Abstract
The underlying cause of human retinoblastoma is complete inactivation of both copies of the RB1 gene. Other chromosome abnormalities, with the most common being extra copies of chromosome arm 6p, are also observed in retinoblastoma. The RB protein has previously been shown to interact with TFAP2 transcription factors. Here, we show that TFAP2A and TFAP2B, which map to chromosome arm 6p, are expressed in the amacrine and horizontal cells of human retina. TFAP2A RNA can readily be detected in retinoblastoma cell lines and tumors; however, the great majority of retinoblastoma cell lines and tumors are completely devoid of TFAP2A protein and TFAP2B RNA/protein. Transfection of TFAP2A and TFAP2B expression constructs into retinoblastoma cells induces apoptosis and inhibits proliferation. Our results suggest that a consequence of loss of RB1 gene function in retinoblastoma cells is inactivation of TFAP2A and TFAP2B function. We propose that inability to differentiate along the amacrine/horizontal cell lineages may underlie retinoblastoma tumor formation.
INTRODUCTION
The retina is an evolutionarily conserved multilayered structure derived from the walls of the neural tube destined to become the forebrain. The retina consists of six major classes of neurons (ganglion, amacrine, bipolar, horizontal, cone photoreceptors, and rod photoreceptors) and one glial cell type (Müller) distributed in three nuclear layers (outer or photoreceptor, inner, and ganglion). The appearance of the different retinal cell types follows a specific order, with ganglion cells appearing first, followed by amacrine cells, cone photoreceptors, and horizontal cells and then rod photoreceptors, bipolar cells, and Müller cells (Prada et al., 1991; Cepko et al., 1996).
TFAP2 is a family of transcription factors implicated in many aspects of development. Five TFAP2 genes have been identified in humans: TFAP2A, TFAP2B, TFAP2C, TFAP2D, and TFAP2E. Three of these genes, TFAP2A, TFAP2B, and TFAP2D, have been mapped to 6p24, 6p12, and 6p12.1, respectively (Gaynor et al., 1991; Williamson et al., 1996; Cheng et al., 2002). TFAP2A and TFAP2B are expressed in the amacrine cells of the developing murine and chick retina, with TFAP2B also found in the horizontal cells of chick retina (Bisgrove and Godbout, 1999; Bassett et al., 2007). TFAP2D is expressed in a subset of ganglion cells, whereas TFAP2C and TFAP2E have a poorly defined expression pattern in the retina (Bassett et al., 2007; Li et al., 2008).
The function of TFAP2 has been investigated by generating mouse models targeting individual Tfap2s. To date, four Tfap2 knock-outs have been described: Tcfap2a, Tcfap2b, Tcfap2c, and Tcfap2e. Tcfap2a knock-out mice die perinatally and show severe defects in cranial and body wall closure and skeletal structures (Schorle et al., 1996; Zhang et al., 1996; West-Mays et al., 1999). The severity of the ocular defects, often resulting in the absence of eyes, precludes analysis of the role of TFAP2A during retinal development. Conditional Tcfap2a knock-out mice specifically targeting the retina have no obvious retinal defects, leading to the hypothesis that TFAP2B and possibly other TFAP2s may compensate for the loss of TFAP2A in mouse retina (Bassett et al., 2007). In contrast to Tcfap2a, Tcfap2b knock-out mice have no defects in craniofacial and ocular structures, but die perinatally due to massive apoptosis of renal tubular epithelia (Moser et al., 1997). Tcfap2b−/− mice express reduced levels of noradrenaline and noradrenaline-synthesizing dopamine β-hydroxylase in the peripheral nervous system (Hong et al., 2008). Tcfap2c knock-out mice die after gastrulation due to defective placental development (Auman et al., 2002; Werling and Schorle, 2002). The main phenotype associated with Tcfap2e knock-out mice is olfactory bulb disorganization (Feng et al., 2009).
Retinoblastoma is a childhood tumor derived from the retina. The underlying cause of retinoblastoma is complete inactivation of both copies of the RB1 gene located in chromosome band 13q14. Although other cancers such as breast cancer and prostate cancer commonly have RB1 gene defects, retinoblastoma tumors are unique in their dependence on loss of pRB function to initiate the events required for tumor formation. To this day, the susceptibility of human retinal cells to RB1 gene inactivation remains an enigma and is believed to be related to unique properties of the cell-of-origin of retinoblastoma. However, there is still controversy regarding the cell-of-origin of retinoblastoma (Kyritsis et al., 1984; Chen et al., 2004; Dyer and Bremner, 2005; Glubrecht et al., 2009; Xu et al., 2009).
A number of reports have linked pRB to the TFAP2 family of transcription factors. For example, TFAP2A and TFAP2B interact with pRB in vitro (Wu and Lee, 1998), and transcription of genes such as BCL2 and E-cadherin is mediated by pRB interacting with TFAP2 (Batsche et al., 1998; Decary et al., 2002). To address a possible role for TFAP2 in retinoblastoma, we have studied the expression of TFAP2A and TFAP2B in human retina and in retinoblastoma tumors and cell lines. We have also expressed TFAP2A and TFAP2B in two retinoblastoma cell lines and have examined how TFAP2 expression affects the growth properties of these cells. Our results suggest that a consequence of RB1 gene inactivation in retinoblastoma cells is inactivation of the TFAP2 pathway. Reinstatement of a functional TFAP2 pathway in these cells leads to cell death and altered differentiation potential.
MATERIALS AND METHODS
Retinoblastoma Cell Lines
Y79 and WERI-Rb1 were obtained from the American Type Culture Collection. RB522A, RB778, RB835, RB893, RB1021, RB1581, RB787, RB1335, RB805, RB894, RB898, RB1210, and RB1442 cell lines were established by Dr. Brenda Gallie, Department of Medical Genetics, University of Toronto, Canada (Gallie et al., 1982; Glubrecht et al., 2009). RB(E)1, RB(E)2, RB(E)3, and RB(E)5 were established from retinoblastoma tumor biopsies obtained from the Royal Alexandra Hospital, Edmonton, Canada. Cells were cultured in DMEM supplemented with 10% fetal calf serum, 100 μg/ml streptomycin, and 100 units/ml penicillin. For proteasome inhibitor experiments, RB522A and WERI-Rb1 cells were treated with 10 μM MG-132 (Sigma) for 8 or 24 hr. For demethylation experiments, RB522A and WERI-Rb1 cells were treated with 10 μM 5-azacytidine (Sigma) for 24 or 48 hr.
