Abstract
Background
To our knowledge, the feasibility of therapy with hypomethylating agents (HAs) in patients with renal insufficiency (RI) has not been examined.
Patients and Methods
We reviewed 41 patients with a diagnosis of acute myeloid leukemia (n = 17), myelodysplastic syndromes (n = 15), and chronic myelomonocytic leukemia (n = 9) who had RI and were receiving therapy with azacitidine or decitabine. The median number of administered cycles was 3. Most patients (39; 95%) received a standard dose of the drugs at the initiation of therapy. Nine patients (22%) required treatment interruptions or discontinuation, and 10 patients (24%) required dose reductions.
Results
The overall response rate was 63%, and 4 patients (10%) achieved a complete response. Twenty patients (51%) experienced grade 3 or 4 myelosuppression-related toxicities. Hospitalization was required in 68% of the patients. Among 12 patients with an estimated glomerular filtration rate of 29 mL per minute or less, 6 required dose reductions attributable to myelosuppression (n = 3) or to worsening renal function (n = 3). The overall survival (OS) at 18 months was 12%, and the median OS was 8.6 months.
Conclusion
The use of HA in patients with RI is feasible, but is associated with a higher incidence of toxicity. Dose adjustments and the use of growth factor may be necessary for some patients.
Keywords: Azacitidine, Decitabine, Myelodysplastic syndrome, Serum creatinine
Introduction
Epigenetic therapy with hypomethylating agents (HAs) is considered the standard of care in patients with high-risk myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML).1–4 Renal insufficiency (RI) constitutes a frequent morbidity in elderly patients, ie, the population most affected by MDS. Because many clinical trials exclude patients with a serum creatinine level (sCr) ≥ 1.5 mg/dL, no reports, to the best of our knowledge, have described the efficacy and safety of low doses of azacitidine (AZA) or decitabine (DAC) in patients with MDS who have RI.
The two azanucleotides are cytotoxic at higher doses, and at lower doses, they inhibit DNA methyltransferase activity and reverse the aberrant methylation patterns found in cancer cells.5 The available HAs differ in their pharmacokinetic and pharmacodynamic profiles. Decitabine is an analogue of 23-deoxycytidine that requires phosphorylation by deoxycytidine kinase before being incorporated into DNA.6 Unlike AZA, DAC is not incorporated into RNA. Similar to cytarabine, DAC is deaminated by cytidine deaminase, forming the inactive metabolite 5-aza-23-deoxyuridine. Its excretion is mainly hepatic, and less than 1% of the drug is excreted in the urine.7 In contrast, AZA is a ribonucleoside analogue of cytidine, which is reduced to 5-aza-23-deoxycytidine and then incorporated into both RNA and DNA.8 More than half of the excretion of AZA and its metabolites occurs through the kidneys.9 Patients who received high doses of azacitidine reportedly developed renal tubular acidosis and electrolyte abnormalities, indicating that a considerable proportion of the drug is excreted by the kidneys.10 The current prescribing information for AZA recommends that patients with RI must be closely monitored for signs of toxicity; the prescribing information for DAC warns that the drug was not tested in patients with sCr > 2 mg/dL. In addition, valproic acid, a histone deacetylase inhibitor used in a subset of the patients described in this study, is extensively metabolized by the liver. However, 30%–50% of its glucuronide conjugates and 3% of its unchanged form are excreted by the kidneys. In patients with a glomerular filtration rate (GFR) < 10 mL per minute, a 27% reduction in clearance of unbound valproate is evident. Although no dose adjustment is recommended in patients with renal failure, careful monitoring of these patients for toxicity is advised.11 The doses of valproic acid used in previous study populations were of short duration (7 days) and were not empirically adjusted for RI.
No data are available regarding the response to therapy with HAs or the outcomes of patients with MDS and RI. Thus, determining the toxicity profile of HAs in this population, and identifying whether dose reductions are needed, are important goals. This report summarizes the experience at the University of Texas M. D. Anderson Cancer Center (UT-MDACC) in patients with hematologic malignancies and RI who where treated with HAs.
