Abstract
The histogenesis of retinoblastoma tumors remains controversial, with the cell-of-origin variably proposed to be an uncommitted retinal progenitor cell, a bipotent committed cell, or a cell committed to a specific lineage. Here, we examine the expression of two members of the orthodenticle family implicated in photoreceptor and bipolar cell differentiation, cone-rod homeobox, CRX, and orthodenticle homeobox 2, OTX2, in normal human retina, retinoblastoma cell lines and retinoblastoma tumors. We show that CRX and OTX2 have distinct expression profiles in the developing human retina, with CRX first expressed in proliferating cells and cells committed to the bipolar lineage, and OTX2 first appearing in the photoreceptor lineage. In the mature retina, CRX levels are highest in photoreceptor cells whereas OTX2 is preferentially found in bipolar cells and in the retinal pigmented epithelium. Both CRX and OTX2 are widely expressed in retinoblastoma cell lines and in retinoblastoma tumors, although CRX is more abundant than OTX2 in the differentiated elements of retinoblastoma tumors such as large rosettes, Flexner-Wintersteiner rosettes and fleurettes. Widespread expression of CRX and OTX2 in retinoblastoma tumors and cell lines suggests a close link between the cell-of-origin of retinoblastoma tumors and cells expressing CRX and OTX2.
Keywords: bipolar, cone-rod homeobox, orthodenticle homeobox 2, photoreceptor, proliferation, retina, retinoblastoma
The earliest morphological events associated with eye development are the formation of the optic pit and optic vesicles from the walls of the neural tube destined to become the forebrain. Invagination of the optic vesicles results in the formation of the double-walled optic cup. The outer layer of the optic cup differentiates into the pigment epithelium, the iris and the ciliary body epithelium, whereas the thicker inner layer forms the neural retina. Early in development, the retina consists of neuroectodermal precursor cells that have the potential of differentiating into six major classes of neuronal cells (ganglion, amacrine, bipolar, horizontal, cone photoreceptors, rod photoreceptors) as well as Müller glial cells. The first cells to differentiate in the vertebrate retina are the ganglion cells, closely followed by amacrine cells, photoreceptors and horizontal cells, then bipolar cells and Müller glia (Prada et al. 1991; Cepko et al. 1996). The orderly appearance and differentiation of the different classes of cells in the developing retina results in the formation of three nuclear layers (ganglion, inner nuclear, outer nuclear) separated by two synaptic layers (inner and outer plexiform). Ganglion cells and photoreceptors are found in the ganglion cell layer and outer nuclear layer, respectively, with the four remaining cell types (horizontal, bipolar, Müller and amacrine) forming the inner nuclear layer.
Important insights into the molecular mechanisms underlying retinal differentiation have been gained through analysis of retina-specific or retina-enriched transcription factors. For example, the homeobox gene, Otx2, has been shown to be critical for photoreceptor cell determination in mice. Misexpression of Otx2 in retinal progenitor cells promotes a photoreceptor cell fate, whereas photoreceptors of Otx2−/− mice gain properties of amacrine cells (Nishida et al. 2003). Tissue-specific ablation of Otx2 in mice suggests a role in both photoreceptor and bipolar maturation (Koike et al. 2007). Otx2 is believed to transactivate another homeobox gene, Crx (cone-rod homeobox), which is required for the terminal differentiation and maintenance of photoreceptors (Furukawa et al. 1997; Nishida et al. 2003). Like Otx2, Crx contains a paired-like homeodomain located near its N-terminus (Chen et al. 1997). Crx has been proposed to be a master regulator of photoreceptor gene transcription because transfection of Crx into iris-derived cells results in a photoreceptor-like phenotype and induction of photoreceptor genes such as rhodopsin, recoverin, inter-photoreceptor retinoid-binding protein (IRBP) and arrestin (Haruta et al. 2001; Akagi et al. 2005). Moreover, morphogenesis of rod photoreceptor outer segments is blocked at the elongation stage in Crx−/− mice, (Furukawa et al. 1999; Morrow et al. 2005). Conversely, over-expression of Crx in mouse retina results in an increased number of clones that contain exclusively rod photoreceptors, and a reduced frequency of clones containing amacrine and Müller glial cells (Furukawa et al. 1997). In humans, mutations in CRX are associated with retinal degenerative diseases including cone-rod dystrophies and Leber congenital amaurosis (Freund et al. 1997; Swaroop et al. 1999), whereas mutations and deletions in OTX2 result in severe ocular malformations (Wyatt et al. 2008).
Complete inactivation of the retinoblastoma gene (RB1) in human retinal cells underlies retinoblastoma tumor formation. Although well-understood from a molecular stand-point, there is still controversy regarding the cell-of-origin of retinoblastoma. Retinoblastoma tumors have been postulated to originate in the inner nuclear layer where amacrine, bipolar, horizontal and Müller glial cell bodies are located, although the tumors often demonstrate morphological features more typically associated with photoreceptor cells (e.g. formation of fleurettes and Flexner-Wintersteiner rosettes) (Ts’o et al. 1970; Gallie et al. 1999). In contrast to human retina, loss of retinoblastoma protein function in mouse retina does not lead to retinoblastoma tumor formation; however, murine retinal cells deficient in both pRb and the related protein p107 can develop retinoblastoma as a result of impaired exit of retinal precursors from the cell cycle (Chen et al. 2004). Three death-resistant cell lineages have been identified in mouse retina: amacrine, horizontal and Müller glia, leading to the hypothesis that retinoblastoma tumors arise from death-resistant precursor cells that escape cell differentiation (Chen et al. 2004). Cone-rod homeobox (CRX) expression has been described in two well-established retinoblastoma cell lines, Y79 and WERI-Rb1 (Boatright et al. 1997; Kobayashi et al. 1999; Li et al. 2003) as well as in retinoblastoma tumors (Xu et al. 2009), suggesting that retinoblastoma cells may be derived from cells committed to the photoreceptor lineage or from uncommitted progenitor cells with the potential of differentiating along the photoreceptor lineage. To further address gene expression in retinoblastoma cells and the cell-of-origin of retinoblastoma, we examine the spatial and temporal distribution of CRX and orthodenticle homeobox 2 (OTX2) in the developing human retina, retinoblastoma cell lines and retinoblastoma tumors.
