1). Forty-one participants (active, 21; wait list, 20) enrolled in the study (see Table 1 for participant characteristics). This study was approved by the Stanford University Institutional Review Board, and all participants provided informed consent.
Figure 1.
Study Schema
Table 1.
Participant Characteristics
| Active (n = 21) |
Wait List (n = 20) |
P | |
|---|---|---|---|
| Mean (SD) Age, y | 55 ± 7 | 56 ±6 | .72 |
| Mean (SD) Education, y | 16 ± 2 | 16 ± 3 | .80 |
| Mean (SD) Time After Therapy, ya | 6 ± 3 | 6 ± 3 | .88 |
| % Radiation | 70 | 63 | .74 |
| % Hormonal Therapy (Tamoxifen)b | 60 | 63 | 1.0 |
| % Disease Stage 1, 2, 3 | 25, 50, 25 | 26, 42, 32 | .34 |
| % Postmenopause | 71 | 65 | .74 |
Chemotherapy and/or radiation.
Six participants in each group were still taking tamoxifen at the time of enrollment and all continued to take it throughout the study.
EF Training Program
The online, computerized training program was administered by using the participant’s home computer. No personal health information was entered or collected by the program. There is no study to date that systematically investigates the critical minimum number of sessions required for successful training of EFs. The length and number of training sessions in previous studies vary widely depending on the study population, sample size, training tasks, and training setting (eg, laboratory vs. home). Studies with healthy older adults and patients with mild cognitive impairments usually used 1 to 6 months of training, with training sessions 15 to 90 minutes in duration.37–43 The curriculum in the present study included 48 sessions, each 20 to 30 minutes in duration, involving various combinations of 13 different exercises designed to improve core EF. This curriculum was selected by a clinical neuropsychologist (S.K.) using existing cognitive exercises created by Lumos Labs Inc (San Francisco, CA). Exercises and training durations were chosen based on clinical experience in addition to relevant literature that pertains to EF training. The participants were required to login to their individual online account and complete a session of 5 exercises 4 times per week for 12 weeks. Each time the participant logged in, the program automatically delivered the exercises assigned for that session. The curriculum and schedule were hard coded into the program by Lumos Labs and thus were the same for each participant. Due to constraints of the Lumos Lab Web site, the curriculum had to be divided into 2 courses. Participants completed course 1, followed immediately by course 2. Each exercise began with the option to start the exercise or view written and animated instructions for the exercise. General instructions for completing the cognitive training program were provided verbally and in writing to each participant during her first assessment appointment.
The exercises were designed to train and practice the following EF skills: cognitive flexibility, working memory, processing speed, and verbal fluency. In summary, the training tasks were composed of switching games (eg, based on the spatial location of the stimulus, participants responded to either a specific number or a specific letter of the stimulus), mental rotation games (eg, navigate a rotating maze), n-back memory games (eg, determine if the current picture or symbol matched the one shown 1 or 2 screens back), spatial sequencing memory games (eg, recall the location of coins and then find them in the order of their value), word stem completion games (eg, use various word stems such as “cog” to produce as many different words as possible), route planning (eg, navigate a maze by using the fewest number of moves possible), and rule-based puzzle solving (eg, determine if groups of figures follow an implicit rule). All exercises involved visual stimuli that required a motor response (key press or mouse click). Exercises were adaptive to individual ability, increasing in difficulty level as the participants progressed. Initial difficulty level was very low (eg, simple stimuli, longer time limits, cued or scaffolded items, illustrations and/or explanations of correct responses) and then, as the participant’s performance improved, difficulty level was increased (more complex stimuli, shorter time limits, no cuing or explanations). Changes in difficulty level were determined by using proprietary algorithms that considered both intra- and intersession performance, including speed and accuracy. The program provided immediate visual and auditory feedback, and reinforcement regarding performance.