Patients and Methods
Study Group
All patients with a diagnosis of MDS, AML, and CMML with RI who were treated with HAs at UT-MDACC were considered for this analysis. Patients were included if they fulfilled the following criteria: (1) age 16 years or older; (2) a diagnosis of MDS with an intermediate or high-risk score according to the International Prognostic Scoring System, or a diagnosis of AML or CMML; (3) a bilirubin level of 2 mg/dL or less; and (4) impaired renal function, ie, a GFR of 59 mL per minute or less. A diagnosis of MDS, AML, or CMML was established before 2001 according to the French-American-British (FAB) morphologic classification. After 2001, the World Health Organization classification or the FAB classification was used. To estimate RI according to the Kidney Disease Outcomes Quality Initiative-Kidney Disease Improving Global Outcomes definition and stratification, renal function, in the form of GFR, was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) formula, which may represent the most accurate choice, especially in patients with chronic kidney disease (CKD). For patients with weight > 25% beyond the calculated ideal body weight, we applied the formula of Cockcroft and Gault, using lean body weight12 to determine a creatinine clearance (CrCl) value, an estimate of GFR. Renal insufficiency at the initiation of treatment was defined as an estimated GFR ≤ 59 mL per minute/1.73 m2, or a CrCl of ≤ 59 mL per minute. Severe renal insufficiency was defined as an estimated GFR ≤ 29 mL/per minute/1.73 m2, or a CrCl of ≤ 29 mL per minute.
Treatment
All patients gave signed, informed consent according to our institutional guidelines. They were treated in various clinical studies or in keeping with our standard of care. Protocols were approved by our Institutional Review Board, and patients’ eligibility criteria were previously reported.2–4,13 Patients received different doses and schedules of HA according to different protocols. The drugs were given every 4 weeks as long as no significant grade 3 or 4 myelosuppression-related toxicities occurred. Bone marrow aspiration and biopsy (including cytogenetics, if findings were abnormal before therapy) were performed before every course until attainment of a complete response (CR), and every 2–3 courses thereafter. Therapy was classified as failed if no response was documented after patients received at least 3 courses of therapy, or if they had progressed to AML (for patients with MDS or CMML). Response to therapy was evaluated using the International Working Group criteria for MDS14 and the standard criteria for AML, as in previous reports.15 Dose reductions were allowed as previously reported.4 Dose reductions of 25%–30% were considered for grade 3 or 4 nonhematologic toxicities, for severe myelosuppression-associated complications (infections or bleeding), and for prolonged myelosuppression (defined as hypocellular marrow, ie, a cellularity of 5% or less, lasting longer than 42 days). Larger dose reductions (eg, a 50% starting dose) were considered for severe complications. The use of erythropoiesis-stimulating agents and granulocyte colony-stimulating factors was permitted at the discretion of the treating physician. Antibiotic prophylaxis and therapy for infections were performed according to institutional guidelines. Toxicities were evaluated using the National Cancer Institute Common Toxicity Criteria, version 3.0.
Statistical Methods
Survival curves were estimated using the Kaplan-Meier method. Fisher exact tests or χ2 tests were used to compare response rates. One-way analysis of variance (ANOVA) was used to assess renal function outcomes. Overall survival (OS) was determined in terms of the time treatment began until death from any cause. Surviving patients were censored at last follow-up. For patients with MDS and CMML, the time to transformation was determined from the time the treatment began until transformation to AML.
