Abstract
Background and Purpose
Impaired hand function decreases quality of life after stroke. The purpose of this study was to pilot a novel two-week upper extremity sensorimotor training program. This case series describes the training program and highlights outcome measures used for documenting behavioral change and neural reorganization.
Case Description
Sensorimotor evaluation identified behavioral changes, activity induced neural reorganization was examined using sensory fMRI, diffusion tensor tractography, and brain volume measurement. Participant 1 was a 75-year-old right-handed man one year post right hemisphere stroke with severe sensory impairment across domains in his left hand, he reported limited left hand/arm use. Participant 2 was a 63-year-old right-handed woman who had experienced a left hemisphere stroke 9 months earlier resulting in mild sensory impairment across domains in her right hand, as well as mild motor deficit.
Intervention
Participants trained 4 hours per day, 5 days per week for two weeks. Training tasks required sensory discrimination of temperature, weights, textures, shapes and objects in the context of active exploration with the involved hand. Random multi-modal feedback was used.
Outcomes
Both participants had improved scores on the Wolf Motor Function Test after training. Participant 1 had no measureable change in sensory function, while Participant 2 improved in touch perception, proprioception and haptic performance. Sensory fMRI suggested neural reorganization in both participants; Participant 1 had a small increase in brain volume, while superior thalamic radiation white matter connectivity was unchanged in either participant.
Discussion/Summary
Participating in sensorimotor training focused on sensory discrimination during manual manipulation was feasible for both participants. Future research to determine efficacy and identify optimal measures of sensory function and neural reorganization is recommended. Video Abstract available (see Video, Supplemental Digital Content 1) for more insights from the authors.
Introduction
Stroke survivors consistently express dissatisfaction with upper extremity recovery. Even when persisting impairments are mild, these impairments negatively influence health-related quality of life. 1 Up to 89% of people with hemiparesis demonstrate upper extremity sensory deficits when tested across domains of touch, temperature, weight, roughness, texture, and/ or shape discrimination.2 Despite evidence that sensory dysfunction predicts the magnitude of recovery from movement focused training protocols,3 and is unchanged by those protocols,4 stroke rehabilitation protocols continue to focus on the motor impairment, ignoring the contributions of concomitant sensory deficits.2 A 2010 review of poststroke sensory rehabilitation identifies a need for well-designed studies of sensory rehabilitation.5 Sensory training paradigms have commonly focused only on sensation without attention to motor recovery,6, 7, 8 however, there is intriguing evidence to suggest that training, using tasks that require active hand exploration and sensory discrimination, facilitates both sensory and motor recovery.9, 10, 11
Neural reorganization associated with motor recovery has been well documented. Functional magnetic resonance imaging (fMRI) has been used to study natural recovery from stroke and training-induced changes.12,13,14,15, 16, 17 Decreases in task-related brain activation in primary and non-primary motor regions15 and a reduction in contralesional activity with a concurrent increase in ipsilesional activity, are associated with better recovery.18 More complete motor recovery has also been associated with a more typical ipsilesional activation pattern, involving fewer brain regions than less complete recovery.13, 14 Interestingly, an increase in activation volume in secondary somatosensory area in the stroke hemisphere correlated with better Fugl-Meyer scores at 3 months poststroke,19 reinforcing the relevance of sensory processing to motor control.
Few studies document neural reorganization associated with recovery of sensory function after stroke. A serial fMRI case study reported a reemergence of activation in the ipsilesional primary and bilateral secondary somatosensory cortices.18 A study of thalamic stroke, identified recovery of touch perception during bilateral stimulation was associated with increased activation in the primary somatosensory cortex of the ipsilesional hemisphere.20 Proprioceptive training in persons with acute stroke results in ventral premotor and parietal cortex activation changes in the contralesional hemisphere during sensorimotor fMRI; unfortunately sensory function was not measured. 21 Limited knowledge of the neural reorganization that accompanies sensory recovery after stroke points to the need for research in this area.
Structural connectivity refers to the physical link between brain regions created by axons, dendrites, and synapses.22 After stroke, a loss of connectivity may occur as a result of direct damage to the axons, or through degeneration of axons proximal or distal to the lesion. Diffusion tensor tractography (DTT) is a method of modeling white matter connections in the human brain in vivo. DTT has been used primarily in cross-sectional studies to explore the relationship between infarct location and sensorimotor pathways,23 and to quantify damage to the corticospinal tract.24 Several studies confirm a strong correlation between structural integrity of the corticospinal tract and poststroke motor function; 24,25, 26,27,28 however, there is little direct evidence of white matter remodeling after stroke.29 The sensory component of the superior thalamic radiation (sSTR) includes afferent connections to the somatosensory cortex, and thus, is the functional analogue of the corticospinal tract. 30 Stroke related structural changes to sSTR have relevance to sensory function in chronic left-stroke,31 however, it is unclear whether DTT measures of the sSTR will be sensitive to reorganization due to training.
The purpose of this study was to pilot a novel two-week upper extremity sensorimotor training program for individuals with stroke. The training was specifically designed to require high level sensory processing, across multiple sensory domains, in tasks that involved manual manipulation of objects. We hypothesized that the training would result in improved upper extremity motor and sensory function that would be accompanied by functional and structural neural reorganization. Two participants are described, who highlight the outcome measures used for documenting behavioral change and neural reorganization.
Methods
During this study, six individuals with chronic stroke completed a two-week upper extremity sensorimotor training program. Inclusion criteria were: 1) one clinical stroke diagnosed greater than six months prior and 2) aged 21-85, 3) contralesional hand function sufficient to grasp and release a small cylinder (such as a 6 ounce frozen juice can) ≥75 times in one hour. Exclusion criteria were: 1) Mini Mental Status Exam ≤ 24, 2) other medical condition that would impair sensation in the upper extremity, 3) significant aphasia or neglect. We used convenience sampling. Our goal was to enroll participants with poststroke sensory impairment. Given the absence of a brief, valid, screening tool for higher level sensory impairment and the frequency of poststroke sensory dysfunction, no inclusion criteria for sensory impairment was used. Participants provided written informed consent, approved by The Ohio State University’s Institutional Biomedical Review Board. A comprehensive sensorimotor evaluation and functional and structural MRI imaging were completed during the week before and after training. The descriptions of the sensorimotor tests and measures are given in Table 1. Behavioral outcomes for each participant are given in Table 2. Measures which most clearly present participant function are discussed in the narrative case descriptions. Sensory fMRI, diffusion tensor tractography, and brain volume measurement were examined for evidence of neural reorganization. Two participants, whose outcome measures demonstrate the broad range of sensory function and recovery in our sample, are described.
Table 1.