We paid Lumos Labs a fee per participant to use the cognitive training curriculum. We do not have any financial relationships with Lumos Labs and have no other conflicts of interest related to the cognitive training program. The exercises that we used are currently commercially available to the public (http://www.lumosity.com/). A previous study from our group demonstrated positive effects of easier versions of our curricula on EF in child and adolescent survivors of cancer.44
Adherence
The online program automatically recorded and stored the time and date of each exercise, which exercises were completed, the performance for each exercise, and the duration of each exercise and session. These parameters were used to determine adherence. Adherence was examined in terms of completing training sessions on schedule (ie, 4 sessions per week) as well as with respect to performance (accuracy). It was expected that subjects would demonstrate an overall positive linear trend in exercise performance if they were actively engaging in the program and a flatter slope if they were not engaging. Because the program was adaptive, even participants who were truly engaging, but struggling with the exercises would show overall improvement. Specifically, the program reduced the difficulty level if performance was low so that those subjects could improve with effort. To enhance adherence, research staff contacted participants via telephone or e-mail once per week to remind them to complete the exercises.
Outcome Measures
The effects of the EF training program on cognitive function were measured by using psychometrically validated and standardized cognitive tests. These tests were administered at baseline before the training program and then again after the participant completed the training program. The participants were required to begin the training program no more than 3 days after completing their baseline cognitive testing. They also were required to return for posttesting no more than 3 days after having completed the EF training program (see Figure 1). Testing was administered by trained research staff members who were blinded to the intervention assignment and time point of the participants. Alternate test forms were used when available to reduce the effects of practice, administered in a standardized order for every participant (ie, A-B). The primary outcome measure was the Wisconsin card sorting test (WCST). The WCST is a well-validated, widely used neuropsychologic measure of EF that measures cognitive flexibility or the ability to generate alternate solutions to problems and fluidly shift mental set.45 The WCST has been shown to be robustly sensitive to abnormalities in executive-prefrontal neurocircuitry.45,46 In addition, results of our previous studies indicated that this measure discriminates between BC survivors who were treated with chemotherapy and noncancer controls.2
Secondary outcome measures included the letter fluency test from the Delis-Kaplan Executive Function System, a measure of EF and language,47 the Hopkins Verbal Learning Test Revised (HVLT-R) for verbal memory,48 the digit span and symbol search subtests of the Wechsler Adult Intelligence Scale 4th edition, measures of working memory and processing speed, respectively,49 and the Global Executive Composite score of the Behavioral Rating Inventory of Executive Function (BRIEF), a self-report measure of EF.50 Although the EF training program did not explicitly train verbal memory, the HVLT-R was included to examine downstream effects of EF on verbal declarative memory. The HVLT-R and verbal fluency tests have previously been shown to discriminate between BC patients treated with chemotherapy and survivors, and are among the tests recommended by the International Cognition and Cancer Task Force for harmonizing studies of cancer-related cognitive deficit.8,51 Digit span also has been shown to discriminate between BC survivors and controls.52,53 The BRIEF not only discriminates between BC and controls but also correlates significantly with deficits in prefrontal cortex among BC survivors.2,54
In addition, we administered the Clinical Assessment of Depression (CAD),55 a measure of psychiatric distress, including depression, anxiety, and cognitive fatigue. CAD was not used as an outcome measure but as a means of evaluating and controlling for effects of any psychiatric distress on cognitive outcome measures. The CAD score did not differ significantly between the intervention groups at either time point (P = .45, P = .93, respectively).
Statistical Analyses
For the primary hypothesis, we used a fixed effects analysis of covariance (ANCOVA) with postintervention WCST as the response, group as the factor, and baseline WCST as the primary co-variate. Age, education level, radiation, hormonal therapy, CAD score, and time since chemotherapy were additional covariates. Secondary hypotheses were tested by using the same ANCOVA model as described above with Bonferroni correction (α = .0125). As noted above, the global score for the BRIEF was used in secondary analyses; however, the BRIEF consists of several subscales that represent specific EF domains. Therefore, exploratory analyses of the BRIEF sub-scales also were conducted by using ANCOVA models (uncorrected) to determine if any individual self-rated EFs were impacted by the intervention.
