Abstract
Polymorphonuclear leukocytes (PMNs) have increased oxidative metabolism during phagocytosis and emit light (chemiluminescence, CL) as a result of metabolic activation. The present study examined PMN CL in the absence of phagocytosis using sodium fluoride (NaF), a nonparticulate agent and known stimulator of cellular oxidative metabolism. Normal human and canine PMNs were assayed in a CL spectrometer which permitted continuous sample mixing and constant temperature regulation during CL measurement. PMNs treated with 20 mM NaF demonstrated maximum CL responses of 10,000-20,000 cpm above background, 13-17 min after addition of NaF at 37°C. Temperature regulation of reaction mixtures was found to be a critical factor in assaying PMN CL responses to NaF, because a small decrease in temperature (i.e. 1.5°C) substantially depressed and delayed the CL response. Superoxide anion production correlated closely with CL responses in NaF-treated human PMNs. CL responses were completely suppressed in the presence of the oxidative metabolic inhibitors, iodoacetamide, and N-ethylmalemide; and were partially suppressed in the presence of either superoxide dismutase or sodium azide.
CL responses of NaF-treated PMNs were significantly lower than responses generated by PMNs phagocytizing opsonized yeast. When NaF was evaluated for its effect on light generation from a singlet oxygen dependent CL reaction, it was found that NaF did not quench singlet oxygen light. This study demonstrates that PMN CL can occur in the absence of phagocytosis, and it proposes that a nonphagocytic PMN CL assay may be useful in evaluating leukocyte metabolic defects.
Full text
PDF




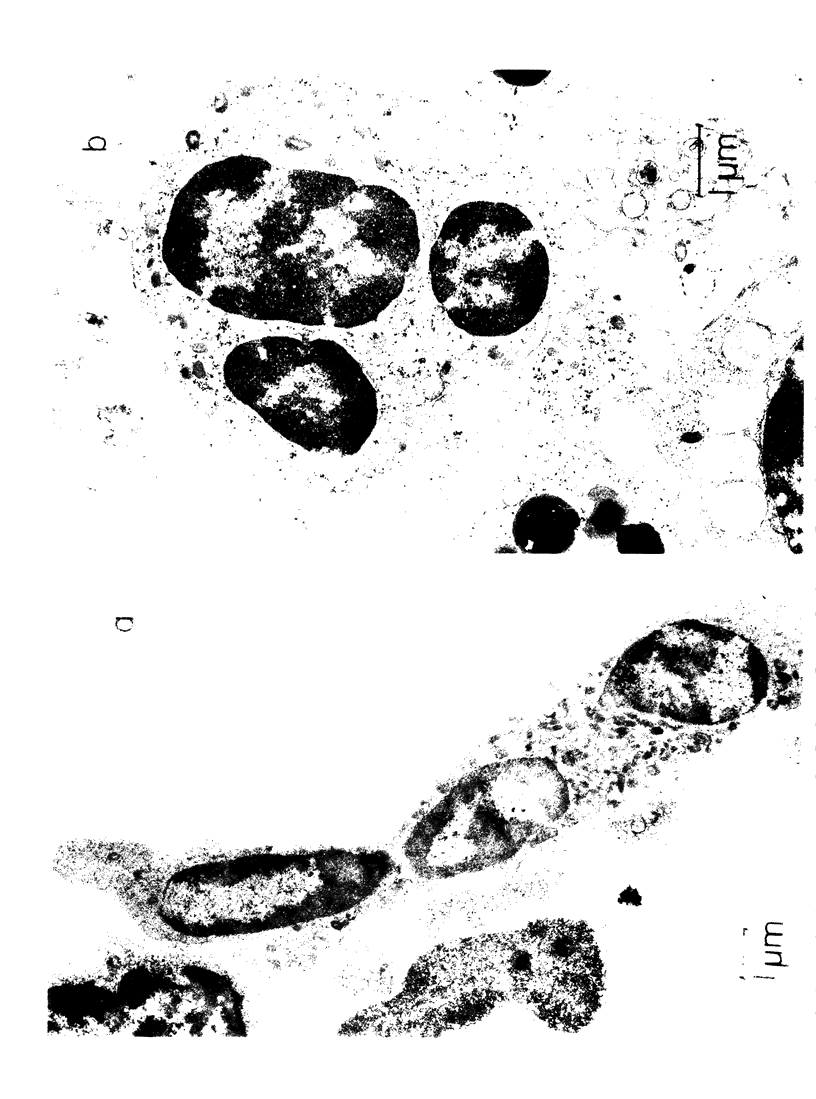
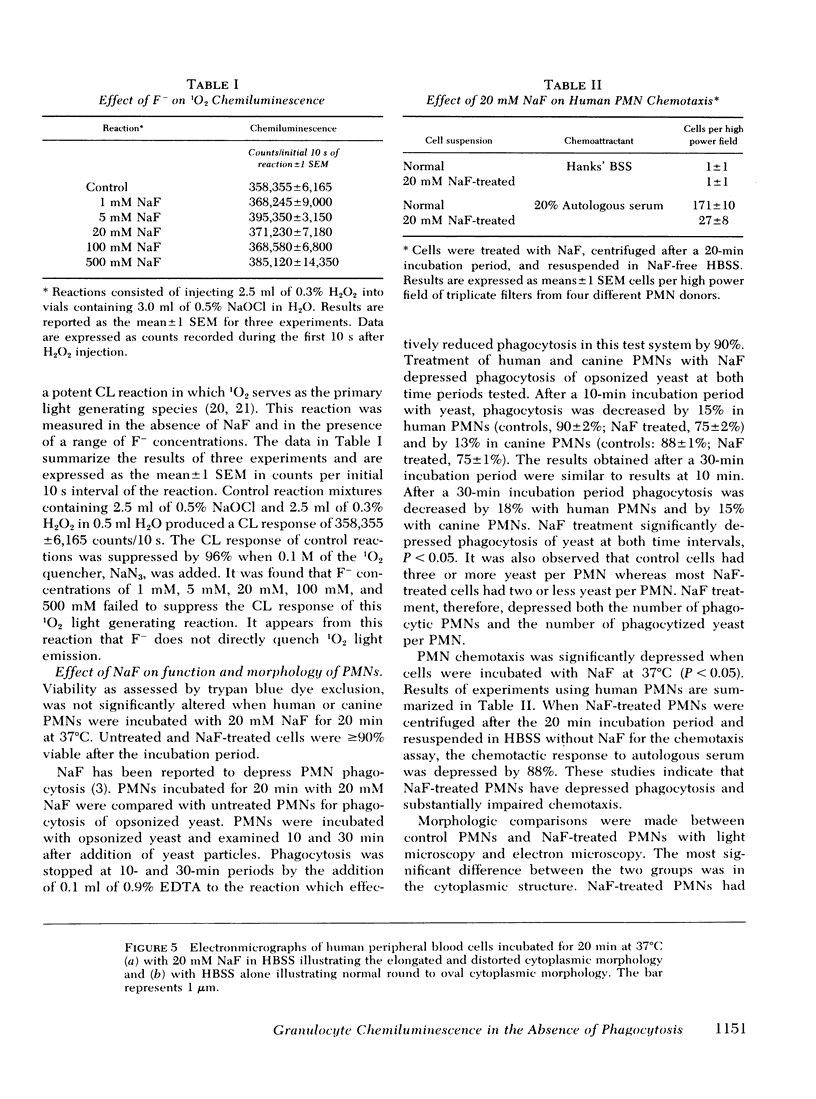