RT-PCR and Northern Blot Analysis
For RT-PCR, 1 μg of poly(A)+ RNA from different retinoblastoma cell lines or 3 μg of total RNA from human fetal retina at 10–11 weeks gestation were reverse transcribed using oligo d(T) and Superscript reverse transcriptase (Invitrogen). Single-strand cDNA was PCR-amplified using the following primers: 5′-ACCTAGCCAGGGAC TTTG-3′ (top strand) and 5′-GTCACTGCTTT TGGCGTTGTT-3′ (bottom strand) for TFAP2A; 5′-ACGTCCAGTCAGTTGAAGATG-3′ (top strand) and 5′-TATCCTCGAGTCATTTCCTGTGTTTCTC-3′ (bottom strand) for TFAP2B. PCR products of 395 bp (1148–1542) and 844 bp (699–1542) were generated for TFAP2A and TFAP2B, respectively.
For northern blotting, poly(A)+ RNAs isolated from the indicated retinoblastoma cell lines were electrophoresed in a 6% formaldehyde-1.5% agarose gel in MOPS buffer and transferred to nitro-cellulose. The filter was hybridized to 32P-labeled cDNA probes specific to TFAP2A, TFAP2B, and actin and washed under high stringency. To ensure that the same amount of RNA was loaded into each lane, the filter was stripped, and rehybridized with 32P-labeled actin cDNA.
Western Blot Analysis
Retinoblastoma cells were lysed in RIPA buffer and proteins separated in a 8% or 10% acrylamide–SDS gel. Proteins were transferred to a nitro-cellulose membrane and TFAP2A detected using mouse anti-TFAP2A antibody (1:250 dilution) (3B5; Developmental Studies Hybridoma Bank), rabbit anti-TFAP2B (1:500 dilution) (H87; Santa Cruz), mouse anti-actin antibody (1:100,000 dilution) (Sigma), rabbit anti-β-catenin antibody (1:1000 dilution, a gift from Dr. Manijeh Pasdar, University of Alberta), and mouse anti-syntaxin antibody (1:2000 dilution) (HPC-1; Sigma).
In Situ Hybridization
Human fetal retina tissue at 10–11 weeks gestation was obtained in accordance with guidelines specified by the Alberta Cancer Board Research Ethics Board and University of Alberta Health Research Ethics Board (protocol ETH-99-11-18/17561). The tissue was fixed in 4% paraformaldehyde, cryoprotected in sucrose, and embedded in OCT (Tissue-Tek). The TFAP2A antisense probe was derived from the 3′ end of the cDNA and encompasses nucleotides 1138–1983 region (846 nt). The TFAP2B probe was derived from the 5′ end of the cDNA and encompasses nucleotides 157–1565 (1409 nt). Antisense riboprobes labeled with digoxigenin (DIG) were synthesized by in vitro transcription with T3 or T7 RNA polymerase (Roche) according to the manufacturer’s instructions. Tissue sections (6–7 μm) were pre-hybridized at 55°C in 40% formamide, 10% dextran sulfate, 1× Denhardt’s solution, 4× standard saline citrate, 10 mM dithiothreitol, 0.5 mg/ml yeast tRNA, and 0.5 mg/ml heat-denatured herring testis sperm DNA. Riboprobes were hybridized to tissue sections overnight at 55°C. Tissue sections were washed and incubated with alkaline-phosphatase-conjugated anti-DIG antibody. The signal was detected with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. Pictures were taken using Zeiss Axioskop 2 plus microscope with a 20× objective.
Immunohistochemistry and Immunofluorescence Analysis of Tissue Sections
Human fetal retina at 10–11 weeks gestation was processed as described earlier for in situ hybridization. Slides of human fetal retina at 7 months gestation were purchased from Pantomics (Richmond CA). Formalin-fixed paraffin-embedded eyes with retinoblastoma tumor tissue were obtained from Dynacare Kasper Medical Laboratories (Edmonton, Alberta, Canada) following the guidelines established by the Alberta Cancer Board Research Ethics Board and the University of Alberta Health Research Ethics Board (protocol ETH-22411). Regions of the retina distal from the tumor were considered normal. Tissues were deparaffinized in xylene, rehydrated, and microwaved in a pressure cooker for 20 min in 10 mM citrate/0.05% Tween-20; pH 6 for antigen retrieval.
For immunohistochemistry, sections were stained with mouse anti-TFAP2A (1:200 dilution) (3B5), rabbit anti-TFAP2B (1:800 dilution) (H87), and mouse anti-Ki-67 (1:1,000 dilution) (MIB-1; DakoCytomation) antibodies, respectively. The signal was detected using the DakoCytomation EnVision+ secondary system. Tissues were counterstained with the nuclear stain hematoxylin. Images were captured with a Zeiss Axioskop 2 plus microscope and 20× objective.
For immunofluorescence analysis, tissue sections were stained with anti-TFAP2A and anti-TFAP2B antibodies, followed by secondary antibodies conjugated to Alexa 488 and Alexa 555. Sections were counterstained with the nuclear fluorescent dye Hoechst 33342 (1:1000). Images were captured using a Zeiss LSM 510 confocal microscope.
Cell Transfections and Immunofluorescence Analysis
The entire coding region of TFAP2A was generated by PCR using primers flanking the start (italicized) (5′-CTAGGAATTCAATGCTTTGGAAATTGACGGA-3′) and stop codons (italicized) (5′-CTAGGGTACCTCACTTTCTGTGCTTCTCC-3′) (253–1,567 bp). The coding region of TFAP2B was generated by PCR using primers flanking the start (5′-CTAGGAATTCAATGCACTCACCTCCTAGAG-3′) and stop codons (5′-CTAGGGTACCTCATTTCCTGTGTTTCTCCT-3′) (167–1,549 bp). The PCR products were inserted into pEGFP-C1 and verified by sequencing.