Materials and methods
Retinoblastoma cell lines
Y79 and WERI-Rb1 were obtained from the American Type Culture Collection. RB522A, RB778, RB893, RB898, RB1021, RB1037, RB1210, RB1224, RB1355, RB1442, RB1518 cell lines were established by Dr. Brenda Gallie, Department of Medical Genetics, University of Toronto, Canada. RB(E)-3 and RB(E)-5 were established from retinoblastoma tumor biopsies obtained from the Royal Alexandra Hospital, Edmonton, Canada. Cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal calf serum, 100 μg/mL streptomycin and 100 U/mL penicillin.
RT-PCR, northern and western blot analysis
Poly(A)+ RNA was isolated from retinoblastoma cell lines and calf retina using the hot phenol method followed by oligo(dT)-cellulose chromatography. Total RNA was isolated from human fetal retina at 10–11 weeks gestation using the hot phenol method. For RT-PCR, 1 μg poly(A)+ RNA from each cell line was reverse-transcribed using an oligo d(T) primer and Superscript reverse transcriptase (Invitrogen, Carlsbad, CA, USA). PCR amplification was carried out using 1/50 of the cDNA generated from these reactions. The following primers were used for PCR amplification of rhodopsin: 5 -ATGGTCCTAGGTGGCTTCACC-3 and 5 -CATGATGGCATGGTTCTCCCC-3 (annealing temperature 55 C; 35 cycles). As a control, we used β-actin primers 5 -CTGGCACCACACCTTCTAC-3 and 5 -CATACTCCTGCTTGCTGATC-3 (annealing temperature 55 C; 28 cycles).
For northern blot analysis, two μg poly(A)+ RNA isolated from retinoblastoma cell lines were electrophoresed in a 6% formaldehyde-1.5% agarose gel in MOPS buffer (20 mM morpholine-propanesulfonic acid, 5 mM sodium acetate, 1 mM EDTA; pH 7.0) and transferred to nitrocellulose. 32P-labelled DNA fragments used as probes were: (i) 500 bp human CRX cDNA, (ii) 500 bp human OTX2 cDNA, (iii) 450 bp human IRBP cDNA, (iv) 400 bp human phosphodiesterase beta subunit (PDEB) cDNA, (v) 450 bp human arrestin1 (S-antigen, rod arrestin, visual arrestin) cDNA, and (vi) 450 bp human cone arrestin (arrestin3, X-arrestin, ARRX) cDNA. 32P-labelled β-actin served as the positive control. Filters were washed at 60°C in 0.1× sodium chloride sodium citrate (SSC) solution, 0.1% sodium dodecyl sulfate (SDS) and visualized by autoradiography.
For western blot analysis, whole cell lysates from retinoblastoma cell lines were prepared by resuspending cells in 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 10% glycerol, 0.1% Triton X-100 and protease inhibitors (phenylmethylsulfonyl fluoride and Complete protease inhibitor cocktail from Roche Molecular Biochemicals, Indianapolis, IN, USA), followed by syringing through a 23G needle. Cell lysates (50 μg/lane) were electrophoresed through a 12% polyacrylamide-SDS gel and proteins transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were immunostained with anti-CRX antibody prepared against the C-terminal half (aa 166–285) of the protein (1 : 4000 dilution) (sc-30150, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-OTX2 antibody prepared against the C-terminal half (aa 152–286 of isoform a) of the protein (1 : 10 000) (HPA000633, Sigma, St Louis, MO, USA), and goat anti-actin antibody (1 : 300 000) (A5441, Sigma). The signal was visualized using the ECL Advance (GE Healthcare, Baie d’Urfe, Quebec, Canada) or Immobilon Western (Millipore Corporation, Bedford, MA, USA) chemiluminescence detection systems. For analysis of anti-CRX and anti-OTX2 antibody specificity, HeLa cells were transfected with pcDNA3 expression constructs containing the entire open reading frame of either human CRX or human OTX2. Cells transfected with empty vector served as the control for these experiments. Duplicate blots containing lysates from control, CRX and OTX2 transfectants were immunostained with anti-CRX (sc-30150, Santa Cruz) or anti-OTX2 (HPA000633, Sigma) antibodies. For competition experiments using bacterially-expressed recombinant protein, PCR-generated cDNAs encoding the C-terminal halves of CRX (amino acids 166–285) and OTX2 (amino acids 166–285) were introduced into the pGEX vector and glutathione S-transferase (GST)-fused recombinant proteins purified with glutathione-Sepharose 4B beads (GE Healthcare). GST-fused recombinant proteins were electrophoresed in a 12% polyacrylamide-SDS gel and immunostained with anti-CRX or anti-OTX2 antibodies incubated with the appropriate CRX or OTX2 peptides. For competition experiments using endogenous protein, lysates (50 μg per lane) prepared from WERI-Rb1 were electrophoresed in a 12% poly-acrylamide-SDS gel and immunostained with anti-CRX or anti-OTX2 antibodies incubated with the C-terminal halves of the CRX or OTX2 proteins.