Model terms that were insignificant at the .05 level were removed from the model to improve parsimony. The significance of the intervention effect (as well as the other fixed effects) were determined by an F test. Effect sizes were calculated by using parameter estimates and the estimated population SD (ie, adjusted mean difference divided by population SD square root). We also calculated a “corrected effect” by subtracting the control within-group (post-training vs. pre-training) Cohen d effect size from that of the active group to represent the results corrected for practice effects. Because there were very few dropouts, we analyzed the available data and did not apply missing data analysis techniques.
Results
Feasibility and Adherence
Analysis of the data suggested that the intervention was safe (no adverse events were associated with the intervention) and feasible to implement. Target accrual was met within 2 months and participation proceeded at a rate of 8 to 10 per month, limited only by study resources. The subjects in the active arm showed 95% compliance, as defined by completing the program (1 active-arm participant dropped out at week 8 due to a family emergency). Two participants in the wait list arm dropped out before completing their Time two assessments; one due to an injury and the other due to health reasons, unrelated to the study.
As noted above, adherence was defined as completing sessions on schedule (ie, 4 sessions per week) as well as performance slope. Participants completed an average of 4 ± 0.42 sessions per week and required an average of 13.0 ± 0.92 weeks, (range, 12–15 weeks) to complete the entire study, including both cognitive assessments and the training program and/or waiting period. There was no difference between the active (mean, 13.1 ± 0.95 weeks; range, 12–15 weeks) and the wait list (mean, 13.0 ± 0.91 weeks; range, 12–15 weeks) group in terms of study completion time (P = .84). In addition, the participants showed high positive performance correlations across sessions and exercises (mean r, 0.72 ± 0.13), which suggests that they were actively engaged and putting forth a strong effort. These correlations reflect performance slope or the correlation between session number and exercise accuracy (a measure of effort), averaged across participants. Taken together, analysis of these data suggests strong intervention adherence.
Cognitive Outcomes
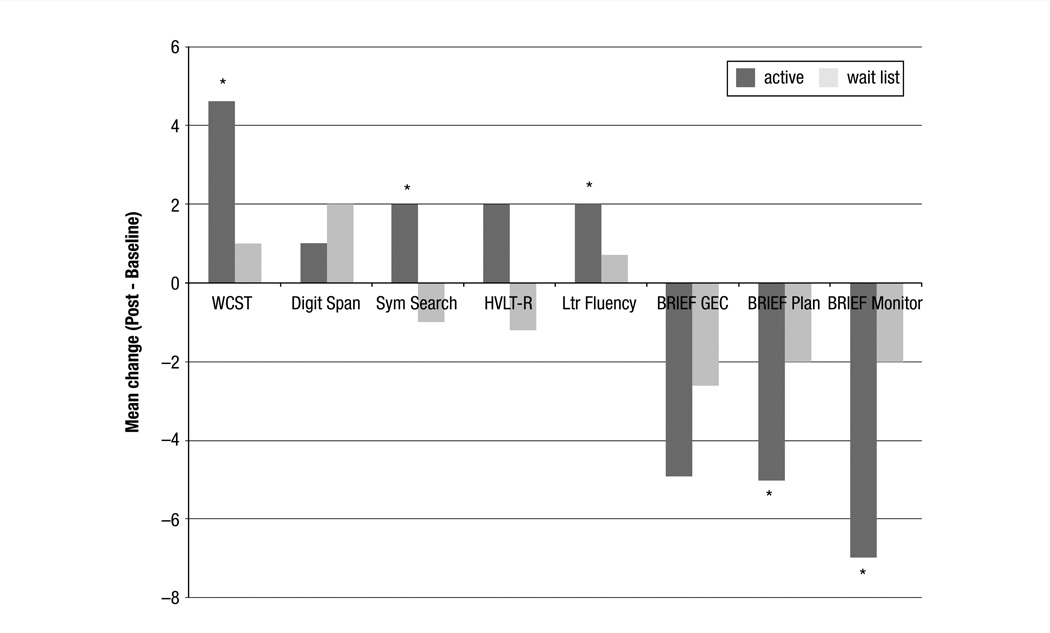
As shown in Table 2 and Figure 2, the active group demonstrated significant improvement in the WCST score compared with the wait list group (effect size, 0.58; P = .008). In addition, the active group showed significant improvement on the letter fluency (effect size, 0.82; P = .003), and symbol search (effect size, 0.87; P = .009), and a trending improvement on the HVLT-R (effect size, 0.56; P = .07). Digit span scores were not significantly improved (effect size, 0.14; P = .57). Although global BRIEF scores were reduced (which indicate improvement), they were not significant (effect size, 0.26; P =.22). However, exploratory analyses suggested significant improvements in BRIEF subscales, including planning and/or organization (effect size, 0.44; P = .02) and task monitoring (effect size, 0.43; P = .03). There were no significant effects of age, education, radiation, or hormonal therapy in any of the models. However, the CAD score showed a significant effect in the BRIEF models only (P < .02).