Retinoblastoma cells RB522A, WERI-Rb1, or HeLa cells were plated onto cover slips. Transfections were carried out with Fugene HD transfection reagent (Roche Applied Science) according to the manufacturer’s protocol. Forty-eight hours after transfection, cells were fixed in 4% paraformaldehyde for 10 min and permeabilized in 0.2% Triton X-100 for 5 min. The cells were labeled with rabbit anti-active Caspase 3 (1:50 dilution) (AB3623; Chemicon), mouse anti-Ki-67 (1:1,000 dilution) (MIB-1; Dakocytomation), or mouse anti-CRX (1:2,000 dilution) (4G11; Abnova) antibodies, respectively. The signal was detected using donkey anti-rabbit or donkey anti-mouse secondary antibodies conjugated with Alexa 555. Cells were counterstained with DAPI.
We used Metamorph software to determine the percentage of GFP-positive cells with reduced MIB-1 and CRX staining. Briefly, the average staining intensity of cells immunostained with MIB-1 (only cells with the higher levels of MIB-1 were included) or CRX, in untransfected cells, was used as the base level. GFP-expressing cells, which showed a fivefold decrease in staining intensity compared to base level, were considered to have reduced MIB-1 or CRX staining.
RESULTS
Distribution of TFAP2 RNA and Protein in Human Fetal Retina
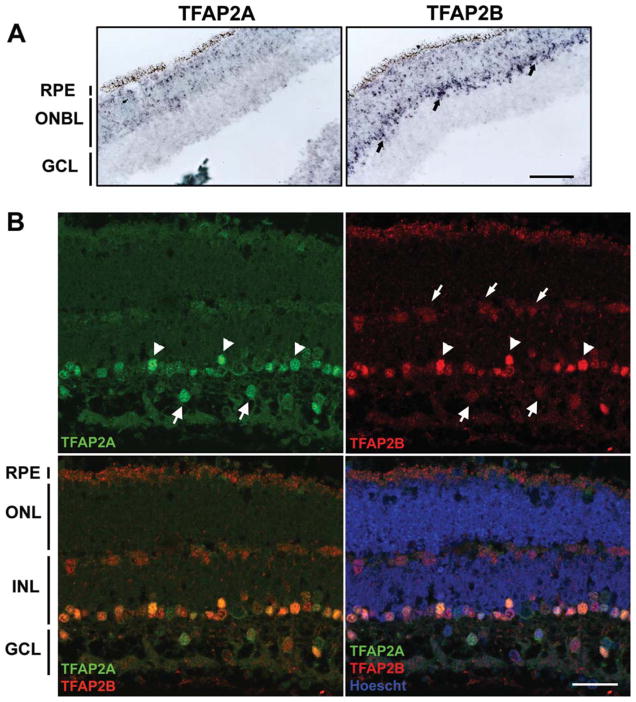
We have previously shown that TFAP2A and TFAP2B are restricted to two cell lineages in the developing chick retina: amacrine and horizontal (Bisgrove and Godbout, 1999). To investigate the expression of these two AP2s in human retina, we carried out in situ hybridization of normal human fetal retina tissue at 10–11 weeks gestation using probes specific to TFAP2A and TFAP2B. At this developmental stage, there are two distinct layers in the developing retina: (i) the outer neuroblastic layer, which consists of proliferating cells and emerging amacrine, bipolar, photoreceptor cells, and (ii) a ganglion cell layer. TFAP2A and TFAP2B-positive cells were detected in the outer neuroblastic layer, with TFAP2B-positive cells clearly concentrated in the inner part of the outer neuroblastic layer where differentiated amacrine cells are located (Fig. 1A).
Figure 1.
In situ hybridization and immunofluorescence analysis of TFAP2A and TFAP2B in human fetal retina. A: Tissue sections from human fetal retina at 10–11 weeks gestation were hybridized to DIG-labeled TFAP2A and TFAP2B antisense RNA probes. The signal was detected using an alkaline-phosphatase-coupled antibody, generating a purple color. Arrows point to the amacrine cells in the outer neuroblastic layer. Photographs were taken with a 20× lens using a Zeiss Axioskop 2 plus microscope. Scale bar = 100 μm. B: Tissue sections from human fetal retina at 7 months gestation were double-stained with mouse monoclonal anti-TFAP2A and rabbit polyclonal anti-TFAP2B antibodies, followed by donkey anti-mouse and donkey anti-rabbit secondary antibodies conjugated with Alexa 488 and Alexa 555, respectively. Sections were counterstained with Hoechst 33342 to label the nuclei. The arrowheads indicate the layer of amacrine cells positive for both TFAP2A and TFAP2B. The large arrows indicate displaced amacrine cells that are positive for both TFAP2A and TFAP2B. The small arrows indicate the layer of horizontal cells that are positive for TFAP2B. Photographs were taken with a Zeiss LSM 510 confocal microscope equipped with a 40× objective. Scale bar = 25 μm. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; ONBL, outer neuroblastic layer; RPE, retinal pigment epithelium. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To further address the distribution pattern of TFAP2A and TFAP2B in human retina, we carried out immunofluorescence analysis of normal retinal tissue at 10–11 weeks gestation and 7 months gestation. Immunostaining results closely mimicked those obtained by in situ hybridization at 10–11 weeks gestation (Supporting Information Fig. S1). At 7 months gestation, the human retina is well-differentiated, consisting of three well-defined nuclear layers separated by the outer and the inner plexiform layers. As shown in Figure 1B, both TFAP2A and TFAP2B are expressed in 7-month fetal retina, with TFAP2A specifically found in amacrine cells and TFAP2B present in both amacrine and horizontal cells. Cells indicated by the large arrows are likely displaced amacrine cells based on their location immediately adjacent to the inner plexiform layer. The majority of amacrine cells expressed both TFAP2A and TFAP2B proteins, consistent with the distribution patterns of TFAP2A and TFAP2B in embryonic chick retina (Bisgrove and Godbout, 1999).