Immunohistochemical and immunofluorescence analysis of tissue sections
Human fetal retinal tissue at 10–11 weeks gestation was obtained in accordance with guidelines specified by the Alberta Cancer Board Research Ethics Board and University of Alberta Health Research Ethics Board (protocol ETH-99-11-18/17561). The tissue was fixed in formalin and embedded in paraffin. Slides of human fetal retina at 7 months were purchased from Pantomics Inc. (Richmond, CA, USA). Formalin-fixed paraffin-embedded eyes with retinoblastoma tumor tissue were obtained from Dynacare Kasper Medical Laboratories (Edmonton, Alberta, Canada) following the guidelines established by the Alberta Cancer Board Research Ethics Board and Health Research Ethics Board (protocol ETH-22411). Regions of the retina distal from the tumor were considered normal. Tissues were deparaffinized in xylene, re-hydrated and microwaved for 20 min in 10 mM citrate/0.05% Tween-20 epitope retrieval buffer pH 6. For immunohistochemistry, sections were stained with rabbit anti-CRX antibody (1 : 75) (sc-30150, Santa Cruz), anti-OTX2 antibody (1 : 75) (HPA000633, Sigma), rabbit anti-CRX15pep antibody prepared using the 15 amino acid CRX peptide CTYNPHDPLDYKDQS (amino acids 279–292) (1 : 1000) obtained from Dr. Cheryl Craft (Zhu and Craft 2000), mouse anti-protein kinase C alpha (PKCα) antibody (1 : 4000) (610107, BD Transduction Laboratories, Lexington, KY, USA), rabbit anti-arrestin1 (1 : 1500) (PA1-731, Affinity Bioreagents Inc., Golden, CO, USA) and rabbit anti-cone arrestin (arrestin3) (1 : 40 000) obtained from Dr. Cheryl Craft (Li et al. 2003). The signal was detected using the Dako-Cytomation EnVision+ anti-rabbit or anti-mouse secondary system. Tissues were counter-stained with the nuclear stain hematoxylin.
For immunofluorescence analysis, sections were double-stained or triple-stained with rabbit anti-CRX antibody (1 : 75) (sc-30150, Santa Cruz), rabbit anti-OTX2 antibody (1 : 75) (HPA000633, Sigma), sheep anti-Ceh-10 homeodomain containing homolog (CHX10) antibody (1 : 3000) (obtained from Dr. Rod Bremner), mouse anti-PKCα antibody (1 : 3000) (P16520, BD Biosciences, San Jose, CA, USA), mouse anti-Ki-67 antibody (clone MIB-1) (1 : 1000) (Dako, Carpinteria, CA, USA), mouse anti-glutamine synthetase antibody (1 : 500) (G45020, BD Biosciences), followed by fluorescent dye-conjugated donkey anti-rabbit, anti-mouse or anti-sheep secondary antibodies. Sections were counter-stained with the fluorescent nuclear stain Hoescht 33342 (Invitrogen) and mounted in FluorSave reagent (Calbiochem, San Diego, CA, USA). Images were collected on a LSM510 confocal microscope (Zeiss, Jena, Germany) with a 40× lens.
Results
Expression of CRX, OTX2 and target genes in retinoblastoma cell lines
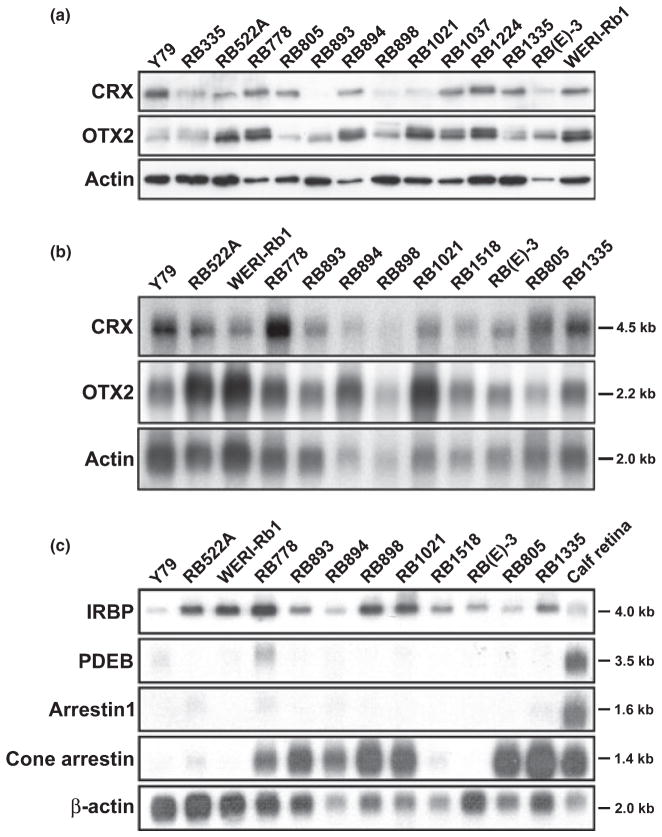
Cone-rod homeobox and/or OTX2 expression has previously been reported in the well-established retinoblastoma cell lines Y79 and WERI-Rb1 (Kobayashi et al. 1999; Boatright et al. 2001; Li et al. 2003). To further document the presence of CRX and OTX2 in retinoblastoma, we carried out western blot analysis of 14 retinoblastoma cell lines using anti-CRX and anti-OTX2 antibodies prepared against the variable C-terminal halves of the respective proteins. CRX and OTX2 were expressed in every retinoblastoma cell line examined, with anti-CRX antibody detecting a band of ~35 kDa and anti-OTX2 antibody revealing two bands migrating at 34– 36 kDa in the majority of retinoblastoma cell lines (Fig. 1a).
Fig. 1.
(a) CRX and OTX2 expression in retinoblastoma cell lines. Western blot analysis of retinoblastoma cell lines using anti-CRX and anti-OTX2 antibodies. Fifty μg of whole cell lysates were loaded in each lane and electrophoresed in a 12% polyacrylamide-SDS gel. Proteins were transferred to PVDF membranes and sequentially immunostained with rabbit anti-CRX, followed by rabbit anti-OTX2 antibody (after stripping the blot), and goat anti-actin antibody. The signal was detected using the ECL or Immobilon detection reagents. (b) Northern blot analysis of CRX and OTX2 in retinoblastoma cell lines. Poly(A)+ RNA was isolated from 12 retinoblastoma cell lines. Two μg poly(A)+ RNA were loaded in each lane. The RNA was electrophoresed in an agarose-formaldehyde gel and the RNA transferred to a nitrocellulose filter. The filter was sequentially hybridized with 32P-labeled CRX, OTX2 and β-actin cDNAs. Sizes of the transcripts are indicated on the side. (c) Northern blot analysis of CRX/OTX2 target genes in retinoblastoma cell lines. Two μg poly(A)+ RNA from each of the indicated retinoblastoma cell lines and calf retina were electrophoresed in a 1.5% agarose-formaldehyde gel and transferred to a nitrocellulose filter. The filter was sequentially hybridized with 32P-labeled IRBP, PDEB, arrestin1, cone arrestin and β-actin cDNAs. The latter served as the loading control. Sizes of the transcripts are indicated on the side.