Results
Study Group
From November 2003 until February 2008, 41 patients with a diagnosis of MDS, AML, or CMML who had RI were treated with HAs at UT-MDACC. Fifteen patients (37%) had MDS, 17 (41%) had AML, and 9 (22%) had CMML. The median age was 73 years (range, 46–94 years). Twenty-nine patients (71%) had an estimated GFR of ≥ 30 to ≤ 59 mL per minute (per 1.73 m2, when using the MDRD formula). Twelve patients (29%) had a GFR ≤ 29 mL per minute (per 1.73 m2, when using the MDRD formula). The estimated GFR range was 4.1–49.9, with a mean of 34.5 ± 10.37 mL per minute (per 1.73 m2, when using the MDRD formula). The baseline characteristics of patients are summarized in Table 1. The underlying renal diseases in 41 patients included CKD attributable to hypertension (n = 14), both hypertension (HTN) and diabetes mellitus (DM; n = 6), extensive atherosclerosis (n = 2), DM alone (n = 1), toxic nephropathy because of previous chemotherapy (n = 3), obstructive etiology with benign prostate hypertrophy (n = 2), secondary glomerular disease attributable to Wegener’s granulomatosis (n = 1), secondary glomerular disease attributable to rheumatoid arthritis (n = 1), end-stage renal disease (ESRD) with hemodialysis (n = 1), ESRD with peritoneal dialysis (n = 1), polycystic kidney disease (n = 1), and CKD of uncertain etiology (n = 8). One patient with CKD secondary to HTN had Behçet’s disease.
Table 1.
Characteristics of Study Cohort (N = 41)
| Parameter | Number of Patients (%) |
|---|---|
| Disease Type | |
| MDS | 15 (37) |
| AML | 17 (41) |
| CMML | 9 (22) |
| Secondary MDS | 6 (40) |
| Bone marrow blasts ≥ 10% | 26 (63) |
| Cytogenetics | |
| Diploid | 18 (44) |
| Abnormalities of chromosome 7 and/or 5 | 17 (41) |
| Other | 6 (15) |
| MDS Classification | 15 |
| RA | 3 (20) |
| RAEB | 12 (80) |
| Hypomethylating Agent | |
| Decitabine | 28 (69) |
| Azacitidine | 13 (31) |
| Glomerular Filtration Ratea | |
| ≥ 30 to ≤ 59 mL per minuteb | 29 (71) |
| ≤ 29 mL per minuteb | 12 (29) |
Estimated by MDRD equation unless obese and then estimated by Cockcroft-Gault equation.
When using MDRD formula, mL per minute/1.73 m2 for estimated GFR.
Abbreviations: AML = acute myeloid leukemia; CMML = chronic myelomonocytic leukemia; MDRD = Modification of Diet in Renal Disease; MDS = myelodysplastic syndrome; RA = refractory anemia; RAEB = RA and excess blasts
Treatment
The dose schedules of DAC and AZA are summarized in Table 2. Overall, 13 (32%) and 28 (68%) patients received AZA and DAC, respectively. The various regimens included single-agent DAC in 24 patients (58%), single-agent AZA in 5 patients (12%), a combination of HA with valproic acid in 6 patients (15%), a combination of AZA, valproic acid, and all-trans-retinoic acid in 5 patients (12%), and DAC with topotecan in 1 patient. As adjuncts of treatment with HA in 38 patients, growth factors such as erythroid-stimulating agents and myeloid growth factors were required in 8 (21%) and 3 (8%) patients, respectively.
Table 2.
Dose Schedules of Therapy with Hypomethylating Agents
| Dose Schedule | Number of Patients |
|---|---|
| Azacitidine | |
| Subcutaneous: 75 mg/m2 per day for 7 days repeated every 4 weeks | 10 |
| Intravenous: 75 mg/m2 per day for 7 days repeated every 4 weeks | 1 |
| Decitabine | |
| Intravenous: 20 mg/m2 per day over 1 hour for 5 days every 4 weeks | 18 |
| Intravenous: 15 mg/m2 per day over 1 hour for 5 days every 4 weeks | 4 |
| Intravenous: 10 mg/m2 per day over 1 hour for 5 days every 4 weeks | 2 |
| Intravenous: 10 mg/m2 per day over 1 hour for 10 days every 4 weeks | 1 |
Data were available for 36 patients.
The median number of courses received was 3 (range, 1–12 courses). The median duration of therapy was 4 months (range, 1–18 months). The median time to granulocyte recovery (≥ 1 × 109/L) was 35 days (range, 3–101 days), and the median time to platelet recovery (≥ 50 × 109/L) was 29 days (range, 4–65 days; Table 3).