Table 2.
Cognitive Outcome and Psychiatric Status Data
| Active Group, Mean (SD) (n = 21) |
Wait List Group, Mean (SD) (n = 20) |
Active vs. Waitlist | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | P | Effect Sizea | Corrected Effectb |
|
| WCST | 46 ± 8 | 51 ±5 | 48 ± 11 | 48 ± 9 | .008 | 0.58 | 0.74 |
| Letter Fluencyc | 12 ± 3 | 14 ± 2 | 11 ±3 | 12 ± 2 | .003 | 0.82 | 0.39 |
| HVLT-Rc | 49 ± 10 | 53 ± 7 | 55 ±6 | 54 ±9 | .07 | 0.56 | 0.59 |
| Digit Span | 11 ± 2 | 12 ± 3 | 11 ± 3 | 13 ± 4 | .57 | 0.14 | −0.17 |
| Symbol Search | 12 ± 3 | 14 ± 3 | 13 ± 3 | 12 ± 3 | .009 | 0.87 | 1.0 |
| BRIEF GECd | 62 ± 15 | 57 ± 16 | 61 ± 12 | 59 ± 11 | .22 | 0.26 | 0.15 |
| BRIEF plan and/or organize | 60 ± 15 | 55 ± 15 | 62 ± 16 | 60 ± 12 | .02 | 0.44 | 0.19 |
| BRIEF task monitor | 63 ± 13 | 56 ± 12 | 62 ± 12 | 60 ± 10 | .03 | 0.43 | 0.38 |
| CADd | 54 ± 12 | 51 ± 13 | 51 ± 11 | 50 ± 13 | .32 | 0.23 | 0.16 |
Abbreviations: BRIEF=Behavioral Rating Inventory of Executive Function; CAD = clinical assessment of depression; GEC = global executive composite; HVLT-R = Hopkins verbal learning test revised; WCST = Wisconsin card sorting test.
Between-group effect size: adjusted mean difference divided by population SD square root.
Corrected effect: active group Cohen d effect size less control Cohen d effect size.
Alternate test forms were used.
For CAD and BRIEF, the higher scores equal increased impairment; for all other tests, lower scores equal increased impairment.
Figure 2.
Mean Change Scores for Cognitive Outcome Measures. The Active Cognitive Training Group Showed Significantly (P < 05) Increased Scores on Measures of EF Outcome, Including WCST, Letter Fluency, Sym Search, and BRIEF Subscales (denoted by asterisks). HVLT-R Was Marginally Improved (P = 07)
Abbreviations: BRIEF = behavioral rating inventory of executive function; EF = executive function; GEC = global executive composite; HVLT-R = Hopkins verbal learning test revised; Ltr Fluency = letter fluency; Sym Search = symbol search; WSCT = Wisconsin card sorting test.