The CRX and OTX2 genes encode proteins of 299 and 297/289 (isoforms a/b) amino acids, respectively. The CRX and OTX2 proteins are 52% identical over their entire open reading frames, with the greatest area of similarity found in the N-terminal halves of the proteins. To ensure that the anti-CRX and anti-OTX2 antibodies used in our experiments were specific to CRX and OTX2, respectively, we carried out three sets of experiments. First, we transfected full-length CRX or OTX2 expression constructs into HeLa cells and used anti-CRX and anti-OTX2 antibodies to immunostain blots containing extracts from control, CRX and OTX2 transfectants. Anti-CRX antibody specifically recognized exogenous CRX whereas anti-OTX2 antibody specifically recognized exogenous OTX2 (Fig. S1a). In addition to exogenous OTX2, anti-OTX2 antibody detected two bands of ~34–36 kDa found in all three transfectants. These two bands likely represent endogenous OTX2 as this protein is expressed in HeLa cells (http://www.proteinatlas.org). Second, we pre-incubated anti-CRX antibody or anti-OTX2 antibody with either GST-CRX or GST-OTX2 recombinant protein prior to immunostaining blots of recombinant GST-CRX or GST-OTX2 proteins. As shown in Fig. S1(b), GST-CRX, but not GST-OTX2, served as an effective competitor for anti-CRX antibody binding, whereas GST-OTX2, but not GST-CRX, effectively competed with anti-OTX2 antibody binding. Third, we pre-incubated anti-CRX antibody or anti-OTX2 antibody with either GST-CRX or GST-OTX2 recombinant protein prior to immunostaining blots of WERI-Rb1 whole cell lysates. CRX protein but not OTX2 protein resulted in a significant decrease in the staining intensity of the protein band detected with the anti-CRX antibody (Fig. S1c). Conversely, OTX2 protein but not CRX protein reduced the staining intensity of the two endogenous OTX2 bands detected with the anti-OTX2 antibody. In addition to western blot analysis, expression of CRX and OTX2 in retinoblastoma cell lines was verified by northern blot analysis (Fig. 1b) and semi-quantitative RT-PCR (data not shown).
A number of putative CRX target genes have been identified, including IRBP, PDEB, visual arrestins (arrestin1/rod arrestin and cone arrestin) and rhodopsin (Boatright et al. 1997; Chen et al. 1997; Furukawa et al. 1997; Bibb et al. 2001; Fujimaki et al. 2004). Furthermore, OTX2 has been shown to be a direct upstream regulator of CRX (Nishida et al. 2003). To determine whether CRX/OTX2 target genes are expressed in retinoblastoma cell lines, we carried out northern blot analysis of IRBP, PDEB, arrestin1, cone arrestin and rhodopsin. Seven of the 12 lines examined had elevated levels of cone arrestin, which is highly expressed in all cone photoreceptors (Craft et al. 1994; Pickrell et al. 2004) (Fig. 1c). IRBP RNA was detected in every cell line, whereas PDEB and arrestin1 transcripts were present at low levels in only a small subset of retinoblastoma cell lines. We were unable to detect rhodopsin RNA in any of the cell lines analysed by either northern blotting or RT-PCR analysis (data not shown).
Expression of CRX and OTX2 in normal human fetal retina
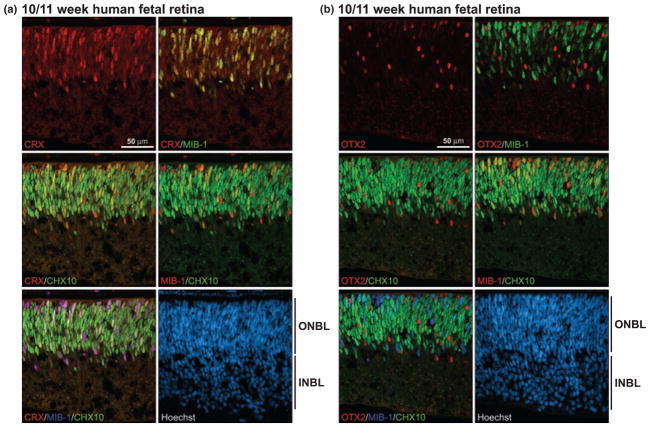
Cone-rod homeobox is classically defined as a photoreceptor-specific transcription factor, although it has also been reported to be expressed in the inner nuclear layer of mammalian and zebrafish retina (Bibb et al. 2001; Liu et al. 2001). To address CRX expression in human fetal retina, sections of a developing eye at 10/11 weeks gestation were immunostained with anti-CRX antibody. CRX-positive cells were observed in the outer neuroblastic layer of the 10/11-week fetal retina, but not in the ganglion cell layer (Fig. 2a), in agreement with O’Brien et al. (2003) who noted CRX transcripts in human fetal eyes at 10 weeks gestation.
Fig. 2.
CRX and OTX2 expression in normal human fetal retina at 10– 11 weeks gestation. Tissue sections from paraformaldehyde-fixed human fetal retina at 10/11 weeks gestation were triple-stained with either rabbit anti-CRX antibody (a) or rabbit anti-OTX2 antibody (b), mouse MIB-1 antibody and sheep anti-CHX10 antibody. The signal was detected using donkey anti-rabbit Alexa 555 secondary antibody (CRX, OTX2), donkey anti-mouse Alexa 647 secondary antibody (MIB-1) and donkey anti-sheep Alexa 488 secondary antibody (CHX10). CRX/MIB-1, CRX/CHX10 and MIB-1/CHX10 co-stained cells range from orange to yellow depending on the relative intensity of the red and green signals. INBL, inner neuroblastic layer; ONBL, outer neuroblastic layer.