Expression of TFAP2 in Retinoblastoma Cell Lines
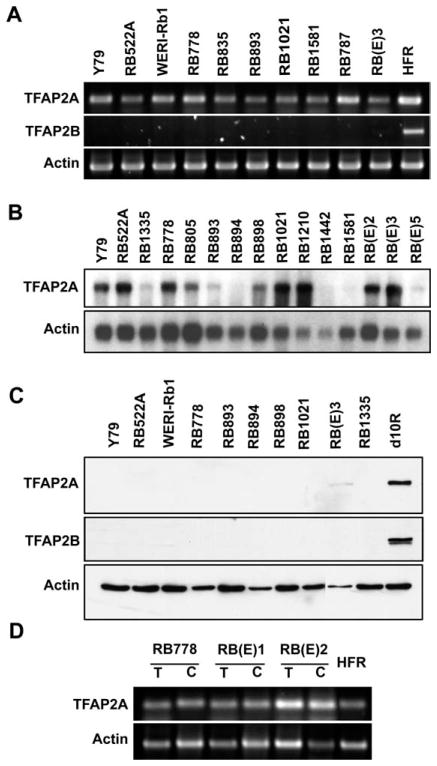
We used both RT-PCR and Northern blot analysis to examine the expression of TFAP2 in retinoblastoma cell lines. RT-PCR analysis revealed TFAP2A transcripts in all 10 retinoblastoma cell lines analyzed as well as in human fetal retina (Fig. 2A). In contrast, TFAP2B RNA was not observed in any of the retinoblastoma cell lines tested although this transcript was easily detected in human fetal retina (Fig. 2A). These data were confirmed by Northern blot analysis where variable levels of TFAP2A RNA were observed in the 15 retinoblastoma cell lines tested (Fig. 2B). TFAP2B transcripts were completely absent in these 15 retinoblastoma cell lines based on northern blots (data not shown).
Figure 2.
TFAP2A and TFAP2B expression in retinoblastoma cells. A: RT-PCR analysis of TFAP2A and TFAP2B using cDNAs from retinoblastoma cell lines, as indicated. Actin served as a positive control for these experiments. HFR, human fetal retina. B: Northern blots were prepared using poly(A)+ RNA purified from retinoblastoma cell lines, as indicated. The filter was sequentially hybridized with 32P-labeled TFAP2A and actin cDNAs. C: Western blot analysis of TFAP2A and TFAP2B using protein lysates from retinoblastoma cell lines, as indicated. Proteins were separated in a 10% polyacrylamide–SDS gel and transferred to a nitrocellulose membrane. The membrane was sequentially immunostained with anti-TFAP2A antibody, anti-TFAP2B antibody, and actin antibody. The signal was detected using the ECL reagent (GE Healthcare). D: RT-PCR analysis of TFAP2A using cDNAs prepared from three sets of fresh tumor cells/established cell cultures, as indicated. Actin served as the positive control. T, tumor; C, cell culture; HFR, human fetal retina.
Growth of retinoblastoma cells in culture may affect expression of differentiation markers. We therefore examined whether TFAP2A RNA was present in fresh tumor tissue before cell culture. Complementary DNA prepared from three paired samples of fresh tumors/cell lines were PCR-amplified using primers specific to TFAP2A. As shown in Figure 2D, TFAP2A RNA was expressed at similar levels in tumor tissue and cell lines, indicating that the presence of TFAP2A transcript in retinoblastoma cell lines is not a tissue culture artifact.
We then carried out Western blot analysis to examine TFAP2 protein in retinoblastoma cell lines. Surprisingly, TFAP2A protein was completely absent in 9 of the 10 lines examined despite the elevated transcript levels in some of these lines (Fig. 2C). Low levels of TFAP2A protein were detected in RB(E)3. As expected based on RNA analysis, there was no TFAP2B protein in any of the retinoblastoma cell lines tested. The presence of TFAP2A RNA in retinoblastoma cell lines suggests that these tumors originate from precursor cells committed to the amacrine lineage. However, the absence of TFAP2A protein and TFAP2B RNA/protein indicates selection against the expression of TFAP2 proteins in retinoblastoma cells.
Absence of TFAP2A in retinoblastoma cell lines could be due to increased protein degradation. We therefore treated two retinoblastoma cell lines, RB522A and WERI-Rb1, with the proteasome inhibitor MG-132 for 8 or 24 hr. Although significant increases in the levels of β-catenin were observed in both RB522A and WERI-Rb1 upon MG-132 treatment (Supporting Information Fig. S2) (Silva et al., 2010), TFAP2A could not be detected in either control or MG-132-treated cells (data not shown). Similarly, absence of TFAP2B transcripts in retinoblastoma cells could be a consequence of silencing of the TFAP2B gene by methylation. However, treatment of RB522A and WERI-Rb1 with 5-azacytidine, an inhibitor of DNA methyltransferases known to induce demethylation and reactivate silenced genes, did not lead to TFAP2B expression (data not shown).
To further address the expression of amacrine cell-specific markers in retinoblastoma cell lines, we examined whether syntaxin, specifically found in the amacrine cells of the retina, was expressed in retinoblastoma cell lines. As shown in Supporting Information Figure S3, six of the nine retinoblastoma cell lines tested expressed syntaxin.
Expression of TFAP2 in Mature Retina and Retinoblastoma Tumor Tissue
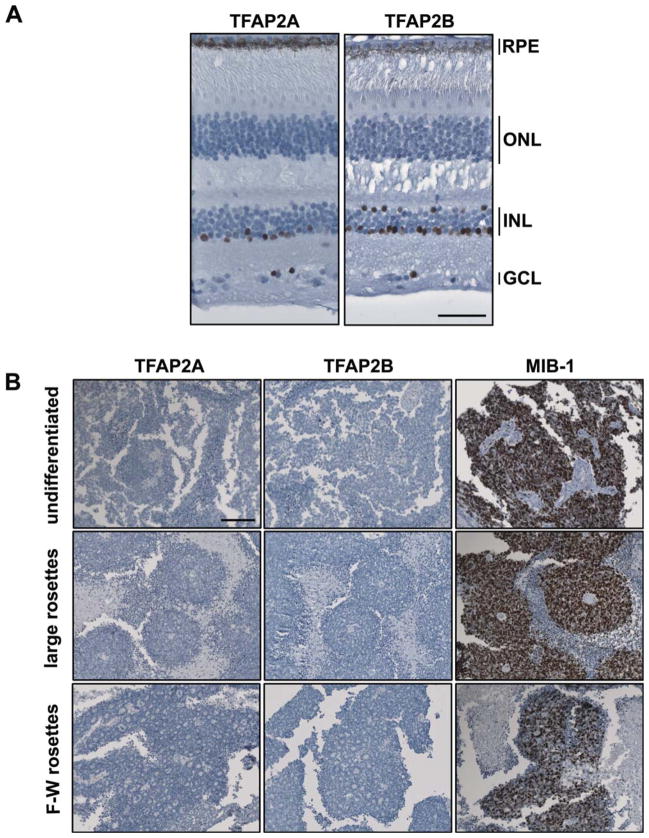
Next, we examined TFAP2A and TFAP2B expression in the noninvolved region of the eye of a 4-yr-old child with retinoblastoma. Tissue sections were immunostained with anti-TFAP2A and TFAP2B antibodies and counterstained with hematoxylin. In agreement with Figure 1B, TFAP2A was found in the inner part of the inner nuclear layer where amacrine cells reside as well as in displaced amacrine cells located in the ganglion cell layer (Fig. 3A). TFAP2B was detected in the inner part of the inner nuclear layer, in displaced amacrine cells, as well as in horizontal cells.