Discussion
Analysis of our results suggests that a computerized, home-based cognitive training curriculum can be feasibly implemented in long-term survivors of BC. The training program was associated with excellent adherence and compliance. We also demonstrated that the training program was an effective method for improving EF. Compared with the wait list control group, participants randomized to the active cognitive training arm demonstrated significantly increased performance on measures of cognitive flexibility, processing speed, and verbal fluency. The active group also showed improvement in self-rated executive behaviors and a trend for improvement in verbal memory, a skill that was not explicitly trained.
Limitations of the study include the small sample size, which restricts the interpretation of the results. Like most other studies, our sample of BC survivors was very heterogeneous in terms of disease and treatment history, and, therefore, the effects of these variables on intervention efficacy could not be addressed due to a lack of statistical power. Importantly, we were not able to include any extended follow-up assessments that may have provided insights regarding the stability of training effects over time. The EF training exercises exclusively involved visual stimuli. There were no options for auditory-based exercises at the time of this study’s inception. Training programs that include both visual and auditory exercises may provide a more comprehensive intervention with larger effects. In addition, we enrolled participants irrespective of baseline cognitive function (or dysfunction). Although this may have prevented participants with subtle, difficult to detect deficits from being excluded, it may also have biased our sample. Including only subjects with a minimal degree of defined impairment may have increased the intervention effects. However, despite these limitations, analysis of our findings suggests that a practical, accessible cognitive training intervention may help improve EF in BC survivors.
Few other studies have examined behavioral interventions for cognitive skills in BC survivors, and, to date, treatment for higher-order EF skills has not been examined. As noted above, previous research has demonstrated the efficacy of memory, attention, and processing speed rehabilitation among BC survivors.35,36 Another study showed no intervention effects after cognitive rehabilitation for attention.56 The participants in this study were only 2 months, on average, after therapy and thus the study results may have been confounded by the control group’s natural neurologic recovery. Rehabilitative cognitive training may be more effective if initiated after the patient has stabilized.57
Animal and human studies indicate that intensive therapy during acute periods of recovery provide no benefit beyond what is associated with spontaneous recovery and can even lead to poorer outcomes in some cases.57 Analysis of the research suggests that patients with BC continue to show cognitive decline within the first 3 to 6 months after therapy,7 and, therefore, cognitive training might be more optimally implemented after this period. Accordingly, cognitive training has been found to be highly effective in the chronic recovery period, even when implemented multiple years after the initial injury.33 Our study required participants to be at least 18 months after therapy to allow for natural neural stabilization.
In fact, our participants, on average, were 6 years after therapy (including chemotherapy and radiation). Previous studies demon strated persistent and even late onset EF deficits in BC survivors8. Our findings suggest that EF can still be improved even after the chronic recovery period after adjuvant therapy for BC. As noted above, the stability of EF improvements over time could not be determined from this pre-post design. Existing evidence from previous studies of other populations suggests potential retention of cognitive training benefits at both short-term (3 months)58 and long-term (5 years)59 postintervention follow-ups. However, further longitudinal assessment after completion of the intervention is necessary to determine if training effects remain stable in BC survivors. It is possible that regular, consistent cognitive exercise, or at least intermittent “booster” sessions, are required to maintain improvements in some or perhaps all patients.
Although participants in the present study were enrolled irrespective of baseline cognitive status, post hoc analyses indicated that their baseline WCST total errors scores were significantly lower than those obtained from a group of 49 comparably aged and educated healthy female controls (P = .03) who were pooled from our previous BC studies.2,60,61 The control WCST mean was 51.0 ± 9.7 and the BC mean (active plus wait list participants) was 46.0 ± 9.6. Thus, our sample of BC survivors was performing lower than their same-aged peers before intervention despite their scores being within the “normal” range. In addition, chemotherapy-related cognitive changes often tend to be quite subtle, and, therefore, mean standardized cognitive scores may not detect them.14,62 Examining cognitive changes over time may be a more sensitive method for identifying these cognitive difficulties.63
A critical issue in cognitive training research is whether training-induced changes transfer or generalize to other skills and/or real-world tasks.64 Cognitive training exercises may simply improve subjects’ ability to perform on cognitive tests. One of the 13 exercises in our training curriculum required participants to determine if groups of figures followed various implicit rules. This concept is similar to that used in the WCST, our primary outcome measure. In addition, the word-stem exercise used in the training program is somewhat similar to the letter fluency test. However, we demonstrated potential transfer effects of the training program to other skills by providing support for the program’s efficacy beyond “training to the test.” Specifically, the BRIEF measures real-world or everyday EF behaviors.50 We did not demonstrate significant improvement in the global BRIEF score, although exploratory analyses indicated that some of the BRIEF subscales, including those that measure planning, organization, and task monitoring, were significantly improved after the intervention. We also demonstrated a potential transfer to verbal memory, which was not explicitly trained. Verbal memory was associated with a moderate effect size, although the statistical significance did not reach our threshold.