Retinal cells committed to a specific lineage are non-dividing and post-mitotic (Turner and Cepko 1987). To examine the proliferative state of CRX-positive cells, retina tissue at 10/11 weeks gestation was co-immunostained with anti-CRX and MIB-1 (cell proliferation marker) antibodies. As shown in Fig. 2(a), virtually every CRX-expressing cell also expressed MIB-1. CHX10, a bipolar cell marker, was broadly expressed throughout the outer neuroblastic layer. A significant percentage of CHX10-positive cells expressed CRX, and most of these cells were also MIB-1-positive (as demonstrated in the triple-strained MIB-1/CHX10/CRX panel in Fig. 2a). As CHX10 is found in both bipolar progenitors and mature bipolar cells during retinal development (Liu et al. 1994), these data indicate that, in the human fetal retina, CRX is first expressed in proliferating cells and in emerging bipolar cells. In contrast to CRX, none of the OTX2-positive cells co-localized with MIB-1-expressing cells in the 10- to 11-week retina (Fig. 2b). OTX2 was also excluded from CHX10-positive cells indicating that OTX2 is not found in bipolar progenitors or mature bipolar cells at 10–11 weeks gestation.
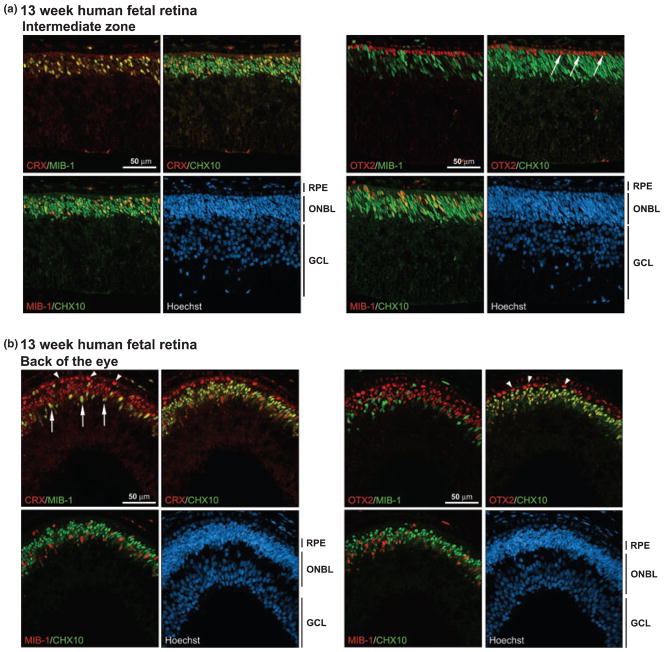
At 13 weeks gestation, well-differentiated horizontal, bipolar and amacrine cells are present in the outer neuroblastic layer which is well-demarcated from the ganglion cell layer (O’Brien et al. 2003). There is a single layer of cone photoreceptors immediately next to the retinal pigmented epithelium (RPE) at this developmental stage. We saw regional differences in the CRX and OTX2 distribution patterns at 13 weeks gestation depending on the differentiation state of the retina. Next to the ciliary epithelium, which represents the least differentiated part of the retina, CRX was expressed in MIB-1- and MIB-1/CHX10-positive cells (Fig. S2), as observed in the 10- to 11-week retina. OTX2 was found in a subset of cells located next to the retinal pigmented epithelium as well as in a few cells in the internal part of the outer neuroblastic layer (Fig. S2). In the intermediate differentiation zone (between the ciliary epithelium and the back of the eye), CRX remained positive in MIB-1- and in MIB-1/CHX10-expressing cells (Fig. 3a). In contrast, OTX2 was specifically found in a single layer of non-CHX10-expressing cells (see arrows) located immediately next to the RPE. The position of these cells indicates that they represent the single layer of photoreceptors characteristic of this developmental stage. RPE cells were also positive for OTX2. In the back of the eye, where the retina is most differentiated, CRX was expressed in a few MIB–1-positive cells mostly located in the inner part of the outer neuroblastic layer (arrows), in all CHX10-positive cells (yellow color), as well as in the layer of photoreceptor cells located next to the RPE (arrowheads) (Fig. 3b). OTX2 distribution was virtually identical to that of CRX in the back of the eye except that OTX2 was not expressed in MIB-1- positive cells.
Fig. 3.
CRX and OTX2 expression in normal human fetal retina at 13 weeks gestation. Tissue sections from paraformaldehyde-fixed human fetal retina at 13 weeks gestation were triple-stained with either rabbit anti-CRX antibody or rabbit anti-OTX2 antibody, MIB-1 antibody and sheep anti-CHX10 antibody. Two different regions of the retina are represented: (a) intermediate differentiation zone and (b) back of the eye, the most differentiated part of the retina. The signal was detected using donkey anti-rabbit Alexa 555 secondary antibody (CRX, OTX2), donkey anti-mouse Alexa 647 secondary antibody (MIB-1) or donkey anti-sheep Alexa 488 secondary antibody (CHX10). The arrows in (a) point to the single layer of OTX2-positive photoreceptor cells. The arrows in (b) point to the CRX/MIB-1-positive cells and the arrowheads indicate the CRX-positive photoreceptor cells. Note that there is a layer of OTX2-expressing RPE cells immediately next to the OTX2-positive photoreceptor cells. RPE, retinal pigmented epithelium; ONBL, outer neuroblastic layer; GCL, ganglion cell layer.
CRX and OTX2 expression in mature retina
Next, we examined CRX and OTX2 expression in the noninvolved regions of the eyes of 2- to 4-year-old children with retinoblastoma. CRX was abundantly expressed in the photoreceptors located in the outer nuclear layer with considerably lower levels in the inner nuclear layer (Fig. 4a). In contrast, OTX2 was most abundant in the inner nuclear layer, with lower levels in photoreceptors (Fig. 4b). The nuclei of RPE cells were strongly immunostained with anti- OTX2 antibody and lightly immunostained with anti-CRX antibody (Fig. S3). To ensure that the weak CRX signal observed in the nuclei of retinal pigment epithelial cells was not an artifact of melanin-producing cells, we immunostainined tissue sections with anti-PKCα, a rod bipolar cell marker (Kosaka et al. 1998). RPE nuclei were completely devoid of PKCα (Fig. S3). Thus, while OTX2 and CRX are both expressed in photoreceptors, inner nuclear layer cells and retinal pigmented epithelium, they show complementary staining patterns in these different cell lineages.