Figure 3.
Immunohistochemical analysis of TFAP2A and TFAP2B in mature human retina and retinoblastoma tumor tissues. A: Tissue sections from a formalin-fixed paraffin-embedded eye from a 4-year old child with retinoblastoma (patient No. 35917) were immuno-stained with anti-TFAP2A or anti-TFAP2B antibodies. Signals were detected using DakoCytomationEnvision+ system and counterstained with hematoxylin. The retinal tissue sections shown are from unaffected regions of the eye. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar = 50 μm. B: Tissue sections from undifferentiated regions of a retinoblastoma tumor (patient No. 48292), differentiated regions of retinoblastoma tumors containing large rosettes (patient No. 45376), or Flexner–Wintersteiner (F–W) rosettes (patient No. 45569), were immuno-stained with anti-TFAP2A, anti-TFAP2B or MIB-1 antibodies. The sections were counterstained with hematoxylin to label the nuclei. Photographs were taken with a 20× lens using a Zeiss Axioskop 2 plus microscope. Scale bar = 100 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The absence of TFAP2A and TFAP2B protein in retinoblastoma cell lines could be explained by loss of differentiated properties in cultured cells. We therefore immunostained 10 retinoblastoma tumor biopsies with anti-TFAP2A or anti-TFAP2B antibodies. As shown in Figure 3B, retinoblastoma tumors were completely devoid of TFAP2A and TFAP2B, in undifferentiated regions, as well as in the more differentiated regions characterized by large rosettes or Flexner–Wintersteiner rosettes. In contrast, the proliferation marker Ki-67 (MIB-1) was abundantly expressed in retinoblastoma tumor cells.
TFAP2 Induces Apoptosis in Retinoblastoma Cells
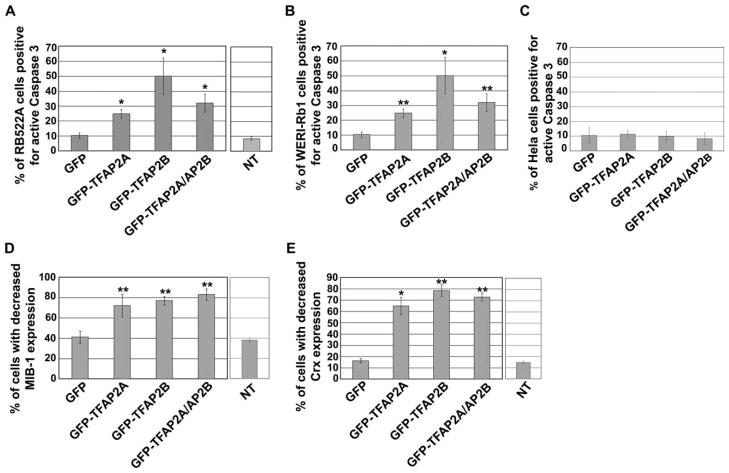
Our attempts to generate retinoblastoma cells stably transfected with TFAP2A and TFAP2B expression constructs have been unsuccessful. We therefore carried out transient transfection of retinoblastoma cells using expression constructs containing GFP-tagged TFAP2A and TFAP2B cDNA under the control of the CMV promoter. The retinoblastoma cell-line RB522A was initially used for these experiments. Over the course of 3 days after transfection, we observed progressively lower number of GFP-positive cells in our GFP-TFAP2 transfectants. No morphological alterations were observed in the GFP-TFAP2-positive cells. To determine whether the cells were undergoing apoptosis, we immunostained RB522A cells transfected with GFP, GFP-TFAP2A, GFP-TFAP2B, or combined GFP-TFAP2A/GFP-TFAP2B expression constructs, with anti-Caspase 3 antibody. This antibody specifically recognizes the cleaved (or active) form of Caspase 3, an early mediator of apoptosis. Approximately 10% of GFP-positive cells in GFP-transfectants was positive for Caspase 3 (Fig. 4A and Supporting Information Fig. S4), similar to the number observed in untransfected RB522A cells. Expression of GFP-TFAP2A in RB522A cells resulted in a significant increase in apoptotic cells, with 25% of GFP-TFAP2A-positive cells expressing the active form of Caspase 3. The percentage of apoptotic cells was even higher in GFP-TFAP2B-positive cells, with 50% of these cells expressing the active form of Caspase 3. No further increase in apoptotic cells was observed in the double GFP-TFAP2A/GFP-TFAP2B transfectants. These results, combined with the reduced number of GFP-positive cells over time in GFP-TFAP2 transfectants, indicate that a significant number of retinoblastoma cells undergo apoptosis when transfected with a GFP-TFAP2 expression construct.
Figure 4.