We did not show any effects on working memory despite the training program, including several exercises specifically focused on this skill. Results of previous studies showed significant improvement in working memory after cognitive training in other populations. 18,65 The reason that these skills were not improved by the present training program is unclear, although it may reflect the choice of outcome measure. The digit span test involves auditory stimuli, whereas a visual working memory test may have elucidated change in this area. It is also possible that the training exercises in working memory domains were not effective or that this sample of BC survivors required more and/or longer training sessions to impact working memory skills. In addition, auditory-based working memory exercises or a combination of visual and auditory tasks may have yielded improved results.
Although CAD scores did not suggest clinically significant depression, anxiety, or fatigue, CAD was a significant covariate in the BRIEF models. This is consistent with previous studies, which show that self-ratings of cognitive function are strongly influenced by psychiatric distress.3,14, In addition, post hoc analysis indicated that the CAD score showed a similar, nonsignificant improvement after the training program (effect size, 0.23; P = .32). Therefore, ongoing subclinical levels of distress may have confounded subjective assessment of cognitive ability after the intervention program. Including relaxation exercises in the curriculum might enhance future cognitive training programs for BC survivors. It is also possible that our study simply lacked the statistical power necessary to detect a change in global BRIEF (and CAD) scores.
Conclusion
Our results demonstrated the preliminary efficacy of online cognitive training to improve EF in BC survivors, including cognitive flexibility, verbal fluency, and processing speed. We also found significant improvement in specific self-rating of everyday EF behaviors and observed some transfer to verbal memory with a strong effect size. Together, the results demonstrate the potential of home-based, computerized EF training for even long-term BC survivors and suggest the significance of targeted training for this population.
The high incidence of BC and increasing survival rates contribute to a large and growing number of individuals who may experience cognitive impairments related to their cancer experience. The National Institutes of Health estimated that, in 2010, the indirect cost of cancer on society due to “illness-related loss of productivity” was $20.9 billion.66 Cognitive difficulties likely contribute significantly to these indirect costs. Our findings demonstrate potential for our standardized, online, home-based cognitive training program to increase commonly impaired EF skills among chemotherapy-treated BC survivors.
Future research should focus on longer duration of cognitive training with more emphasis on metacognitive strategies and a better understanding of the long-term effects of the intervention. Longer training durations may be necessary to ensure adequate transfer effects.67 In addition, an emphasis on metacognitive strategies may help improve transfer to real-world behaviors. The combination of home-based cognitive training with manualized components such as a survivor workbook might retain the practical, accessible elements of home-based cognitive training with more clinic-based instruction. For example, participants could be provided with a workbook that contains instructions for applying cognitive exercises to real-world tasks as well as methods for compensating for certain cognitive difficulties so that they can perform daily living tasks with success. Larger studies should ideally include extended postintervention follow-up assessments to examine the stability of intervention effects after training has ceased. Larger samples also would allow for analyses of variables that might help predict individual differences in response to cognitive training. The inclusion of neuroimaging assessments in future studies could significantly assist with this prediction68 and could also increase our understanding regarding neuroplasticity mechanisms after chemotherapy treatment.