Fig. 4.
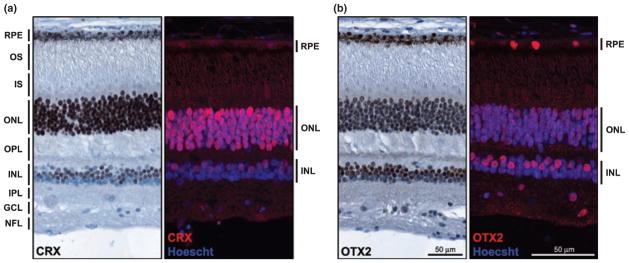
CRX and OTX2 expression in mature retina. Tissue sections from a formalin-fixed paraffin-embedded eye from a 4-year-old child with retinoblastoma (patient No. 35917) were immunostained with anti- CRX antibody (a) or anti-OTX2 antibody (b), and analyzed by immunohistochemistry (brown signal) or immunofluorescence microscopy (red signal). The sections shown are from unaffected, apparently normal retina. For immunohistochemical analysis, the tissue section was counter-stained with the nuclear stain hematoxylin (blue) and the CRX or OTX2 signal detected using the DakoCytomationEnVision+ system. For immunofluorescence analysis, the tissue section was counter-stained with the nuclear stain Hoechst 33342 (blue) and CRX- and OTX2-positive cells (red) detected using Alexa 555-conjugated donkey anti-rabbit secondary antibody. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer (photoreceptor); IS, inner segments; OS, outer segments; RPE, retinal pigmented epithelium.
CRX and OTX2 expression in the inner nuclear layer of the retina
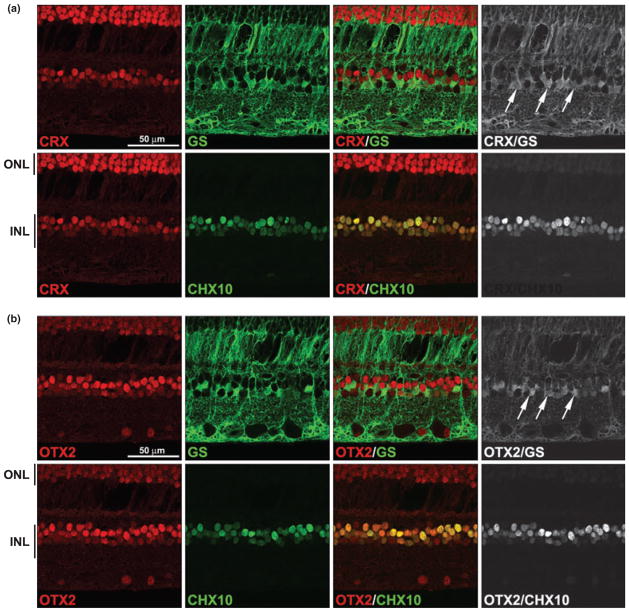
Orthodenticle homeobox 2- and CRX-positive cells are observed in the outer half of the inner nuclear layer where bipolar cells and Müller glial cells are located. To identify which cell types within the inner nuclear layer express OTX2 and CRX, we triple-immunostained retinal tissue sections with either anti-CRX or anti-OTX2 antibodies, anti-glutamine synthetase (GS) (for Müller glial cells) and anti-CHX10 (for bipolar cells) antibodies. As expected, both CRX and OTX2 were found in bipolar cells, with every cell co-stained for CRX or OTX2 and CHX10 (Fig. 5). Surprisingly, CRX, OTX2 (and by inference CHX10) were also expressed in GS-staining Müller glial cells. To more clearly demonstrate co-expression of CRX and OTX2 with GS-staining cells, only co-immunostained regions of the retina are shown in the panels on the right.
Fig. 5.
Co-immunostaining of CRX and OTX2 with markers of bipolar and Müller glial cells. A tissue section from an unaffected region of the eye of patient 35917 was triple-stained with rabbit anti-CRX antibody (a) or rabbit anti-OTX2 antibody (b), mouse anti-glutamine synthetase (GS) antibody and sheep anti-CHX10 antibody. Signals were detected with Alexa 555-conjugated donkey anti-rabbit (CRX, OTX2), Alexa 647-conjugated donkey anti-mouse (GS) and Alexa 488-conjugated donkey anti-sheep secondary antibodies (CHX10). Merging the CRX (a) or OTX2 (b) (red) and CHX10 (green) signals demonstrates their co-expression in bipolar cells (indicated by the yellow color). Merging the CRX (a) or OTX2 (b) (red) and GS (green) signals demonstrates their co-expression in Müller glial cells. Non-co-localized signals have been removed in the panels on the right in order to highlight sites of co-expression. Arrows point to CRX/GSand OTX2/GS-positive cells. INL, inner nuclear layer; ONL, outer nuclear layer (photoreceptor).
To address the possibility that expression of CRX, OTX2 and CHX10 in Müller glial cells might be a consequence of tumor formation, normal fetal retina tissue at 7 months gestation was co-immunostained with anti-CHX10 and anti-GS antibodies. As shown in Fig. 6, CHX10 and GS were expressed in distinct non-overlapping populations of cells in the inner nuclear layer. Although we were not able to compare age-matched retinas from normal children and retinoblastoma patients, the absence of CHX10 in the Müller glial cells of well-differentiated normal fetal retina suggests that expression of neuronal markers in the Müller glial cells of retinoblastoma patients may be a consequence of tumor growth.
Fig. 6.