Table 3.
Outcomes and Side Effects
| Parameter | Number of Patients |
|---|---|
| Number of Patients | 41 |
| Complete Response, Number (%) | 4 (10) |
| Median Number of Courses (Range) | 3 (1–12) |
| Median Duration of Therapy, Months (Range) | 4 (1–18)a |
| Median Follow-up Time, Months (Range) | 8.3a |
| Median Days to Granulocyte Recovery to 1 × 109/L or More | 35 |
| Median Days to Platelet Recovery to 50 × 109/L or More | 29 |
| Median Days to Delivery of Subsequent Courses | 35 |
| Number of Courses Requiring Hospitalization (%) | 61 (33) |
Data were available for 36 patients.
Response and Outcomes
After a median follow-up of 8.3 months, 4 patients (10%) achieved CR, 2 patients (5%) had a partial response (PR), 2 patients (5%) had a marrow CR (MCR), 4 patients (10%) had an MCR with hematologic improvements (HIs), 5 patients (12%) had single-lineage HI (2 erythroid responses, 1 neutrophil response, and 2 platelet responses), and another 9 patients (22%) achieved HIs of 2 or 3 lineages. These responses are summarized in Table 4.
Table 4.
Outcomes of Patients According to Renal Status
| Parameter | A | B |
|---|---|---|
| Number of Patients | 41 | 12 |
| Complete Response | 4 | 0 |
| Partial Response | 2 | 0 |
| Marrow CR | 2 | 1 |
| MCR + Other HIs | 4 | 1 |
| Hematologic Improvement | ||
| Single lineage | 5 | 0 |
| Two or three lineages | 9 | 5 |
| Overall Response (%) | 26 (63) | 7 (58) |
Abbreviations: A = patients with GFR ≤ 59 mL per minute (/1.73 m2 when using MDRD formula); B = patients with GFR ≤ 29 mL per minute (/1.73 m2 when using MDRD formula); CR = complete remission; HI = hematologic improvement; MCR = marrow complete remission; MDRD = Modification of Diet in Renal Disease
The overall response rate (ORR) among patients who received AZA was no different from those who received DAC (61% vs. 60%). The responses of 13 patients receiving AZA included CR (n = 1), MCR (n = 1), MCR + HI (n = 2), single-lineage HI (n = 1), 2–3–lineage HI (n = 3), and no response (n = 5). The responses of 28 patients receiving DAC included CR (n = 3), PR (n = 2), MCR (n = 1), MCR + HI (n = 2), single-lineage HI (n = 3), 2–3–lineage HI (n = 6), and no response (n = 11).
Among 12 patients (29%) with severe RI, 7 achieved a response, including HIs of 2–3 lineages (n = 5), and MCR or MCR with HI in 1 patient each, for an ORR of 58%. Three patients (23%) treated with AZA had a GFR = 29 mL per minute (per 1.73 m2, when using the MDRD formula) compared with 9 patients (32%) treated with DAC (P = .7).
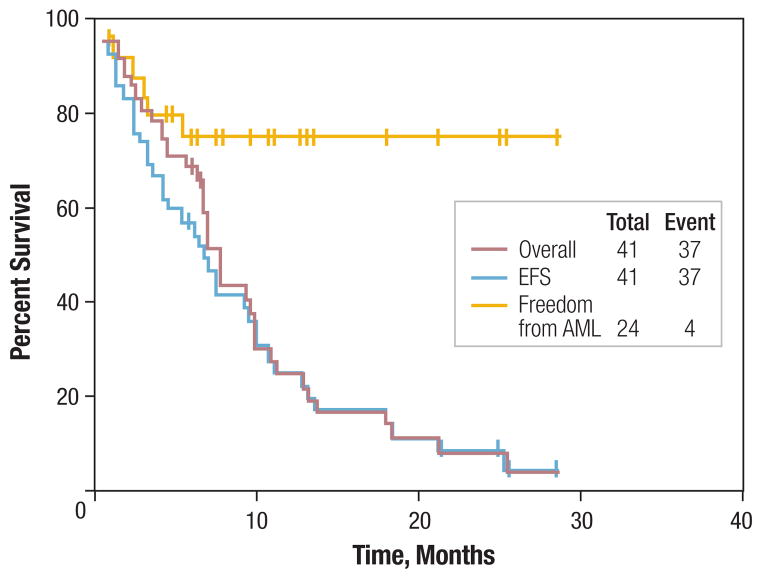
The estimated 18-month survival rate for all patients was 12%. The estimated 18-month event-free (where the events were AML or death) survival rate was 12% (Figure 1). The median OS was 8.6 months. The estimated rate of transformation to AML after 18 months was 25%. The median OS in patients with CMML, MDS, and AML was 12.8, 9, and 5 months, respectively.