Clinical Practice Points
Cognitive difficulties are one of the most common quality-of-life complaints among BC survivors. However, there currently are very few treatment options available. Pharmacologic trials have been hampered by null effects on cognitive function69 as well as harmful consequences, including reduced survival rates.70
Cognitive training represents a behavioral, nonpharmacologic method for improving cognitive function after chemotherapy treatment.
Our results indicate that even long-term survivors can benefit from these interventions. Training programs such as the online curriculum used in this study are practical, accessible, inexpensive, and easily disseminated to survivors as part of survivorship care plans due to their self-contained and computerized platform.
Acknowledgments
This work was supported by grants from the National Institutes of Health New Innovator Award (1DP2 OD004445-01) (S.K.). We thank Della Koovakkattu and Mika Pritchard-Berman for their assistance with project coordination and data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 2.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68:1447–1553. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 4.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stilley CS, Bender CM, Dunbar-Jacob J, et al. The impact of cognitive function on medication management: three studies. Health Psychol. 2010;29:50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 8.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 9.de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 12.Yamada TH, Denburg NL, Beglinger LJ, et al. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res. 2009;151:387–419. doi: 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- 15.Morris J. Cognitive rehabilitation: where we are and what is on the horizon. Phys Med Rehabil Clin N Am. 2007;18:27–42. doi: 10.1016/j.pmr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Serino A, Ciaramelli E, Di Santantonio A, et al. A rehabilitative program for central executive deficits after traumatic brain injury. Brain Cogn. 2006;60:213–214. [PubMed] [Google Scholar]
- 17.Jean L, Bergeron ME, Thivierge S, et al. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18:281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- 18.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 21.Mowszowski L, Batchelor J, Naismith SL. Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique? Int Psychogeriatr. 2010:1–12. doi: 10.1017/S1041610209991748. [DOI] [PubMed] [Google Scholar]
- 22.Fisher M, Holland C, Subramaniam K, et al. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorell LB, Lindqvist S, Bergman Nutley S, et al. Training and transfer effects of executive functions in preschool children. Dev Sci. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 24.Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Front Hum Neurosci. 2010;4:16. doi: 10.3389/neuro.09.016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelinski EM, Spina LM, Yaffe K, et al. Improvement in memory with plasticity-based adaptive cognitive training: results of the 3-month follow-up. J Am Geriatr Soc. 2011;59:258–265. doi: 10.1111/j.1532-5415.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- 26.Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther. 2007;31:119–127. doi: 10.1097/NPT.0b013e31814a63c2. [DOI] [PubMed] [Google Scholar]
- 27.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkin JJ, Leuchter AF, Cook IA, et al. Executive dysfunction predicts nonre-sponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 29.Tarter RE, Kirisci L, Reynolds M, et al. Neurobehavior disinhibition in childhood predicts suicide potential and substance use disorder by young adulthood. Drug Alcohol Depend. 2004;76(suppl):S45–S52. doi: 10.1016/j.drugalcdep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Woods SP, Weinborn M, Posada C, et al. Preliminary evidence for impaired rapid verb generation in schizophrenia. Brain Lang. 2007;102:46–51. doi: 10.1016/j.bandl.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf TJ. Executive function in the workplace. Works. 2010;36:371–372. doi: 10.3233/WOR-2010-1038. [DOI] [PubMed] [Google Scholar]
- 32.Jaeggi SM, Buschkuehl M, Jonides J, et al. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92:519–530. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Ownsworth T. A metacognitive contextual approach for facilitating return to work following acquired brain injury: three descriptive case studies. Works. 2010;36:381–388. doi: 10.3233/WOR-2010-1041. [DOI] [PubMed] [Google Scholar]