TFAP2A and TFAP2B induce cell death, inhibit cell proliferation, and reduce CRX expression in retinoblastoma cells. The retinoblastoma cell lines RB522A (A) and WERI-Rb1 (B), and HeLa cells (C), were transfected with pEGFP-C1, pEGFP-C1-TFAP2A, pEGFP-C1-TFAP2B, or both pEGFP-C1-TFAP2A and pEGFP-C1-TFAP2B. A: Percentages of transfected RB522A cells positive for active Caspase 3. A total of 418, 207, 431, and 299 GFP-positive cells (obtained from four independent experiments) were analyzed for GFP, GFP-TFAP2A, GFP-TFAP2B, and GFP-TFAP2A/AP2B-transfected cells, respectively. For comparison, the percentage of active Caspase 3-positive cells in nontransfected (NT) RB522A cells is indicated in the column on the right. B: Percentages of transfected WERI-Rb1 cells positive for active Caspase 3. A total of 413, 403, 446, and 519 GFP-positive cells (from four independent experiments) were analyzed for GFP, GFP-TFAP2A, GFP-TFAP2B, and GFP-TFAP2A/AP2B-transfected cells, respectively. C: Percentages of transfected HeLa cells positive for active Caspase 3. Percentages were derived from at least four independent experiments. A total of 260, 235, 295, and 211 GFP-positive cells were analyzed for GFP, GFP-TFAP2A, GFP-TFAP2B, and GFP-TFAP2A/AP2B transfected cells, respectively. D: Percentages of transfected RB522A cells with reduced MIB-1 expression. The Ki-67 proliferation marker was visualized by staining with MIB-1 antibody. A total of 484, 536, 529, and 436 GFP-positive cells (from four independent experiments) were analyzed for GFP, GFP-TFAP2A, GFP-TFAP2B, and GFP-TFAP2A/AP2B-transfected cells, respectively. Cells were considered to have a reduced MIB-1 signal when the signal intensity was decreased by at least five-fold as measured by Metamorph software. E: Percentages of transfected RB522A cells with reduced CRX expression. Cells were immunostained with anti-CRX antibody. A total of 419, 206, 429, and 300 GFP-positive cells (from four independent experiments) were analyzed for GFP, GFP-TFAP2A, GFP-TFAP2B, and GFP-TFAP2A/AP2B-transfected cells, respectively. Cells were considered to have reduced CRX expression if the signal intensity was decreased by at least five-fold as measured using Metamorph software. The asterisks indicate that the data are significantly different (single asterisk, P < 0.05; double asterisks, P < 0.01) compared to GFP-transfected cells.
To ensure that TFAP2-induced apoptosis was not specific to RB522A, we transfected WERI-Rb1 with GFP and GFP-TFAP2 expression constructs. As shown in Figure 4B, ~10% of GFP-expressing cells was positive for Caspase 3. Expression of GFP-TFAP2 in WERI-Rb1 resulted in a significant increase in apoptotic cells, with 32% of GFP-TFAP2A-expressing cells positive for Caspase 3 and 43% of GFP-TFAP2B-expressing cells positive for Caspase 3. These results indicate that TFAP2-induced apoptosis is not specific to RB522A. We also transfected HeLa cells with GFP, GFP-TFAP2A, GFP-TFAP2B, or combined GFP-TFAP2A/GFP-TFAP2B expression construct. Expression of GFP-TFAP2A and GFP-TFAP2B in HeLa cells did not affect the number of apoptotic cells, with 8–12% of GFP-positive cells staining positive for active Caspase 3, regardless of expression construct used (Fig. 4C). These data indicate that GFP-TFAP2 expression is not generally toxic to cells.
As a complementary approach, we examined whether proliferation was affected in retinoblastoma cells expressing TFAP2. There was significant variation in MIB-1 intensity in RB522A cells transfected with the GFP expression construct as well as in untransfected RB522A cells, with 40% of cells showing reduced staining (Fig. 4D and Supporting Information Fig. S5). This difference in MIB-1 intensity likely reflects cell-cycle variation. Importantly, the percentage of cells with reduced MIB-1 reactivity increased to 70–80% upon GFP-TFAP2 expression. Furthermore, the relative decrease in MIB-1 signal intensity was much more dramatic in GFP-TFAP2 transfectants compared to GFP transfectants or untransfected controls. Thus, expression of TFAP2A and TFAP2B in retinoblastoma cells significantly inhibits their proliferation.
TFAP2 Reduces the Levels of Differentiation Marker CRX in Retinoblastoma Cells
CRX is a cone-rod homeobox transcription factor expressed in the photoreceptor and bipolar lineages in human retina (Glubrecht et al., 2009). CRX is widely distributed in retinoblastoma cell lines and is found throughout tumor biopsies (Glubrecht et al., 2009; Xu et al., 2009). Expression of TFAP2A and TFAP2B in RB522A cells had a major impact on CRX expression (Fig. 4E and Supporting Information Fig. S6), with 65–80% of GFP-TFAP2A- and/or GFP-TFAP2B-positive retinoblastoma cells showing significantly reduced levels of CRX. In comparison, only 15% of untransfected RB522A cells and 18% of cells transfected with the GFP expression construct had reduced levels of CRX. As the percentage of CRX-reduced cells in GFP-TFAP2 transfectants is higher than the percentage of active Caspase 3-positive cells, these results suggest that not only does TFAP2 expression induce apoptosis, but it may also affect the differentiation state of retinoblastoma cells.
DISCUSSION
In contrast to other cancers, inactivation of both copies of the RB1 gene is the underlying cause of human retinoblastoma tumor formation, suggesting that the retinoblastoma cell-of-origin is particularly susceptible to loss of pRB function. In mice, retinoblastoma-like tumors form only when both the Rb1 gene and another member of the Rb gene family are inactivated (Chen et al., 2004; MacPherson et al., 2004). We propose that the unique susceptibility of human retinal cells to RB1 gene inactivation is a consequence of a dual role played by pRB in the developing retina; that is, control of cell proliferation and control of cell differentiation.
Over the years, there have been numerous investigations addressing the cellular origin of human retinoblastoma. Evidence has been presented in support of a neuroectodermal, neuronal, or photoreceptor cell-of-origin (Ts’o et al., 1970; Kyritsis et al., 1984; Boatright et al., 1997; Li et al., 2003). In support of the latter, three recent reports indicate that the photoreceptor marker CRX is highly expressed and widely distributed throughout retinoblastoma tumors and cell lines (Glubrecht et al., 2009; Santagata et al., 2009; Xu et al., 2009). Inconsistent with a photoreceptor cell-of-origin, examination of early-stage retinoblastoma foci suggests that tumors originate from the inner nuclear layer of the retina rather than from the outer nuclear (photoreceptor) layer (Gallie et al., 1999). Furthermore, murine retinoblastoma-like tumors appear to originate from death-resistant retinal cells such as amacrine, horizontal, and Müller glial cells (Chen et al., 2004; MacPherson et al., 2004).