Description of Sensorimotor Tests and Measures
| Measurement tool | Dependent Variable | Description | Directions | Reliability/Validity |
|---|---|---|---|---|
| Weinstein Enhanced Sensory Test (WEST)32 | Touch threshold | Monofilament aesthesiometer, applied to the tip of index finger. Ladder method used to identify threshold. 32 | Indicate when you feel a touch. | Inter-rater reliability for monofilaments (healthy controls) ICC=0.965.33 |
| Wrist Position Sense Test (WPST)34 | Wrist proprioception | Wrist is flexed/extended passively to a predetermined angle, average error in estimate across 20 trials recorded.34 | Align the arrow with your wrist position. (protractor visible only to the tester) | Test-retest reliability(person’s with stroke) r = 0.92 session 1-2, r=0.88 session 2-3. Valid poststroke.34 |
| Hand Active Sensation Test (HASTe)35 (See video abstract in Supplemental Digital Content) | Weight and texture discrimination | 18 item match-to-sample forced choice task without visual assistance. | Which of the three is an exact match to the test object? | Test-retest reliability ICC=0.77. Valid poststroke.35 |
| Haptic Object Recognition Test (HORT)36, 37 | Haptic performance | Seventeen novel objects constructed from Legos. are matched to one of five sample objects using manual exploration. | With your hand in the bag select one test object, find its match from the 5 displayed. No visual verification was permitted. | SEM=1.3 to 1.8 (errors), sensitive to training effect in healthy elderly (n=22).36 |
| Nine Hole Peg Test (NHP)38 | Fine motor performance | Square board has 9 holes, evenly spaced and 0.64 × 3.2 cm pegs. The average time to place a peg was calculated. | Use one hand to place all the pegs in the hole and remove them one at a time as quickly as possible. | Test-retest reliability (persons with stroke) r=0.83-0.99.39 |
| Box and Blocks Test (BBT)40 | Upper extremity functional performance | A timed test of grasp and release of 2.54 cm square blocks over a 14.3 cm barricade. Blocks/60 seconds was recorded.41 | Move as many blocks as possible to the other side one at a time. | Test-retest reliability ICC=0.89-0.97.42 |
| Wolf Motor Function Test (WMFT) | Upper extremity motor function | 15 timed and 2 strength items. A maximum of 120 seconds is allotted per task. | Specific instructions of each task are read twice, no practice is allowed. First attempt is timed. | Inter-rater reliability=0.97-0.99.43 Test-retest reliability for performance time (chronic stroke) r=0.90.44 |
| Motor Activity Log (MAL) | Subjective report of upper extremity function | 28 activities of daily living, scored on a 0-5 scale for amount of use (AOU) and quality of movement (QOM) | Use the rating scale to describe how much (or how well) you use your weaker arm while you are doing the specific activities. | Test-retest reliability (sub-acute stroke) QOM: ICC=0.82, AOU: ICC=0.79.45 |
Subjects were measured at baseline and after completion of training, inter-testing interval 3 weeks.
Table 2.
Behavioral Outcomes
| Measure | Normal/impaired criteria | Participant1 | Participant 2 | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| WEST (index, in grams) | Normal threshold for the index and pinky fingers for men/women older than 55 is 0.385/ 0.15 grams. 89 | 220(0.07) | 220(0.07) | 0.2(0.07) | 0.07(0.07) |
| WPST (degrees) | Criterion of impairment ≥11° average error. | 140.6 | 17.9 | 13.5 | 9 |
| HASTe (matches/18) | Criterion of impairment <13/18.35 | 8(7) | 7(9) | 14(15) | 13(16) |
| HORT (errors/17) | Mean errors for healthy elderly 2.71±1.45 errors.36 | 13 | 13 | 7 | 1 |
| MAL -How much(0-5 scale) | Proposed MCID is 10%.73 | 1.2 | 1.6 | 3.6 | 4.7 |
| MAL-How well (0-5 scale) | 1.2 | 1.8 | 3.4 | 4.7 | |
| WMFT (mean in seconds) | MCID (for acute stroke) -19 s (or -1.3 s change in mean).72 | 7.88 | 5.93 | 3.57 | 2f1 |
| NHP (sec/peg) | Normal range for men/women (60-75+years) is 2.25 – 2.9/ 2.04 – 2.73 s/peg. MCID for the more/less affected hand is 32.8/6.2 s overall.90 | 43 | 24 | 3.1 | 2.2 |
| BBT (blocks/min) | Normal for older adults is 67 blocks.40 MCID for the more/less affected hand is 5.5/7.8 blocks per minute.90 | 26(54) | 28(55) | 52(NT) | 51(57) |
(Scores for uninvolved upper extremity, where available, in parentheses) NT=Not Tested, s=seconds
Imaging methods
A 3 Tesla magnetic resonance imaging scanner (Philips Achieva; Best, Netherlands) with volume transmit and 8 channel receiver coil was used to collect structural and functional MRI scans. T1-weighted Magnetization Prepared Rapid Field Echo anatomical images were acquired of the entire brain for spatial normalization to a standard atlas. Blood oxygen level-dependant (BOLD) T2* weighted functional MRIs in the transverse plane TR/TE =3000/35ms, flip angle=90°, field of view (FOV)= 23cm×23cm2, matrix = 80×80 interpolated to 128×128) were obtained using Gradient Echo-Echo Planar Imaging with parallel imaging and a sensitivity encoding (SENSE) reduction factor of 2. Diffusion tensor images were acquired using single-shot echo-planar imaging spin-echo sequence (flip angle=90 degree, TR/TE=9750/92 ms). The acquisition matrix was 128 × 128 with a field of view (FOV) of 256 × 256 (mm2), which resulted in a 2.0 mm isotropic in-plane resolution. The slice orientation was axial with 2.0 mm thickness without gap. Diffusion-weighting gradient was applied along 32 independent axes with b-factor of 1000 s/mm2 with a minimally weighted image (b factor of 0 s/mm2). SENSE reduction factor of 2.2 was used to reduce geometric distortion caused by EPI-based sequence and susceptibility-related artifacts. Total scan time was 6 minutes. Following acquisition, the raw diffusion-weighted images were corrected for potential subject motion and eddy current using affine transformation of the automated image registration. The elements of the diffusion tensor were calculated voxel-wise across the whole brain. Three eigenvalues and corresponding eigenvectors were calculated. The eigenvector associated with the largest eigenvalue was considered as the principal fiber orientation. Diffusion tensor imaging (DTI) metrics were derived using DTI Studio.46 Fractional anisotropic index (FA), the most commonly used anisotropy measure, is the fraction of the tensor that can be assigned to anisotropic diffusion. Fiber density (FD) is a quantitative description of the white matter fiber integrity expressed as the mean number of ‘fibers’ per voxel in the bundle.47 Fiber density, like other measures of diffusion data, is based on average water diffusion within a voxel, a direct relationship to axonal density cannot be assumed.