Figure 1. Outcomes of Study Group With Hypomethylating Agents.
Overall survival is indicated by red line, event-free survival (EFS; where events comprise AML or death) is indicated by blue line, and duration of freedom from AML is indicated by yellow line in patients with MDS and CMML.
Abbreviations: AML = acute myeloid leukemia; CMML = chronic myelomonocytic leukemia; MDS = myelodysplastic syndrome
Toxicity
Grade 3 or 4 drug-related nonhematologic toxicities were infrequent and most commonly involved fatigue (7%), bone pain (5%), and liver-enzyme abnormalities (5%; Table 5). Grade 3 or 4 myelo-suppression-related toxicities occurred in 21 patients (51%). The rate of hospitalization was no different from that in previous studies4: 29 patients (71%) and 61 courses (33%) of therapy required hospitalization. The median number of days to subsequent courses was 35. Nine patients (22%) required treatment delay or discontinuation, among whom were 6 patients with a GFR of ≤ 29 mL per minute (per 1.73 m2, when using the MDRD formula). Three patients had a delay of 2.5 weeks or more before proceeding to the next course, and 6 patients discontinued therapy after a median of 3 cycles. Dose reductions were required in 10 patients (24%). Seven had a GFR of ≤ 29 mL per minute (per 1.73 m2, when using the MDRD formula). Reasons for dose reductions included grade 3 or 4 nonhematologic toxicities (n = 3), grade 3 or 4 myelosuppression-associated complications (n = 4), and prolonged myelosuppression (n = 3). One patient on hemodialysis and treated with AZA was hospitalized 13 times during her courses of therapy. This may have skewed the number of hospitalizations of patients receiving AZA into appearing higher than those treated with DAC.
Table 5.
Side Effects Reported According to Each Hypomethylating Agent
| Toxicity, n | Hypomethylating Agent | |||
|---|---|---|---|---|
| Azacitidine | Decitabine | |||
| Grades 1–2 | Grades 3–4 | Grades 1–2 | Grades 3–4 | |
| Extramedullary Toxicitiesa | ||||
| Fatigue | 1 | 2 | 9 | 1 |
| Bone aches | 1 | 1 | 1 | |
| Liver dysfunction | 2 | – | 2 | 1 |
| Skin rashes | 1 | – | 2 | 1 |
| Nausea and vomiting | 1 | 1 | 3 | 1 |
| Diarrhea | 2 | 1 | 1 | 1 |
| Other | – | 1 | 2 | 3 |
| Myelosuppression-Related Toxicitiesa | ||||
| Fever of unknown origin | 1 | 1 | – | 2 |
| Documented Bacterial Infections | ||||
| Sepsis alone | – | 4 | 1 | 4 |
| Minor infections | 1 | – | 4 | – |
| Pneumonias With or Without Other Infections | 1 | 2 | 1 | 2 |
| Fungal Infections | ||||
| Confirmed | – | – | – | 2 |
| Suspected | – | – | – | 1 |
| Bleeding | 2 | 1 | 4 | 2 |
| Hospitalizationb | NA | 26 | NA | 35 |
N = 41 patients.
N = 187 courses.