Attempts to elucidate the cell-of-origin of retinoblastoma based on expression of differentiation markers are confounded by the natural plasticity of retinal cells, which allows them to differentiate along a different lineage when prevented from differentiating along the correct lineage. For example, cells committed to the photoreceptor lineage gain properties of amacrine cells in the absence of OTX2, a transcription factor that activates CRX (Nishida et al., 2003). Moreover, mis-expression of CRX in mouse retina results in an increased number of photoreceptor cells and a decreased number of amacrine cells (Furukawa et al., 1997). As an additional confounding factor, retinal progenitor cells (and other ocular progenitor cells) appear to have a propensity to differentiate along the photoreceptor lineage as a default mechanism (Haruta et al., 2001; Hatakeyama and Kageyama, 2004; Poche and Reese, 2009).
In this study, we demonstrate that TFAP2A and TFAP2B are specifically expressed in amacrine (both TFAP2A and TFAP2B) and/or horizontal (TFAP2B) cells in human retina. As TFAP2A and TFAP2B are first detected in the migrating cells of the retina, this suggests a role for TFAP2 in the commitment of progenitor cells to the amacrine and horizontal cell lineages. Importantly, TFAP2A RNA, but not TFAP2A protein, is widely expressed in retinoblastoma cell lines and tumors. The presence of TFAP2A RNA in these tumor cells indicates that the regulatory machinery required for TFAP2A transcription is intact in retinoblastoma cells, suggesting a link between retinoblastoma cells and the amacrine lineage. Alternatively, retinoblastomas may be derived from nonamacrine cells that express TFAP2A RNA but not TFAP2A protein. Although we cannot categorically eliminate the latter possibility, we have found no evidence for distinct populations of cells expressing TFAP2A RNA versus TFAP2A protein in human retina based on in situ hybridization and immunostaining analysis. Furthermore, the majority of retinoblastoma cell lines tested also express the amacrine cell marker syntaxin. Yet another possibility is that retinoblastomas are derived from retinal cells that aberrantly express markers of different lineages as a consequence of the tumorigenic process.
The retinoblastoma cell lines and tumors examined in this study express both amacrine/horizontal cell markers (TFAP2A RNA and syntaxin protein) and photoreceptor/bipolar cell markers (CRX and OTX2 RNA and protein) (Glubrecht et al., 2009). It is noteworthy that the expression of CRX (normally found in proliferating retinal cells as well as bipolar and photoreceptor cells) and OTX2 (found in bipolar and photoreceptor cells) is entirely compatible with retinoblastoma tumor growth. In contrast, retinoblastoma cells express neither the TFAP2A protein nor the TFAP2B protein, suggesting either an incompatibility with or a selection against TFAP2 activity in these tumor cells. Intriguingly, CRX and OTX2 expression is induced in Müller glial cells of morphologically normal retinas obtained from patients with retinoblastoma, suggesting some plasticity in the regulation of these two genes (Glubrecht et al., 2009).
pRB has long been known to play a critical role in cell-cycle progression by binding to the S-phase transcription factor E2F. There is also strong evidence supporting a role for pRB in cell differentiation. For example, pRB regulates the activity of transcription factors MyoD and C/EBP, which play important roles in muscle cell differentiation and adipocyte differentiation, respectively (Gu et al., 1993; Chen et al., 1996). We propose that pRB plays a similar role in amacrine cell differentiation, through the TFAP2 transcription factors. In support of this hypothesis, TFAP2A has already been shown to physically interact with pRB in vitro and associates with pRB in vivo (Wu and Lee, 1998). Furthermore, pRB regulates the transcription of E-cadherin and BCL2 in epithelial cells through interaction with TFAP2 (Batsche et al., 1998; Decary et al., 2002).
Absence of TFAP2A protein in retinoblastoma cells suggests that the TFAP2A protein is either not produced or is highly unstable in the absence of its pRB binding partner. Although treatment of retinoblastoma cells with the proteasome inhibitor MG-132 did not result in detectable TFAP2A protein, it is still possible that the TFAP2A protein is highly unstable in these cells. In turn, the complete absence of both TFAP2B RNA and TFAP2B protein in retinoblastoma cells suggests a hierarchical relationship between the TFAP2A and TFAP2B, with perhaps TFAP2A required for TFAP2B transcription.
Introduction of TFAP2A and TFAP2B into retinoblastoma cells induces apoptosis, inhibits cell proliferation, and reduces CRX expression, indicating that the reactivation of a functional TFAP2 pathway in retinoblastoma cells is not compatible with tumor cell growth and may affect the differentiation state of the tumor cells. We propose that expression of at least one of the TFAP2A and TFAP2B transcription factors in the developing retina is required for commitment and/or differentiation along the amacrine and horizontal lineages. Absence or reduction of both these transcription factors prevents amacrine and horizontal cell differentiation. As cone photoreceptors, amacrine, and horizontal cells are born around the same time in the developing retina (Cepko et al., 1996), inability to differentiate into amacrine or horizontal cells may result in activation of genes (such as CRX) associated with differentiation along a parallel lineage.
Karyotypic analysis of retinoblastoma tumors has revealed a number of common chromosome abnormalities, with the most distinctive and frequent abnormalities involving extra copies of chromosome arm 6p, usually in the form of an isochromosome 6p (Corson and Gallie, 2007). To date, no gene on 6p has been directly implicated in retinoblastoma tumor formation or progression. It is noteworthy that both the TFAP2A and TFAP2B genes are located on 6p. Given our finding that TFAP2A and TFAP2B protein are not expressed in retinoblastoma cells, it seems unlikely that having extra copies of the TFAP2 genes could provide a selective advantage to the tumor itself. However, extra copies of the TFAP2A and TFAP2B genes may provide a benefit, albeit a temporary one, to the developing retina in the context of precancerous RB+/− or RB−/− cells.
In summary, we report that both TFAP2A and TFAP2B are expressed in the amacrine cells of the developing human fetal retina and mature retina, with TFAP2B also observed in horizontal cells. TFAP2A RNA is expressed in retinoblastoma cell lines and tumors; however, neither the TFAP2A protein nor the TFAP2B RNA/protein is detected in retinoblastoma cells. We propose that TFAP2A and/or TFAP2B are required for differentiation of retinal cells along the amacrine and horizontal lineages and that the TFAP2 pathway is disrupted in RB1−/− retinal cells resulting in uncontrolled proliferation accompanied by the expression of default lineage genes such as photoreceptor marker CRX. Reactivation of the TFAP2A/B pathway in retinoblastoma cells induces apoptosis, decreases cell proliferation, and inhibits the expression of photoreceptor marker CRX.