Lesion analysis
Stroke lesion location and volume were determined from T2 Fluid attenuated inversion recovery (FLAIR) images. Lesion volume was calculated after manually outlining signal abnormality slice-by slice in the axial plane.48 The location of lesions was determined by visualization of anatomical structures in the T2 image and comparison to a brain atlas.49
fMRI paradigm
Block-design fMRI, with 21-second stimulation epochs, alternating with rest, was obtained for a brush discrimination task on the paretic hand. Tactile stimulation of the index finger was generated by manual brushing applied to the distal phalanx at the rate of 1 Hz, timed with an auditory metronome heard only by the examiner. Brushing has previously been used for analysis of sensory perception with fMRI.20
fMRI data analysis
FMRIB Software Library (FSL) tools were used.50 Standard pre-statistic processing was applied to individual participants data: motion correction,51 non-brain removal,52 spatial smoothing =5mm, and mean based intensity normalization of all volumes by the same factor. High-pass temporal filtering and time-series statistical analysis was carried out using a linear model with local autocorrelation correction.53 Functional images from each participant were co-registered with their high-resolution image and standard (MNI 152, 2mm) images using linear image registration,54 then optimized using non-linear registration.55 First-level analysis of functional scans, relative to rest, were carried out with Z>3.0, and a cluster significance threshold of P = 0.01. Anatomical areas were defined based on the Harvard Oxford Structural Atlas (implemented in FSLView version 3.1.2). 56 The reliability of between-session fMRI in persons poststroke has been tested, using a complex visual-motor hand task; good reliability (ICC=0.58, voxel count method) was found, giving confidence to fMRI test/retest designs poststroke.57 A longitudinal study of texture discrimination fMRI in healthy adults found a consistent location of activation over time, suggesting changes in location of activation can be monitored confidently.58
Diffusion Tractography Reconstruction
Tractography was based on the Fiber Assignment Continuous Tracking (FACT) algorithm.59 The sSTR was isolated using a multi-region of interest (ROI) approach in DTI Studio.60 ROI’s were located on the FA color map, according to the brain anatomy of each participant. The first ROI included the entire posterior limb of the internal capsule at the axial level in which the fornix can be identified as a single intense structure. From the reconstruction result of this ROI the bundle that reaches the post-central gyrus was isolated at the level of cleavage of the central sulcus. In this method, only the “fibers” between both ROI’s are selected using the ‘cut’ and ‘cut+’ functions in DTI Studio. Tracking was started and stopped in voxels with a FA of 0.2, and a tract turning angle of ≥ 40 degrees.60
Brain volume
Two time-point percentage brain volume change was estimated with SIENA61 using MPRAGE images. This method has an error rate of 0.2%.62
Case description
Participant 1 was a 75-year-old right-handed man one year after stroke in the right internal capsule, the lesion extended into the lenticular nucleus, thalamus, and insular cortex. Lesion volume, measured by signal abnormality in a T2 FLAIR, was 14.3 cm3. (Figure 1a.) Sensorimotor Summary: Participant 1 had hemiparesis and sensory loss in the contralesional, non-dominant, left hand, including absent hot/cold discrimination (identified during training) and impaired touch perception and discrimination and haptic performance. Motor function measured by the Wolf Motor Function Test (WMFT) and performance measured by the Box and Blocks Test (BBT) and Nine-hole Peg Test (NHP) were also impaired on objective measures, and he reported limited arm/hand use on the Motor Activity Log (MAL amount of use = 1.2 /5.0). In his ipsilesional, right hand, touch perception was normal and, hot/cold discrimination was functional; however, he had impairments of proprioception and discrimination (see Table 2).
Figure 1.
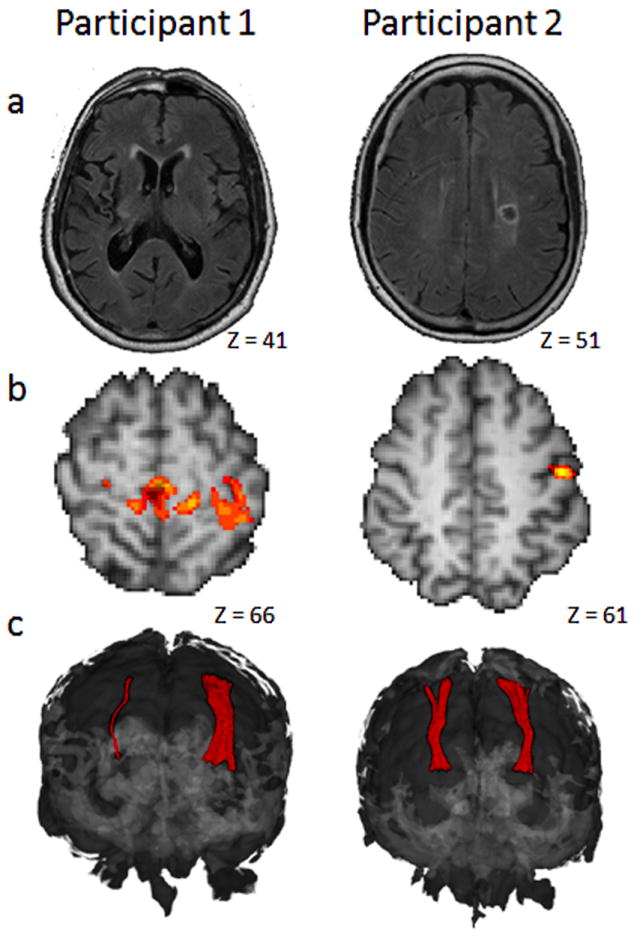
a)T2 Fluid attenuated inversion recovery image of axial slice in which stroke lesion volume is largest b)Statistical parametric map of post-pre contrast of impaired index finger brush discrimination was thresholded at z=3.0, p.05 and overlaid on individual high resolution images. Participant 1 had statistically greater activation in bilateral (though L>R) sensorimotor cortical areas after training. (shown) Participant 2 had significantly greater activation in the left post-central gyrus (shown) and in the right inferior frontal gyrus and left supplementary motor area. c) Coronal view of bilateral sSTR model shown in the 3D brain. Quantification is found in table 4. Images are in radiological convention in which the right brain is shown on the left side of the image. MNI coordinates of axial slices are indicated.
Participant 2 was a 63-year-old right-handed woman 9 months after a stroke localized to the left internal capsule and corona radiata. Lesion volume was 2.1 cm3. (Figure 1a) Sensorimotor Summary: Participant 2 had functional bilateral hot/cold discrimination; touch perception was impaired. Haptic performance and proprioception were impaired in the right hand; weight and texture discrimination was normal bilaterally. Motor testing identified a mild deficit on both objective and self-reported right hand use (3.6/5.0 on the MAL amount of use (see Table 2).