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Brendzel A. M., Lint T. F. Chemiluminescence spectra of human myeloperoxidase and polymorphonuclear leukocytes. Infect Immun. 1977 Jul;17(1):62–66. doi: 10.1128/iai.17.1.62-66.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B. R., Brendzel A. M. Use of a unique chemiluminescence spectrometer in a study of factors influencing granulocyte light emission. J Immunol Methods. 1978;19(2-3):279–287. doi: 10.1016/0022-1759(78)90187-4. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Debelak-Fehir K. M., Epstein R. B. Effect of bone marrow suppression on granulocyte opsonin levels. Proc Soc Exp Biol Med. 1976 Jan;151(1):105–109. doi: 10.3181/00379727-151-39153. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall G. D., Repine J. E., Hoidal J. R., Rasp F. L. Chemiluminescence by human alveolar macrophages: stimulation with heat-killed bacteria or phorobol myristate acetate. Infect Immun. 1977 Jul;17(1):117–120. doi: 10.1128/iai.17.1.117-120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Epstein R. B., Waxman F. J., Bennett B. T., Andersen B. R. Pseudomonas septicemia in neutropenic dogs. I. Treatment with granulocyte transfusions. Transfusion. 1974 Jan-Feb;14(1):51–57. doi: 10.1111/j.1537-2995.1974.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner J. V., Mills E. L., Gray G. H., Quie P. G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977 Jan;89(1):153–159. [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Durack D. T., Rosen H., Clark R. A. Functional studies on human peritoneal eosinophils. Infect Immun. 1977 Jul;17(1):167–173. doi: 10.1128/iai.17.1.167-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SELIGER H. H. A photoelectric method for the measurement of spectra of light sources of rapidly varying intensities. Anal Biochem. 1960 Jun;1:60–65. doi: 10.1016/0003-2697(60)90019-1. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Mendelson D. S., Metz E. N. The effect of sodium azide on the chemiluminescence of granulocytes--evidence for the generation of multiple oxygen radicals. J Lab Clin Med. 1977 Jun;89(6):1333–1340. [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]



