Abstract
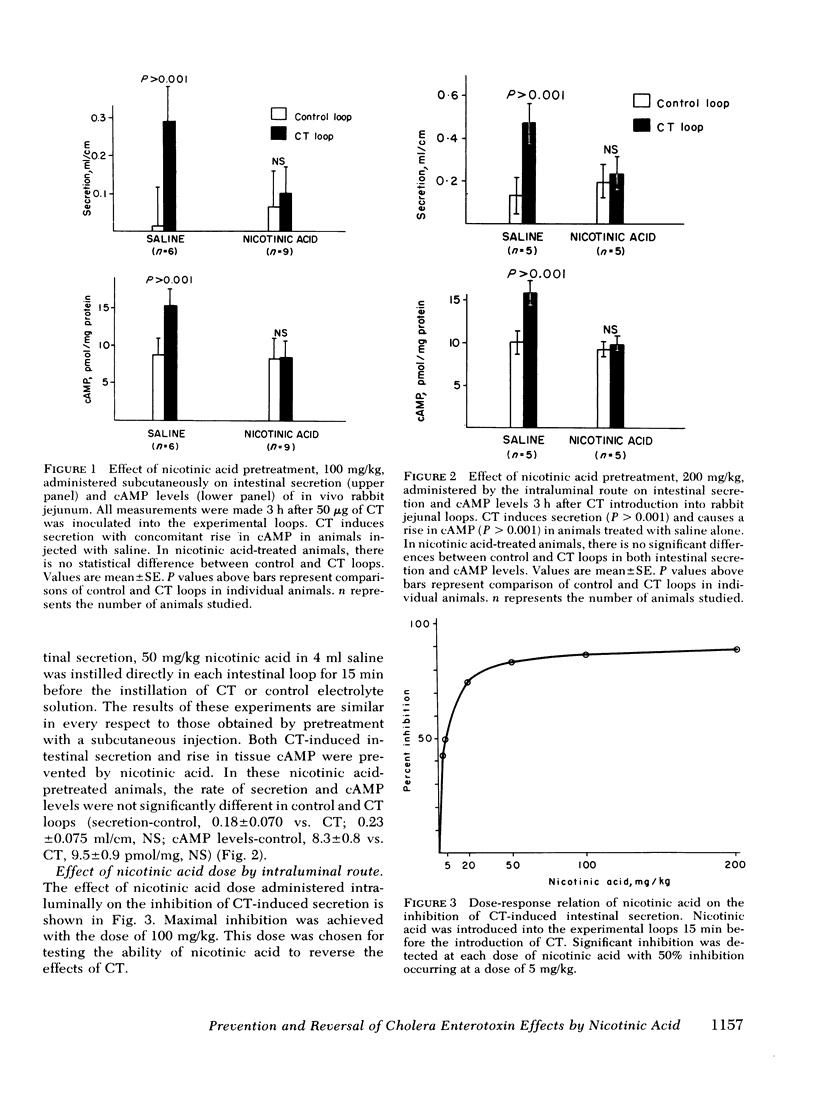
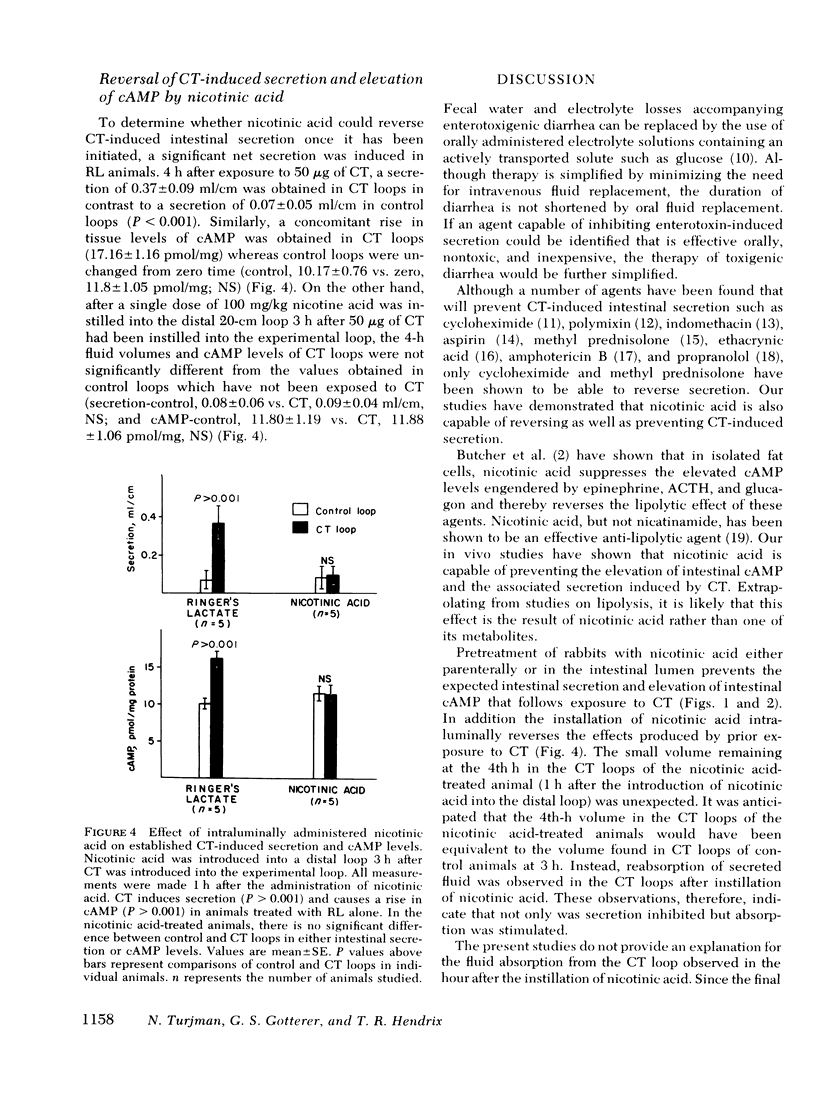
The cholera enterotoxin produces intestinal secretion associated with an elevation of tissue levels of cyclic adenosine 3′,5′-monophosphate levels of cyclic adenosine 3′,5′-monosphosphate (cAMP). The objectives of this study were to determine whether intestinal secretion and cAMP elevation induced by cholera toxin could be prevented, or once initiated, reversed by nicotinic acid, an agent known to lower tissue levels of cAMP. In rabbits, four jejunal loops were constructed as alternating control (3-ml isotonic electrolyte solution) and cholera toxin (same solution containing 50 μg purified cholera toxin) loops. Net intestinal secretion was determined by measuring fluid accumulation, after which intestinal biopsies were taken for cAMP assay. The animals were pretreated either subcutaneously with 50 mg/kg nicotinic acid in saline 3 h and 1 h before the introduction of cholera toxin, or intraluminally with 200 mg/kg nicotinic acid in Ringer's lactate solution 15 min before the instillation of cholera toxin. Under these conditions, nicotinic acid blocked the cholera toxin-induced secretion and the rise in cAMP measured 3 h after the loops were exposed to cholera toxin. The effect of the nicotinic acid administered within the lumen on net intestinal secretion was studied. Maximal inhibition of net intestinal secretion was achieved with an intraluminally administered dose of nicotinic acid of 100 mg/kg. This dose was chosen for testing the ability of nicotinic acid to reverse the effects of cholera toxin. When nicotinic acid was instilled into a fifth loop constructed distally to the four experimental loops 3 h after exposure of these loops to cholera toxin, both intestinal secretion and elevation of cAMP were reversed. These results suggest that nicotinic acid can prevent and reverse the secretory effects of cholera toxin and may have a role in the therapy of cholera and other cAMP-associated diarrheal diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- CARLSON L. A. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med Scand. 1963 Jun;173:719–722. doi: 10.1111/j.0954-6820.1963.tb17457.x. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Curlin G. T., Greenough W. B. Response of canine Thiry-Vella jejunal loops to cholera exotoxin and its modification by ethacrynic acid. J Infect Dis. 1969 Sep;120(3):332–338. doi: 10.1093/infdis/120.3.332. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney A. N., Donowitz M. Prevention and reversal of cholera enterotoxin-induced intestinal secretion by methylprednisolone induction of Na+-K+-ATPase. J Clin Invest. 1976 Jun;57(6):1590–1599. doi: 10.1172/JCI108429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Guerrant R. L., Rohde J. E., Casper A. G. Effect of amphotericin B on sodium and water movement across normal and cholera toxin-challenged canine jejunum. Gastroenterology. 1973 Aug;65(2):252–258. [PubMed] [Google Scholar]