Intervention
The participants trained 5 days per week for 2 weeks. Daily sessions lasted 4 hours, and breaks were provided upon request. Both participants completed all 10 days of training. Trainers recorded time on task for each participant on every task. For Participants 1 and 2, respectively, mean time on task per day was 203 minutes and 215 minutes, mean number of tasks per day was 9.1 tasks and 7.6 tasks, while the mean time per task was 22 minutes and 28 minutes.
The intervention was based on the following principles. Treatment intensity was adapted from constraint- induced movement therapy (CIMT).63 Tasks were primarily unimanual, based on the concept of forced-use,64 but occasionally bimanual (<10%), dependent on participant ability and task structure. To force a sensory demand, most tasks were performed with vision of task objects obscured by a curtain hung between the participant and the activity.65 If obscuring vision resulted in a task being too difficult or vision had no relevance to the task (i.e. hot/cold discrimination) the trainer would remove the curtain. A variety of tasks were used (listed in Table 3). They were developed in our lab with the goal of requiring sensory discrimination of temperature, weights, textures, shapes and objects in the context of active exploration with the involved hand. 9, 10, 11 (see Video Abstract in Supplemental Digital Content for an example task description). Task variety has been associated with maintaining motivation66 and attention.67, 68 Moreover, across domains, higher level sensory processing shares a parietal-prefrontal-premotor network as suggested by evidence from neuroimaging studies. 31, 69-71 Tasks were progressed in difficulty, based on performance and participant-specific impairments. Tasks were modified and/or participants were assisted by the trainer when necessary to accommodate for motor impairment. Random verbal feedback was provided by the trainers; participants also used the non-paretic hand and/or vision for feedback. Student Physical Therapists, trained to administer this protocol, provided one-on-one or one-on-two guidance to the participants.
Table 3.
Sensorimotor Training Tasks
| Task | Description |
|---|---|
| Weighted Eggs | Sort plastic eggs filled with 1, 2, or 3 oz weights and place into egg carton. |
| Weighted balloons | Sort balloons filled with flour by either grasping whole balloon with gross grasp or with pincer grasp on knot of balloon. 1 or 2 oz difference in weight can be used. |
| Plastic Ice Cubes | Frozen and room temperature plastic ice cubes are sorted by temperature with vision occluded. Cubes are removed individually from one container and placed in separate containers. |
| Immersion bath | Fill 2 tubs with water of equal amounts, one warm, one cold. Discriminate temperature by submerging arm, hand or finger. Confirm temperature with other hand. |
| Hot pack / Cold pack | Select pack of certain temperature from within a bag with multiple packs of varying temperature (warm, room, cold). Remove pack and place on table. |
| Fabric Texture discrimination | Match to sample with wooden 3 × 3 × ½ inch tiles covered with textured fabrics (20 total with 2 of each fabric). Matching can be to visual presentation with the match objects behind a curtain or by comparison of match objects between the paretic and non-paretic hands, vision occluded. Can vary the number of choice tiles per trial. |
| Texture dowels | Wooden dowels with center openings are created with different thickness and/or different textures. Create a target object with 3 dowels on a rod. After manual exploration, participant is asked to recreate the same pattern on another rod. Dowel widths and textures can be varied. |
| Textured peg board | Create peg board with large pegs (3″×1 ½″). Cover bottom of peg holes and top of pegs with textures. Place pegboard on table and pegs behind curtain. With vision or touch of pegboard, select correct peg from behind curtain through manual manipulation. |
| Beans and Therapeutic putty | Hide beans within a ball of putty. Remove beans and place in container. Vision may be occluded. |
| Clay modeling | Create simple shape out of clay; participant manually explores object behind curtain and then recreates the shape with his/her clay in front of the curtain. |
| Lego™ Rebuilding | Trainer constructs an object of Legos that is manipulated behind a curtain; reconstruction of the object is completed on the visualized side of the curtain. Can alter the number, orientation and size of blocks used. |
| Silverware sorting | Silverware is laid out behind curtain. Identification is made via manual exploration and then sorted into a silverware holder on the visualized side of the curtain. |
| Stereognosis | A variety of household objects are placed behind a curtain. Manual exploration is used to identify objects. |
| Dominoes | Place dominoes in a bag. Play a standard game of dominoes; the participant must find the right match by manual exploration of the dots on the dominoes within the bag. |
| Magnetic letters | Place magnetic letters in a bag sufficient to spell a word. Letters must be pulled from bag and placed on card with a word written on it. Extra letters can be included to increase difficulty. |
| Wooden alphabet blocks | Wooden blocks with letters engraved in the sides are placed in a bag. Manual manipulation is used to find a target block. Blocks are stacked as they’re removed. |
| Geometric puzzle | Wooden geometric shapes are placed in a bag. A geometric puzzle diagram is placed on the table. Targeted shapes are manually identified and placed on the puzzle diagram. |
| Puzzle edges | Puzzle pieces are placed in a bag. Individually, all of the edge pieces are manually removed and placed on the table. They can be connected to shape the border of the puzzle. |
| Shape sorting | Using a standard infant shape sorter, place shapes in a bag and have a targeted shape manually retrieved from the bag and placed in the sorter. |
| Byl-Chenai | With vision occluded, a finger is used to feel a small Lucite block fitted with Brad nails that form a pattern. The perceived shape is visually matched to a printed sheet of shape options. |
| Perfection™ | The shapes from the game Perfection are verbally identified and then retrieved from a bag and placed into the game board. |
| Stone / bean sorting | Different sized stones/beans are placed in a bag. A given size is designated as the target. All stones / beans of matching size are retrieved from the bag. |
| Coin sorting | Multiple coins are placed in a bag. A particular coin is designated and removed from the bag and placed in a bank. |
| Bottle caps | Place caps from a variety of plastic bottles of different sizes in a bag. The participant manually explores and identifies the appropriate cap for a designated bottle and then places it on the bottle. |
| Nuts and bolts | A board, fitted with various-sized bolts and matching nuts is placed behind a curtain. Through manual exploration each nut is removed and placed in a bowl; nuts are then screwed back onto the bolts behind the curtain. |
Outcomes
Sensory and motor outcomes for both participants are provided in Table 2.