Abbreviation: NA = not applicable
Outcomes of Renal Function
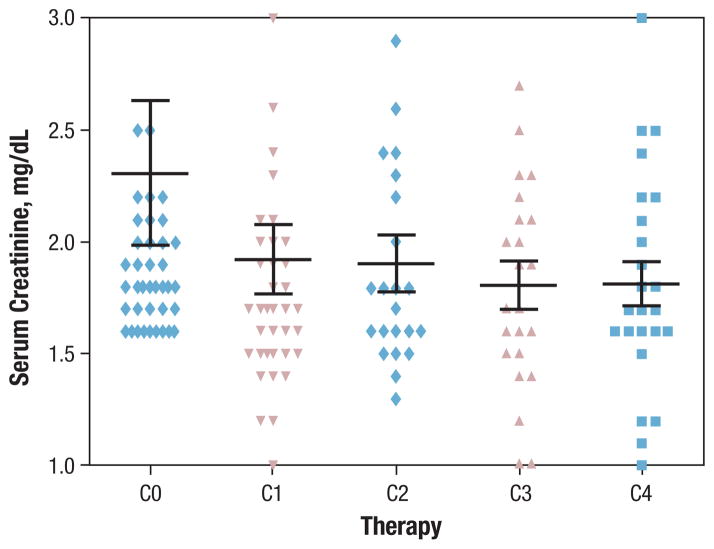
The sCr levels in all patients at the start of therapy and after each of the first 4 cycles are shown in Figure 2. No statistically significant decline occurred in renal function among patients (n = 13) who received more than 5 cycles of HA (one-way ANOVA, P = .66).
Figure 2. Renal Status of Study Group With Hypomethylating Agents.
C0 = start date of therapy, C1 = end date of first cycle, C2 = end date of second cycle, C3 = end date of third cycle, and C4 = end date of fourth cycle
While receiving therapy, only 2 patients required dialysis. One of them was on dialysis at the start of therapy and was hospitalized 13 times for therapy-related toxicities while receiving 12 cycles of AZA. The trend of sCr was analyzed in 37 patients whose records of administered doses were complete. Ten patients required a dose reduction, among whom 2 were treated with AZA. Only 2 patients had a dose reduction of 25% at the start of the therapy because of their abnormal renal function. One of them was on dialysis, and the other exhibited no worsening of renal function at the end of therapy. Dose reductions during treatment occurred in 8 patients, and in 3 of them, dose reductions were attributable to worsening renal function by 30%, 20%, and 15%, respectively. The first patient was treated with AZA, whereas the other 2 were treated with DAC. The remaining 27 patients never required a dose reduction, but 1 experienced a decline in renal function by 40%. Possible aggravating causes of renal function during therapy with HAs included sepsis and the use of nephrotoxic drugs in 18 (47%) and 5 (13%) patients, respectively.
Discussion
This is the first analysis, as far as we are aware, to assess the response and toxicity of treatment with HAs in patients with RI. In our patients, treatment with HAs was feasible, and most patients were able to tolerate them at standard doses. Dose reductions and treatment interruptions were required in some patients, particularly in those with severe RI. The response rates were comparable to those in previous reports of AZA and DAC in MDS. However, the interpretation of these results must take into account the limitations of the retrospective nature of our study.
Our results show that treatment with HAs is relatively well-tolerated in patients with MDS/AML/CMML and RI. Toxicity rates were comparable to those in previous reports of AZA and DAC in similar populations of patients without RI. Our cohort experienced an increased rate of hospitalization, and patients with severe RI experienced more treatment-related toxicities and required frequent dose reductions. The pharmacokinetic parameters of current HAs are unavailable in the medical literature on patients with RI. Although renal excretion differs between AZA9 and DAC,7 at > 50% and < 1%, respectively, we observed no difference in response rates or renal outcomes among patients treated with AZA or DAC. In fact, renal function remained stable in most patients, and a few patients experienced a treatment-associated decline in renal function. The reason for this worsening of renal function was not clearly defined.