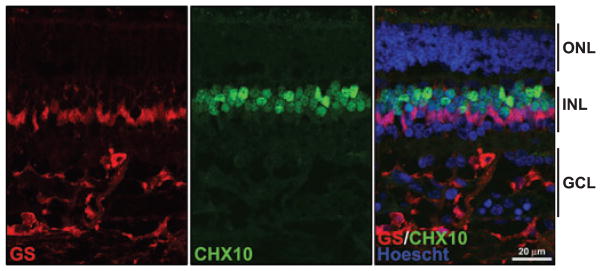
GS and CHX10 localization in normal human fetal retina at 7 months gestation. Slides containing tissue sections from normal human fetal retina at 7 months gestation were co-immunostained with anti- GS antibody (red) and CHX10 antibody (green). The merged figure demonstrates that there is little or no overlap in the staining pattern of GS and CHX10 in Müller glial cells. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
CRX and OTX2 expression in retinoblastoma tumors
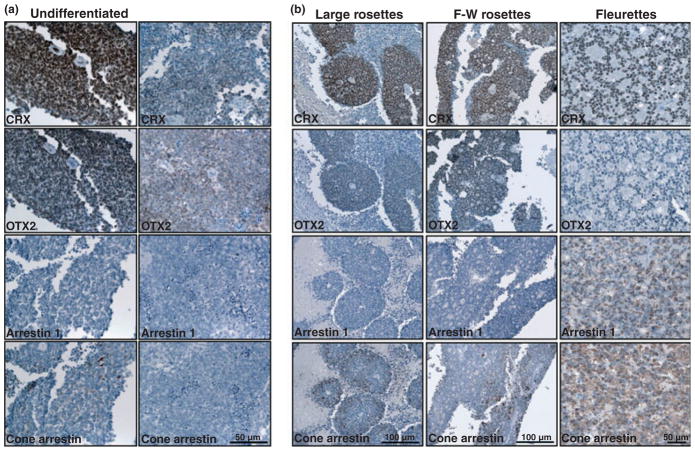
Immunostaining of ten paraffin-embedded retinoblastoma tumor biopsies with anti-CRX and anti-OTX2 antibodies revealed expression of CRX and OTX2 in all ten tumors. CRX was found throughout the tumors, whether they had an undifferentiated (Fig. 7a) or a differentiated appearance (large rosettes, Flexner-Wintersteiner rosettes, fleurettes) (Fig. 7b). OTX2 had a similar distribution pattern as CRX, except that OTX2 was expressed at lower levels than CRX in some of the large rosettes, Flexner-Wintersteiner rosettes and fleurettes (Fig. 7b). In contrast to CRX and OTX2, arrestin1, a proposed target of CRX, was expressed in only a subset of tumors and in selected regions of these tumors (Fig. 7a and b). Arrestin1 was not detected in large rosettes or in Flexner-Wintersteiner rosettes, but was abundantly expressed in fleurettes, suggesting rod photoreceptor properties for the latter. Cone arrestin-positive cells were observed in apparently undifferentiated regions of retinoblastoma tumors (Fig. 7a) as well as in some of the rosettes and fleurettes (Fig. 7b), suggesting that both the undifferentiated and the differentiated elements of retinoblastoma tumors may have cone photoreceptor properties.
Fig. 7.
Expression of CRX, OTX2, arrestin1 and cone arrestin in retinoblastoma tumors. (a) Tissue sections from two undifferentiated regions of a retinoblastoma tumor (patient 48292) were immunostained with anti-CRX antibody, anti-OTX2 antibody, anti-arrestin1 antibody and anti-cone arrestin antibody. Strong staining (brown color) is observed for both anti-CRX and OTX2 antibodies. No signal was detected using the anti-arrestin1 antibody whereas some staining was observed with anti-cone arrestin in the tumor on the left. (b) Tissue sections from differentiated regions of retinoblastoma tumors were immunostainined with anti-CRX antibody, anti-OTX2 antibody, anti-arrestin1 antibody and anti-cone arrestin antibody. Regions containing large rosettes (patient 15884) were strongly stained with anti-CRX antibody and moderately stained with anti-OTX2 and anti-cone arrestin antibody. Regions containing Flexner-Wintersteiner (F-W) rosettes (patient 45569) were strongly stained with anti-CRX antibody and moderately stained with anti-OTX2 and anti-cone arrestin antibody. Regions containing fleurettes (patient 48842) were strongly stained with anti-CRX antibody, anti-arrestin1 antibody and anti-cone arrestin antibody.
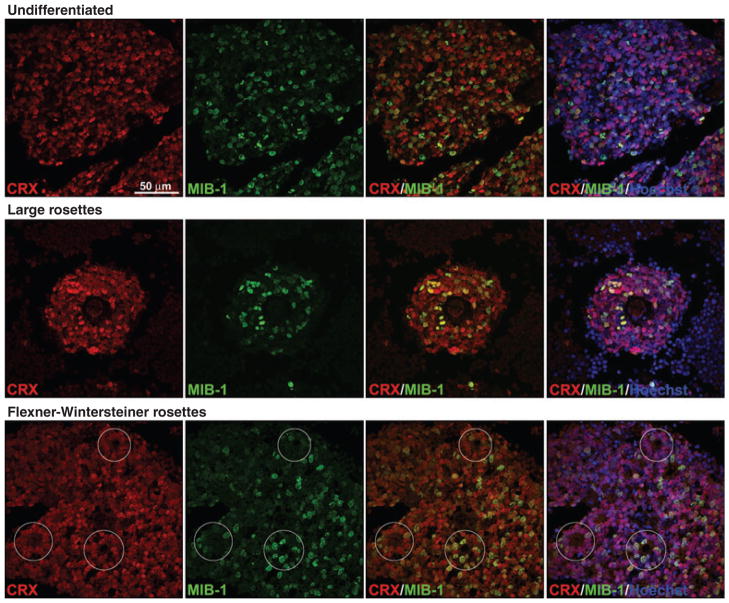
Co-immunostaining experiments revealed co-localization of the proliferation marker MIB-1 with both CRX and OTX2. CRX/MIB-1-positive and OTX2/MIB-1-positive cells were observed in undifferentiated retinoblastoma tumor tissue, in large rosettes as well as in Flexner-Wintersteiner rosettes (see Fig. 8 for CRX and Fig. S4 for OTX2). Although not every cell within a tumor expressed CRX or OTX2, these two proteins were found in a higher percentage of tumor cells than MIB-1, with virtually every MIB-1-positive cell being positive for CRX/OTX2 (indicated by the yellow color in the CRX/MIB-1 and OTX2/MIB-1 merged panels).