Participant 1 had no increase in sensory scores in either hand after training. His WMFT scores met the criteria for minimal clinically important difference (MCID); importantly, the MCID was established in a study of acute stroke.72 His change in MAL was not clinically meaningful.73 Imaging Outcomes: Participant 1 had low cortical activation overall, during contralesional, left index brush discrimination at pre-test and post-test imaging time points. In a post-minus-pretest contrast of impaired hand brush discrimination statistic parametric maps, Participant 1 had a statistically significant increase in activation that peaked in the contralesional pre-central and post-central gyri after training; this resulted from less deactivation in these areas at post-test compared to pretest (see Figure 1b). The diffusion tractography models of the right and left sSTR’s for Participant 1 are asymmetrical, consistent with attenuation of the right sSTR due to right hemisphere internal capsule stroke. The post-test sSTR models are depicted in Figure 1c; as no significant difference was observed between pre-test and post-test, only the post-test sSTR models are shown for both Participants. For Participant 1, FA and FD values for the left (contralesional) sSTR were stable across imaging time points; while at post-test in the right sSTR, FA appears to have increased while FD appears to have decreased (see Table 4). It should be noted that this variability is likely due to the relatively small number of voxels in the right sSTR model. Reliability of DTT has not been established for small white matter projections such as this.60 Brain volume change was + 0.4%, which is slightly above the published error rate of this method of 0.2%.74
Table 4.
Quantification of the Sensory Component of the Superior Thalamic Radiation (sSTR)
| Participant 1 | Participant 2 | ||||
|---|---|---|---|---|---|
| Pre | Post | Pre | post | ||
| Right Bundle | Volume | 46 | 38 | 431 | 371 |
| FA | 0.29 | 0.44 | 0.48 | 0.50 | |
| FD | 2.8 | 1.0 | 20.82 | 16.52 | |
|
| |||||
| Left Bundle | Volume | 720 | 870 | 319 | 351 |
| FA | 0.52 | 0.52 | 0.49 | 0.48 | |
| FD | 23.49 | 21.64 | 18.91 | 17.95 | |
FA=fractional anisotropy; FD= Fiber Density (mean fibers/voxel in bundle), Volume=number of voxels in bundle. Pre = pretest; Post= posttest. Lesioned hemisphere is indicated by gray shading.
Participant 2 had a positive change in sensory scores for touch perception, proprioception and haptic performance while weight and texture discrimination was in the range of normal at pre-test and post-test. Right index finger touch perception improved from a threshold of 0.2 grams at pre-test to within the range of normal (0.07 grams) at post-test.75 Mean normal error for the WPST is 11 degrees, while standard error of measurement is 2.8 degrees; 8.4 degrees is considered ‘genuine change’.34 Participant 2 had a 4.5 degree decrease in error in the WPST after training, which represents a 33% decrease in error and a shift from above normal to below normal error. For healthy elderly, the average number of errors on the Haptic Object Recognition Test (HORT) is 4.7±2.5.36 Participant 2 decreased from 7 errors at pre-test to 1 error at post-test. Following training, MAL scores for Participant 2 approached pre-stroke levels of use at 4.7/5, change in MAL scores exceeded the arbitrarily established MCID of 10%.73 WMFT scores also exceeded the MCID.72 Participant 2 had a 29% decrease in time per peg on the NHP and was within the range of normal (18-20 seconds)38 at post-test. Her BBT score was 76% of normal at both time-points. Imaging Outcomes: Analysis of post-minus-pretest contrast of brush discrimination for Participant 2 indicated a statistically significant increase in activation in clusters, peaking in the left post-central gyrus (Figure 1b), the supplementary motor area, and in the right inferior frontal gyrus after training. The diffusion tractography models of the right and left sSTR’s for Participant 2 were symmetrical, FA and FD pre and post-training values were stable showing typical variability associated with these measures.60 (Table 4) Percent brain volume change was 0.17%.
Discussion
Poststroke tactile sensory dysfunction is common. This paper detailed a novel, intensive training program and associated outcome measures, emphasizing sensory discrimination and manual manipulation of objects, which may be a useful framework from which a clinical trial of sensorimotor training after stroke could be designed. During training, the participants were engaged and able to participate at relatively similar levels, as indicated by time on task, despite differences in hand function. Tasks were easily adapted to meet the functional level of each individual and progressed to challenge emerging skills. Further, this study provides an example of extending measurement to neural reorganization of the sensorimotor system that accompanies functional improvement.
Both participants surpassed the MCID in arm function after training, measured by the WMFT. Their recovery paralleled that achieved from motor-focused training paradigms, such as constraint-induced movement therapy.63 It should be noted that the MCID on the WMFT of -19 seconds, referred to here, was established in a study of participants with acute stroke.72 Therefore, caution should be used when interpreting the importance of this change for person’s with chronic stroke. Sensory recovery was more variable and difficult to evaluate. Participant 2, with initial mild sensory impairment, demonstrated improvement in touch perception, wrist proprioception and shape discrimination. Participant 1, with severely impaired sensation at pretest, had not improved in sensory measures by post-test. It is possible that the 2-week intervention was not adequate to show change on the sensory measures when impairment is severe. It is also possible that the more severe motor impairment of Participant 1 limited the amount of sensory improvement.
This protocol used a variety of behavioral tests to evaluate function. Review of the results and observation regarding their application yielded several key ideas. The Weinstein Enhanced Sensory Test, although easily completed, did not allow sufficient discrimination of touch perception; thus, the Semmes-Weinstein Monofilaments are a preferable measure.75 The HORT is very challenging, especially for older adults, due to the cross-modal nature (i.e. haptic exploration with visual comparison of unfamiliar objects). It requires visual imagery, perhaps to a greater extent than other shape discrimination measures; 36 use of another measure of shape discrimination is recommended. The Hand Active Sensation Test discriminates high and low function well but is lengthy to administer; other measures of texture and weight discrimination should be explored. The WPST adequately identify proprioception deficits and was sensitive to change. Finally, our participant with no sensory recovery after training could not discriminate hot and cold. Hot/cold discrimination screening is economical and time-efficient. Future researchers may wish to include temperature discrimination with other measures examined to predict sensory recovery. Motor measures used here were effective in discriminating motor behavior. The largest pre-post test differences were documented using WMFT and MAL. We included both the NHP and BBT to provide a greater focus on hand and finger function; however, the WMFT seems to differentiate function as effectively as these other measures. In summary, the motor outcome measures used in this case series are expected to capture change due to training in larger studies using this protocol. Continued evaluation of sensory measures to identify those that provide the best profile of sensory behavior poststroke is recommended.