Most patients did not require dose reductions at the beginning of treatment. Because pharmacokinetic studies were not performed and we analyzed 2 agents with different metabolic and excretion pathways, we were unable to determine which subset of patients needed dose adjustments at the start of therapy because of renal impairment. However, we think it is relatively safe to administer this therapy at standard low doses in patients with RI, provided they are closely monitored and that dose reductions are implemented as necessary. A prospective study would better define the pharmacokinetics of HAs in patients with renal insufficiency and could determine which patients, if any, would need dose adjustments at the beginning of treatment.
The ORR in our patients was similar to that in previous reports. Patients with severe RI also had comparable ORRs. However, no CRs or PRs occurred. This may be attributable to the delayed delivery of treatment because of worsening renal function or an increase of the frequency of grade 3 or 4 myelosuppression-related complications.
Patients with severe RI experienced more frequent dose reductions. Overall, grade 3 or 4 extramedullary toxicities were uncommon but were higher than previously reported. Therefore, these agents must be administered with caution in patients with an estimated GFR of ≤ 29 mL per minute. A trial (NCT00652626) is currently recruiting patients with MDS, AML, and other hematologic malignancies and solid tumors, to study the effects of renal impairment on the pharmacokinetics of AZA.
Conclusion
Patients with RI and hematologic malignancies who are treated with HAs may have ORRs comparable to those of patients without RI. Dose adjustments at the start of therapy may not be required in most patients with RI, but a subset will require dose reductions because of worsening renal function and increased toxicity. A prospective study may be warranted to define the pharmacokinetics of HAs in RI and the characteristics that would indicate dose adjustments at the start of the therapy.
Footnotes
Disclosures
Elias Jabbour has received research funding from and been on a Speakers’ Bureau for Bristol-Myers Squibb Company and Novartis Pharmaceuticals Corporation. Farhad Ravandi, Hagop Kantarjian, Jean-Pierre J. Issa, Guillermo Garcia-Manero, and Jorge E. Cortes have received research funding from Eisai Inc. Farhad Ravandi has served as a consultant/advisor and on a Speakers’ Bureau for Eisai Inc. and MGI Pharma. Jean-Pierre Issa has served as a consultant/advisor for Celgene Corporation, Eisai Inc., and MGI PHARMA.
The remaining authors report no relevant potential conflicts of interest.
References
- 1.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional cure regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a (abstract 817). [Google Scholar]
- 3.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 5.Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–6. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 6.Momparler RL, Derse D. Kinetics of phosphorylation of 5-aza-2′-deoxycytidine by deoxycytidine kinase. Biochem Pharmacol. 1979;28:1443–4. doi: 10.1016/0006-2952(79)90454-4. [DOI] [PubMed] [Google Scholar]
- 7.Chabot GG, Momparler RL. Antileukemic activity of 5-aza-2′-deoxycytidine and cytarabine against intracerebral L1210 murine leukemia. Cancer Treat Rep. 1984;68:1483–7. [PubMed] [Google Scholar]
- 8.Lyons J, Bayar E, Fine G, et al. Decitabine: Development of a DNA methyltransferase inhibitor for hematological malignancies. Curr Opin Investig Drugs. 2003;4:1442–50. [PubMed] [Google Scholar]
- 9.Marcucci G, Silverman L, Eller M, et al. Bioavailability of Azacitidine Subcutaneous Versus Intravenous in Patients With the Myelodysplastic Syndromes. J Clin Pharmacol. 2005;45:597. doi: 10.1177/0091270004271947. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BA, Collins AJ, Vogelzang NJ, et al. 5-Azacytidine and renal tubular dysfunction. Blood. 1981;57:182–5. [PubMed] [Google Scholar]
- 11.Aronoff GR, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5. Philadelphia, PA: American College of Physicians; 2007. [DOI] [PubMed] [Google Scholar]
- 12.Demirovic JA, et al. Estimation of creatinine clearance in morbidly obese patients. Am J Health Syst Pharm. 2009;66:642–8. doi: 10.2146/ajhp080200. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson B, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-23-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–9. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]