Supplementary Material
Acknowledgments
Supported by: Canadian Institutes of Health Research and Alberta Cancer Foundation.
We thank Dr. Brenda Gallie for the retinoblastoma cell lines, Dr. Manijeh Pasdar for the β-catenin antibody, and Dr. Laurie Russell and Faye Chambers for their help in collecting the paraffin-embedded retinoblastoma tumor tissue.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2γ is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Pontoriero GF, Feng W, Marquardt T, Fini ME, Williams T, West-Mays JA. Conditional deletion of activating protein 2 alpha (AP-2α) in the developing retina demonstrates non-cell-autonomous roles for AP-2α in optic cup development. Mol Cell Biol. 2007;27:7497–7510. doi: 10.1128/MCB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol. 1998;18:3647–3658. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove DA, Godbout R. Differential expression of AP-2α and AP-2β in the developing chick retina: Repression of R-FABP promoter activity by AP-2. Dev Dyn. 1999;214:195–206. doi: 10.1002/(SICI)1097-0177(199903)214:3<195::AID-AJA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Borst DE, Peoples JW, Bruno J, Edwards CL, Si JS, Nickerson JM. A major cis activator of the IRBP gene contains CRX-binding and Ret-1/PCE-I elements. Mol Vis. 1997;3:15. [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Livne-Bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Chen PL, Riley DJ, Chen Y, Lee WH. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- Cheng C, Ying K, Xu M, Zhao W, Zhou Z, Huang Y, Wang W, Xu J, Zeng L, Xie Y, Mao Y. Cloning and characterization of a novel human transcription factor AP-2 β like gene (TFAP2BL1) Int J Biochem Cell Biol. 2002;34:78–86. doi: 10.1016/s1357-2725(01)00098-x. [DOI] [PubMed] [Google Scholar]
- Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46:617–634. doi: 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- Decary S, Decesse JT, Ogryzko V, Reed JC, Naguibneva I, Harel-Bellan A, Cremisi CE. The retinoblastoma protein binds the promoter of the survival gene bcl-2 and regulates its transcription in epithelial cells through transcription factor AP-2. Mol Cell Biol. 2002;22:7877–7888. doi: 10.1128/MCB.22.22.7877-7888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005;5:91–101. doi: 10.1038/nrc1545. [DOI] [PubMed] [Google Scholar]
- Feng W, Simoes-de-Souza F, Finger TE, Restrepo D, Williams T. Disorganized olfactory bulb lamination in mice deficient for transcription factor AP-2ε. Mol Cell Neurosci. 2009;42:161–171. doi: 10.1016/j.mcn.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Gallie BL, Holmes W, Phillips RA. Reproducible growth in tissue culture of retinoblastoma tumor specimens. Cancer Res. 1982;42:301–305. [PubMed] [Google Scholar]
- Gallie BL, Campbell C, Devlin H, Duckett A, Squire JA. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 1999;59:1731s–1735s. [PubMed] [Google Scholar]
- Gaynor RB, Muchardt C, Xia YR, Klisak I, Mohandas T, Sparkes RS, Lusis AJ. Localization of the gene for the DNA-binding protein AP-2 to human chromosome 6p22.3-pter. Genomics. 1991;10:1100–1102. doi: 10.1016/0888-7543(91)90209-w. [DOI] [PubMed] [Google Scholar]
- Glubrecht DD, Kim JH, Russell L, Bamforth JS, Godbout R. Differential CRX and OTX2 expression in human retina and retinoblastoma. J Neurochem. 2009;111:250–263. doi: 10.1111/j.1471-4159.2009.06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Haruta M, Kosaka M, Kanegae Y, Saito I, Inoue T, Kageyama R, Nishida A, Honda Y, Takahashi M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4:1163–1164. doi: 10.1038/nn762. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Lardaro T, Oh MS, Huh Y, Ding Y, Kang UJ, Kirfel J, Buettner R, Kim KS. Regulation of the noradrenaline neurotransmitter phenotype by the transcription factor AP-2β. J Biol Chem. 2008;283:16860–16867. doi: 10.1074/jbc.M709106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis AP, Tsokos M, Triche TJ, Chader GJ. Retinoblastoma—Origin from a primitive neuroectodermal cell? Nature. 1984;307:471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Li A, Zhu X, Brown B, Craft CM. Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2003;44:996–1007. doi: 10.1167/iovs.02-0434. [DOI] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Mita R, Godbout R. Expression of AP-2δ in the developing chick retina. Dev Dyn. 2008;237:3210–3221. doi: 10.1002/dvdy.21744. [DOI] [PubMed] [Google Scholar]
- MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, Fässler R. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2β. Genes Dev. 1997;11:1938–1948. doi: 10.1101/gad.11.15.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Poche RA, Reese BE. Retinal horizontal cells: Challenging paradigms of neural development and cancer biology. Development. 2009;136:2141–2151. doi: 10.1242/dev.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Santagata S, Maire CL, Idbaih A, Geffers L, Correll M, Holton K, Quackenbush J, Ligon KL. CRX is a diagnostic marker of retinal and pineal lineage tumors. PLoS One. 2009;4:e7932. doi: 10.1371/journal.pone.0007932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Silva AK, Yi H, Hayes SH, Seigel GM, Hackam AS. Lithium chloride regulates the proliferation of stem-like cells in retinoblastoma cell lines: A potential role for the canonical Wnt signaling pathway. Mol Vis. 2010;16:36–45. [PMC free article] [PubMed] [Google Scholar]
- Ts’o MO, Fine BS, Zimmerman LE. The nature of retinoblastoma. II. Photoreceptor differentiation: An electron microscopic study. Am J Ophthalmol. 1970;69:350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2 γ essential for early murine development. Mol Cell Biol. 2002;22:3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2α transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Williamson JA, Bosher JM, Skinner A, Sheer D, Williams T, Hurst HC. Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics. 1996;35:262–264. doi: 10.1006/geno.1996.0351. [DOI] [PubMed] [Google Scholar]
- Wu F, Lee AS. Identification of AP-2 as an interactive target of Rb and a regulator of the G1/S control element of the hamster histone H3.2 promoter. Nucleic Acids Res. 1998;26:4837–4845. doi: 10.1093/nar/26.21.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D, Liu A, Jhanwar SC, Abramson DH, Cobrinik D. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell. 2009;137:1018–1031. doi: 10.1016/j.cell.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.