Participant 1, with severely impaired left hand sensation and no measurable recovery of sensory function, had low activation overall during left index brush discrimination, and most notably, the ipsilesional right parietal cortex had no activation that met threshold before or after training. This likely reflects impaired perception of the stimulus at both time points. A post-test-minus- pre-test contrast identified significantly greater activation bilaterally; however, the majority of voxels were in the left sensorimotor cortical areas after training, suggesting predominantly contralesional neural reorganization. In the absence of measurable change in sensory function we suggest this functional reorganization may represent the effect of practice imagining sensory stimuli.76 However, we cannot rule out the possibility that improved sensory function may have been possible with longer treatment duration or that the post-test activation change might be a precursor for later sensory improvement. Participant 1 had a 0.4% increase in brain volume after training. At just over the published error rate of 0.2% for the method used, the significance of the increase is difficult to interpret. Participant 2’s overall volume and pattern of activation was within the range of previously published control data at both time points,77 evidence that she perceived the stimulus and was performing the task. A contrast of post-test-minus-pre-test right index brush perception identified statistically significantly greater activation in left ipsilesional sensory cortex (Figure 1b), right inferior frontal gyrus and left supplementary motor area after training. If the statistical difference in fMRI in these two participants is taken as evidence of neural reorganization, these findings are in line with others who report better function is associated with a return to contralateral control, as demonstrated by Participant 2, while poorer function is more often associated with ipsilateral and diffuse patterns of activation, as demonstrated by Participant 1.13
Diffusion imaging studies suggest white matter remodeling results from training in healthy children,78 adults79 and elders.80 Animal studies of training induced white matter remodeling identify time-frames as short as one,81 two,82 and six 83 weeks. At present, there is limited evidence of white matter remodeling after stroke in humans. A recent cross-sectional study of the microstructure of the corticospinal tract after stroke suggests motor skill recovery relates to the remodeling of both ipsilesional and contralesional corticospinal tracts.84 Network analysis of poststroke white matter suggests contralesional regions homologous to the lesion are compromised while other regions exhibit positive adaptive changes.85
The Participants in this case series have markedly different structural integrity of the sSTR in their lesioned hemisphere. In these two participants, ipsilesional sSTR integrity appears to correspond to their level of sensory function. While the sSTR’s are nearly symmetrical in Participant 2, in Participant 1 contralesional sSTR has high FA, FD, and bundle volume values that may reflect contralesional remodeling similar to data from Schaechter et al., who suggest evidence of contralesional corticospinal tract remodeling.84 The small volume of white matter obtained in the right sSTR bundle in Participant 1, reflects white matter damage associated with the stroke lesion. Participant 1’s lack of change in sensory function, after training, points to the possibility that a minimum amount of neural substrate may be necessary for sensory recovery. Given this, it is interesting to consider whether a visuomotor training program with enriched feedback, as described by Quaney et al.,86 would have yielded better outcomes for this participant. These ideas are important from a prognostic standpoint; however, additional inquiry is needed to identify the minimum functional activation or substrate required to benefit from sensorimotor or visuomotor training.
The tractography method and diffusion parameters used here did not identify a change in sSTR white matter after training in either participant. The 34% increase in FA in Participant 1 is likely an artifact of low reliability associated with measurement of small white matter tracts.60 Importantly, the tractography method and diffusion parameters used here may not be sensitive to white matter reorganization; alternatively, reorganization may have taken place elsewhere in network. These cases highlight questions for future research on poststroke white matter reorganization, including: 1) what treatment dose is necessary, 2) where microstructural white matter changes might occur, 3) what diffusion parameters are sensitive enough to identify change, and 4) what time-frame is sufficient?
Limitations
This study lacked a multiple baseline design, and while the participants were in the chronic phase poststroke, natural recovery cannot be ruled out. Study participants did not have aphasia, apraxia or neglect. We expect these conditions would affect outcomes of future research. We used different sensory discrimination tasks for fMRI and behavioral testing. Due to incompatibility with the MRI, HASTe scores were not obtained during functional scanning; instead, we used brush texture discrimination, which reliably identifies sensory discrimination dysfunction in stroke survivors.87 Prior experience with MRI may impact activation at post-test, consistent with the observation of less variability in experienced than MRI naive participants. 88 Further research on dose, timing, and duration of training is necessary to generalize this protocol to the greater population of individuals with stroke.
Summary
Sensorimotor training, using a protocol focused on manual manipulation and sensory discrimination, may be an effective method for improving sensory and motor function poststroke and bears further evaluation. Additional research is needed to identify best measures of sensory function that are easily applied and span the breadth of tactile sensory behavior. Conversely, the WMFT and MAL effectively measure change from this and other sensorimotor training paradigms. 63 Finally, the potential for sensory recovery appears strongly related to the integrity of the sSTR; however, future work must look beyond this large white matter tract for structural change in other components of the sensory discrimination network. It is expected that subsequent evaluation of this protocol, in a large clinical trial, will elucidate the neural reorganization that supports sensorimotor recovery.
Supplementary Material
Footnotes
A portion of this work was previously presented at:
APTA CSM 2011:
CONTROL ID: 898623
TITLE: SENSORY RETRAINING IN CHRONIC STROKE: EVIDENCE OF CORTICAL AND BEHAVIORAL CHANGES.
PRESENTATION TYPE: Platform
CURRENT SECTION: Neurology
Society for Neuroscience Annual meeting 2011:
Program#/Poster#: 74.04/LL6
Presentation Title: Sensory retraining in chronic stroke: A case series
List of supplemental digital content.
SCD 1: JNPT_videoabstract.mp4
Contributor Information
Travis Bird, Physical Therapist, Good Samaritan Hospital, Dayton, OH.
Seongjin Choi, Post-Doctoral Researcher, Radiology, College of Medicine, The Ohio State University, Columbus, OH.
Lindsay Goodman, Physical Therapist and Geriatric Residency Mentor at Home Care by Blackstone, Columbus, OH.
Petra Schmalbrock, Associate Professor of Radiology, The Ohio State University, Columbus, OH.
Deborah S. Nichols-Larsen, Director, School of Health and Rehabilitation Sciences, The Ohio State University, Columbus, OH.