- 35.Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buschkuehl M, Jaeggi SM, Hutchison S, et al. Impact of working memory training on memory performance in old-old adults. Psychol Aging. 2008;23:743–753. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- 38.Schmiedek F, Lövdén M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: findings from the COGITO study. Front Aging Neuroscience. 2010;2:27. doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SC, Schmiedek F, Huxhold O, et al. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol Aging. 2008;23:731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- 40.Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 41.Mozolic JL, Long AB, Morgan AR, et al. A cognitive training intervention improves modality-specific attention in a randomized controlled trial of healthy older adults. Neurobiol Aging. 2011;32:655–668. doi: 10.1016/j.neurobiolaging.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozzini L, Costardi D, Chilovi BV, et al. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry. 2007;22:356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- 43.Kurz A, Pohl C, Ramsenthaler M, et al. Cognitive rehabilitation in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2009;24:163–168. doi: 10.1002/gps.2086. [DOI] [PubMed] [Google Scholar]
- 44.Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011;25:101–112. doi: 10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyhus E, Barceló F. The Wisconsin card sorting test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Lie CH, Specht K, Marshall JC, et al. Using fMRI to decompose the neural processes underlying the Wisconsin card sorting test. NeuroImage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol. 2005;27:599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro AM, Benedict RH, Schretlen D, et al. Construct and concurrent validity of the Hopkins verbal learning test-revised. Clin Neuropsychol. 1999;13:348–458. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler D. Wechsler Adult Intelligence Scale Fourth Edition. San Antonio, TX: Psychological Corporation; 2008. [Google Scholar]
- 50.Roth RM, Isquith PK, Gioia G. Behavioral rating inventory of executive function — adult version. Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- 51.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Im-mun. 2013;30(suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 53.Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 54.McDonald BC, Conroy SK, Smith DJ, et al. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. 2013;30(suppl):S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aghakhani A, Chan EK. J Psy-choed Assess. 4. Vol. 25. Odessa, FL: Psychological Assessment Resources; 2007. pp. 416–422. Test reviews: bracken, B.A., and Howell, K 2004. Clinical Assessment of Depression. [Google Scholar]
- 56.Poppelreuter M, Weis J, Bartsch HH. Effectsof specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J Psychosoc Oncol. 2009;27:274–296. doi: 10.1080/07347330902776044. [DOI] [PubMed] [Google Scholar]
- 57.Sohlberg M, Turkstra L. Optimizing Cognitive Rehabilitation: Effective Instructional Methods. New York, NY: Guilford Publications; 2011. [Google Scholar]
- 58.Jolles DD, Grol MJ, Van Buchem MAV, et al. Practice effects in the brain: changes in cerebral activation after working memory practice depend on task demands. NeuroImage. 2010;52:658–668. doi: 10.1016/j.neuroimage.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castellon S, Ganz PA. Neuropsychological studies in breast cancer: in search of chemobrain. Breast Cancer Res Treat. 2009;116:125–127. doi: 10.1007/s10549-008-0211-2. [DOI] [PubMed] [Google Scholar]
- 63.Ouimet LA, Stewart A, Collins B, et al. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neu-ropsychol. 2009;31:73–89. doi: 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- 64.Green CS, Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol Aging. 2008;23:692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salminen T, Strobach T, Schubert T. On the impacts of working memory training on executive functioning. Front Hum Neurosci. 2012;6:166. doi: 10.3389/fnhum.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Cancer Society. [Accessed: November 16, 2011];AC Cancer Facts and Figures; 2010 11/16/11. Available from: http://www.cancer.org/acs/groups/content/nho/documents/document/acspc-024113.pdf.
- 67.Norman G. Teaching basic science to optimize transfer. Med Teach. 2009;31:807–811. doi: 10.1080/01421590903049814. [DOI] [PubMed] [Google Scholar]
- 68.Strangman GE, O’Neil-Pirozzi TM, Supelana C, et al. Regional brain morphome-try predicts memory rehabilitation outcome after traumatic brain injury. Front Hum Neurosci. 2010;4:182. doi: 10.3389/fnhum.2010.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of D-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 70.Crouch Z, DeSantis ER. Use of erythropoietin-stimulating agents in breast cancer patients: a risk review. Am J Health Syst Pharm. 2009;66:1180–1185. doi: 10.2146/ajhp080214. [DOI] [PubMed] [Google Scholar]