- Finck A. D., Katz R. L. Prevention of cholera-induced intestinal secretion in the cat by aspirin. Nature. 1972 Aug 4;238(5362):273–274. doi: 10.1038/238273a0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Grayer D. T., Serebro H. A., Iber F. L., Hendrix T. R. Effect of cycloheximide on unidirectional sodium fluxes in the jejunum after cholera exotoxin exposure. Gastroenterology. 1970 Jun;58(6):815–819. [PubMed] [Google Scholar]
- Harper D. T., Jr, Yardley J. H., Hendrix T. R. Reversal of cholera exotoxin-induced jejunal secretion by cycloheximide. Johns Hopkins Med J. 1970 May;126(5):258–266. [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Kinzie J. L., Ferrendelli J. A., Alpers D. H. Adenosine cyclic 3':5'-monophosphate-mediated transport of neutral and dibasic amino acids in jejunal mucosa. J Biol Chem. 1973 Oct 25;248(20):7018–7024. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maimon H. N., Mitch W. E., Banwell J. G., Hendrix T. R. Inhibition of enterotoxin-induced intestinal secretion by the polypeptide antibiotic, polymyxin. Johns Hopkins Med J. 1976 Mar;138(3):82–90. [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Mitra R. C., Banwell J. G., Brigham K. L., Fedson D. S., Mondal A. Replacement of water and electrolyte losses in cholera by an oral glucose-electrolyte solution. Ann Intern Med. 1969 Jun;70(6):1173–1181. doi: 10.7326/0003-4819-70-6-1173. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore I. F., Schönhöfer P. S., Kritchevsky D. Effects of nicotinic acid and some of its homologues on lipolysis, adenyl cyclase, phosphodiesterase and cyclic AMP accumulation in isolated fat cells. Pharmacology. 1971;6(6):330–338. doi: 10.1159/000136261. [DOI] [PubMed] [Google Scholar]
- Taub M., Bonorris G., Chung A., Coyne M. J., Schoenfield L. J. Effect of propranolol on bile acid- and cholera enterotoxin-stimulated cAMP and secretion in rabbit intestine. Gastroenterology. 1977 Jan;72(1):101–105. [PubMed] [Google Scholar]
- Wald A., Gotterer G. S., Rajendra G. R., Turjman N. A., Hendrix T. R. Effect of indomethacin on cholera-induced fluid movement, unidirectional sodium fluxes, and intestinal cAMP. Gastroenterology. 1977 Jan;72(1):106–110. [PubMed] [Google Scholar]