References
- 1.Carod-Artal J, Egido JA, González JL, Varela de Seijas E. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31(12):2995–3000. doi: 10.1161/01.str.31.12.2995. [DOI] [PubMed] [Google Scholar]
- 2.Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical Rehabilitation. 2008;22(8):758. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- 3.Park SW, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE Trial: Predicting a Clinically Meaningful Motor Activity Log Outcome. Neurorehabil Neural Repair. 2008 Sep;22(5):486–93. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao N, Jellinek HM, Harberg JK, Fryback DG. The art of medicine: subjective measures as predictors of outcome in stroke and traumatic brain injury. Archives of physical medicine and rehabilitation. 1988;69(3 Pt 1):179. [PubMed] [Google Scholar]
- 5.Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst Rev. 2010;6 doi: 10.1002/14651858.CD006331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Archives of physical medicine and rehabilitation. 1993;74(6):602–11. doi: 10.1016/0003-9993(93)90158-7. [DOI] [PubMed] [Google Scholar]
- 7.Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: facilitation of stimulus generalization. Am J Phys Med Rehabil. 2005 Jun;84(6):428–42. doi: 10.1097/01.phm.0000159971.12096.7f. [DOI] [PubMed] [Google Scholar]
- 8.Yekutiel M, Guttman E. A controlled trial of the retraining of the sensory function of the hand in stroke patients. Journal of Neurology, Neurosurgery & Psychiatry. 1993;56(3):241. doi: 10.1136/jnnp.56.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series 1. Archives of physical medicine and rehabilitation. 2003;84(10):1505–14. doi: 10.1016/s0003-9993(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 10.Byl NN, Pitsch EA, Abrams GM. Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke. Neurorehabilitation and neural repair. 2008;22(5):494. doi: 10.1177/1545968308317431. [DOI] [PubMed] [Google Scholar]
- 11.Carey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation A Randomized Controlled Trial. Neurorehabilitation and neural repair. 2011;25(4):304–13. doi: 10.1177/1545968310397705. [DOI] [PubMed] [Google Scholar]
- 12.Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys 10. J Neurophysiol. 1998 Apr;79(4):2119–48. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- 13.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(11):2476. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrien DJ, Strens LHA, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Experimental neurology. 2004;190(2):425–32. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–12. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 16.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125(4):773. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 17.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: an FMRI study. Neurorehabilitation and neural repair. 2006;20(3):398–405. doi: 10.1177/1545968306286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey LM, Abbott DF, Puce A, Jackson GD, Syngeniotis A, Donnan GA. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology. 2002 Sep 10;59(5):749–52. doi: 10.1212/wnl.59.5.749. [DOI] [PubMed] [Google Scholar]
- 19.Nhan H, Barquist K, Bell K, Esselman P, Odderson IR, Cramer SC. Brain function early after stroke in relation to subsequent recovery. Journal of Cerebral Blood Flow & Metabolism. 2004;24(7):756–63. doi: 10.1097/01.WCB.0000122744.72175.9C. [DOI] [PubMed] [Google Scholar]
- 20.Staines WR, Black SE, Graham SJ, McIlroy WE. Somatosensory gating and recovery from stroke involving the thalamus. Stroke. 2002 Nov;33(11):2642–51. doi: 10.1161/01.str.0000032552.40405.40. [DOI] [PubMed] [Google Scholar]
- 21.Dechaumont-Palacin S, Marque P, De Boissezon X, et al. Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: an fMRI study. Neurorehabilitation and neural repair. 2008;22(2):154. doi: 10.1177/1545968307307118. [DOI] [PubMed] [Google Scholar]
- 22.Johansen-Berg H, Behrens TEJ. Diffusion MRI: From quantitative measurement to In vivo neuroanatomy. London: Academic Press; 2009. [Google Scholar]
- 23.Yamada K, Kizu O, Nishimura T. MR tractography for stroke. Elsevier; 2006. pp. 67–72. [Google Scholar]
- 24.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. NeuroImage. 2008;39(3):1370–82. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang DS, Kim DS, Kim YH, Jang SH. Demonstration of Recovery of a Severely Damaged Corticospinal Tract: A Diffusion Tensor Tractography and Transcranial Magnetic Stimulation Follow-Up Study. Journal of computer assisted tomography. 2008;32(3):418. doi: 10.1097/RCT.0b013e31811eba4e. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Mori S, Nakamura H, et al. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003;34(9):e159. doi: 10.1161/01.STR.0000085827.54986.89. [DOI] [PubMed] [Google Scholar]
- 27.Nelles M, Gieseke J, Flacke S, Lachenmayer L, Schild HH, Urbach H. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. American Journal of Neuroradiology. 2008;29(3):488. doi: 10.3174/ajnr.A0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang SH. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neuroscience letters. 2007;426(2):123–7. doi: 10.1016/j.neulet.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Johansen-Berg H, Scholz J, Stagg CJ. Relevance of structural brain connectivity to learning and recovery from stroke. Frontiers in systems neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl P, Mori S. Fiber Tractûbased Atlas of Human White Matter Anatomy1. Radiology. 2004;230(1):77. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 31.Borstad A, Schmalbrock P, Choi S, Nichols-Larsen DS. Neural correlates supporting sensory discrimination after left hemisphere stroke. Brain research. 2012 Jun 15;1460:78–87. doi: 10.1016/j.brainres.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. Journal of hand therapy: official journal of the American Society of Hand Therapists. 6(1):11. [PubMed] [Google Scholar]
- 33.Jerosch-Herold C. Assessment of sensibility after nerve injury and repair: a systematic review of evidence for validity, reliability and responsiveness of tests. The Journal of Hand Surgery: British & European Volume. 2005;30(3):252–64. doi: 10.1016/j.jhsb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Carey LM, Oke LE, Matyas TA. Impaired limb position sense after stroke: a quantitative test for clinical use. Arch Phys Med Rehabil. 1996 Dec;77(12):1271–8. doi: 10.1016/s0003-9993(96)90192-6. [DOI] [PubMed] [Google Scholar]
- 35.Williams PS, Basso DM, Case-Smith J, Nichols-Larsen DS. Development of the Hand Active Sensation Test: reliability and validity. Arch Phys Med Rehabil. 2006 Nov;87(11):1471–7. doi: 10.1016/j.apmr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Kalisch T, Tegenthoff M, Dinse HR. Improvement of sensorimotor functions in old age by passive sensory stimulation. Clinical Interventions in Aging. 2008;3(4):673. doi: 10.2147/cia.s3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalisch T, Kattenstroth JC, Kowalewski R, Tegenthoff M, Dinse HR. Cognitive and Tactile Factors Affecting Human Haptic Performance in Later Life. PLoS One. 2012;7(1):e30420. doi: 10.1371/journal.pone.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole peg test of finger dexterity. Occup Ther J Res. 1985;5(1):24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 39.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. Journal of Neurology, Neurosurgery & Psychiatry. 1987;50(6):714–9. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1985;39(6):386. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 41.Platz T, Pinkowski C, Van Wijck F, Kim IH, Di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clinical Rehabilitation. 2005;19(4):404–11. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 42.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Archives of physical medicine and rehabilitation. 1994;75(7):751–5. [PubMed] [Google Scholar]
- 43.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32(7):1635. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 44.Morris DM, Uswatte G, Crago JE, Cook EW, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Archives of physical medicine and rehabilitation. 2001;82(6):750–5. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 45.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67(7):1189. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, van Zijl P, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. computer methods and programs in biomedicine. 2006;81(2):106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Roberts TPL, Liu F, Kassner A, Mori S, Guha A. Fiber density index correlates with reduced fractional anisotropy in white matter of patients with glioblastoma. American Journal of Neuroradiology. 2005;26(9):2183. [PMC free article] [PubMed] [Google Scholar]
- 48.Bazin PL, Cuzzocreo JL, Yassa MA, et al. Volumetric neuroimage analysis extensions for the MIPAV software package. Journal of neuroscience methods. 2007;165(1):111–21. doi: 10.1016/j.jneumeth.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orrison WW. Atlas of brain function. Thieme Medical Pub; 2008. [Google Scholar]
- 50.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 51.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 52.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 54.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 55.Andersson JLR, Jenkinson M, Smith S. Non-linear registration aka Spatial normalisation FMRIB Technial Report TR07JA2. FMRIB Analysis Group Technical Reports TR07JA02. 2007 Jun [Google Scholar]
- 56.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Experimental Brain Research. 2008;186(1):183–90. doi: 10.1007/s00221-007-1221-8. [DOI] [PubMed] [Google Scholar]
- 58.Carey LM, Abbott DF, Egan GF, Donnan GA. Reproducible activation in BA2, 1 and 3b associated with texture discrimination in healthy volunteers over time. Neuroimage. 2008 Jan 1;39(1):40–51. doi: 10.1016/j.neuroimage.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 59.Mori S, Crain BJ, Chacko VP, Van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999 Feb;45(2):265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007 Jul 1;36(3):630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002 Sep;17(1):79–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 62.Smith SM, Rao A, De Stefano N, et al. Longitudinal and cross-sectional analysis of atrophy in Alzheimer’s disease: cross-validation of BSI, SIENA and SIENAX. NeuroImage. 2007;36(4):1200–6. doi: 10.1016/j.neuroimage.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 63.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296(17):2095. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 64.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Experimental neurology. 1989;104(2):125–32. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 65.Saito D, Okada T, Honda M, Yonekura Y, Sadato N. Practice makes perfect: The neural substrates of tactile discrimination by Mah-Jong experts include the primary visual cortex. BMC neuroscience. 2006;7(1):79. doi: 10.1186/1471-2202-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yekutiel M. Sensory re-education of the hand after stroke. Lavoisier; France: 2000. [Google Scholar]
- 67.Stinear CM, Byblow WD. Task–dependent modulation of silent period duration in focal hand dystonia. Movement disorders. 2005;20(9):1143–51. doi: 10.1002/mds.20514. [DOI] [PubMed] [Google Scholar]
- 68.McDonnell MN, Hillier SL, Miles TS, Thompson PD, Ridding MC. Influence of combined afferent stimulation and task-specific training following stroke: a pilot randomized controlled trial. Neurorehabilitation and neural repair. 2007;21(5):435–43. doi: 10.1177/1545968307300437. [DOI] [PubMed] [Google Scholar]
- 69.Harada T, Saito DN, Kashikura KI, et al. Asymmetrical neural substrates of tactile discrimination in humans: a functional magnetic resonance imaging study. Journal of Neuroscience. 2004;24(34):7524. doi: 10.1523/JNEUROSCI.1395-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Han H, Chui D, Shen Y, Wu J. Prominent activation of the intraparietal and somatosensory areas during angle discrimination by intra–active touch. Human brain mapping. 2011 doi: 10.1002/hbm.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kitada R, Kito T, Saito DN, et al. Multisensory activation of the intraparietal area when classifying grating orientation: a functional magnetic resonance imaging study. The Journal of Neuroscience. 2006;26(28):7491–501. doi: 10.1523/JNEUROSCI.0822-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper extremity measures early after stroke. Archives of physical medicine and rehabilitation. 2008;89(9):1693. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van der Lee JH, Beckerman H, Knol DL, De Vet HCW, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 74.Smith SM, Rao A, De SN, et al. Longitudinal and cross-sectional analysis of atrophy in Alzheimer’s disease: cross-validation of BSI, SIENA and SIENAX. Neuroimage. 2007 Jul 15;36(4):1200–6. doi: 10.1016/j.neuroimage.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 75.Hunter JM, Mackin EJ, Callahan AD. Rehabilitation of the Hand: Surgery and Therapy. Mosby; St. Louis, Missouri: 1995. [Google Scholar]
- 76.Deutsch JE, Fischer S, Liu W, Kalnin A, Mosier K. Representation of imagined and executed sequential finger movements of adults post stroke and healthy controls. Journal of Neurologic Physical Therapy. 2005;29(4):194. [Google Scholar]
- 77.Borstad AL, S P, C S, N-L D. Neural correlates supporting sensory discrimination after left hemisphere stroke. j brainres. 2012 Apr 2; doi: 10.1016/j.brainres.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–31. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lovden M, Bodammer NC, Kohn S, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48(13):3878–83. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 81.Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6(6):e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hihara S, Notoya T, Tanaka M, et al. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia. 2006;44(13):2636–46. doi: 10.1016/j.neuropsychologia.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 83.Ding G, Jiang Q, Li L, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2* WI. Stroke. 2008;39(5):1563–8. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human brain mapping. 2009;30(11):3461–74. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crofts JJ, Higham DJ, Bosnell R, et al. Network analysis detects changes in the contralesional hemisphere following stroke. NeuroImage. 2011;54(1):161–9. doi: 10.1016/j.neuroimage.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quaney BM, He J, Timberlake G, Dodd K, Carr C. Visuomotor training improves stroke-related ipsilesional upper extremity impairments. Neurorehabilitation and neural repair. 2010;24(1):52–61. doi: 10.1177/1545968309341646. [DOI] [PubMed] [Google Scholar]
- 87.Dannenbaum RM, Michaelsen SM, Desrosiers J, Levin MF. Development and validation of two new sensory tests of the hand for patients with stroke. Clin Rehabil. 2002 Sep;16(6):630–9. doi: 10.1191/0269215502cr532oa. [DOI] [PubMed] [Google Scholar]
- 88.Loubinoux I, Carel C, Alary F, et al. Within-Session and Between-Session Reproducibility of Cerebral Sensorimotor Activation: A Test-Retest Effect Evidenced With Functional Magnetic Resonance Imaging. Journal of Cerebral Blood Flow & Metabolism. 2001;21(5):592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Schulz LA, Bohannon RW, Morgan WJ. Normal digit tip values for the Weinstein Enhanced Sensory Test. Journal of hand therapy: official journal of the American Society of Hand Therapists. 1998;11(3):200. doi: 10.1016/s0894-1130(98)80038-0. [DOI] [PubMed] [Google Scholar]
- 90.Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilitation and neural repair. 2009;23(5):435–40. doi: 10.1177/1545968308331146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