Fig. 8.
Co-staining of CRX and MIB-1 in retinoblastoma tumors. Tissue sections from retinoblastoma tumors (undifferentiated tumor from patient 48292 – top panels; large rosettes from patient 48292 – middle panels; Flexner-Wintersteiner rosettes from patient 45569 – bottom panels), were co-stained with anti-CRX and MIB-1 antibodies. Positive signals were detected using Alexa 488-conjugated donkey anti-rabbit (CRX) and Alexa 555-conjugated donkey anti-mouse (MIB-1) secondary antibodies. Sections were counter-stained with Hoescht 33342 to visualize nuclei and mounted with FluorSave reagent. The yellow color in CRX/MIB-1 merged panels demonstrates co-immunostaining. Although CRX-positive cells are more abundant than MIB-1-positive cells, the great majority of MIB-1-positive cells express CRX.
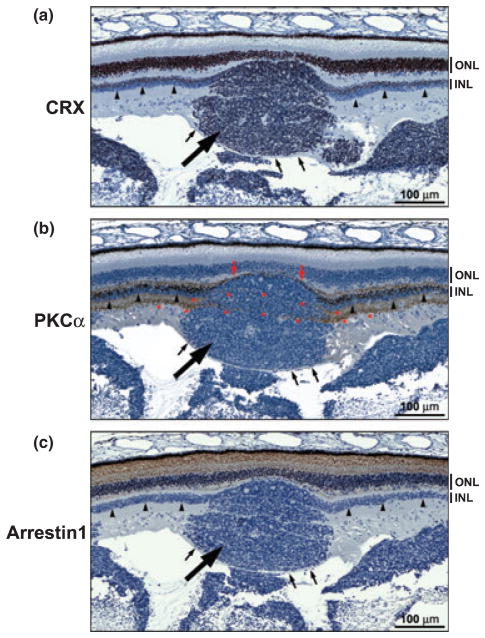
We next examined a region of the retina containing a tumor nodule (large arrow) encased by a fiber layer (thin arrows) suggesting that the tumor nodule is originating from (rather than invading or seeding) this part of the retina. CRX was widely expressed throughout the tumor nodule, with a staining intensity similar to that observed in the inner nuclear layer (Fig. 9a). In contrast to CRX, the rod bipolar marker PKCα was only observed in a thin layer of cells located between the tumor nodule and the outer nuclear layer (Fig. 9b – red arrows). None of the tumor cells appeared to be positive for PKCα. Interestingly, the PKCα signal in the inner plexiform layer on either side of the tumor was found to extend through the tumor itself (asterisks). While arrestin1 was abundantly expressed in the photoreceptor layer of the retina, the tumor nodule appeared completely devoid of this protein (Fig. 9c). The abundance of CRX throughout the tumor nodule, combined with the intensity of the CRX signal in the tumor cells, suggest a link between the inner nuclear layer and retinoblastoma.
Fig. 9.
Expression of CRX, PKCα and arrestin1 in a transitory zone between retinal tissue and tumor. Tissue sections from patient 48842 were immunostained with anti-CRX15pep (a), anti-PKCα (b), and anti-arrestin1 (c) antibodies (DakoCytomationEnVision+ system) and counter-stained with hematoxylin. The tumor nodule is indicated by the thick arrow and the inner nuclear layer of the normal retinal tissue on either side of the tumor nodule is indicated by the arrowheads. The fiber layer overlaying the tumor is indicated by the small arrows. The PKCα-positive inner plexiform layer on either side of the tumor nodule (b) appears to extend through the tumor (as shown by the asterisks). INL, inner nuclear layer; ONL, outer nuclear layer.
Discussion
The debate regarding the cell-of-origin of retinoblastoma has been ongoing for decades. Retinoblastomas were first described as gliomas of the retina (rev. in Nork et al. 1995). Differentiated aspects were noted by Flexner and Wintersteiner in the 1890s, giving rise to the term Flexner-Wintersteiner rosettes to describe the spherical layers of columnar cells often observed in retinoblastoma tumors (rev. in Tajima et al. 1994; Nork et al. 1995). Subsequent ultrastructural analyses revealed morphological features characteristic of photoreceptors (Ts’o et al. 1970). Since the 1970s, the majority of reports addressing the cell-of-origin of retinoblastoma tumors have primarily focused on whether they express markers of photoreceptors, neuronal and/or glial cells.
In humans, inactivation of the RB1 gene is sufficient to initiate the series of events required for retinoblastoma tumor formation. Although inactivation of the Rb gene by itself does not cause retinoblastoma tumors in mice, double knock-out of Rb and p107 results in the formation of sporadic retinoblastoma-like tumors (Robanus-Maandag et al. 1998). Detailed analysis of the retinas of Rb/p107 knock-out mice suggest that murine retinoblastomas are derived from cells committed to the amacrine, Müller glia or horizontal lineages (Chen et al. 2004; MacPherson et al. 2004). Mice in which both the Rb and the p130 genes are inactivated do not form retinoblastoma tumors although cell proliferation is mildly deregulated in the retina (MacPherson et al. 2004). Expression of p107 in Rb−/−;p130−/− retinal cells initially prevents ectopic proliferation of neurons in the inner nuclear layer during development. However, with time, the horizontal cells of Rb−/−; p107+/−;p130−/− mice re-enter the cell cycle and form retinoblastoma tumors (Ajioka et al. 2007), indicating that even apparently fully-differentiated retinal cells cannot be excluded as possible cells-of-origin for retinoblastoma.
Cone-rod homeobox expression has been reported as early as 10.5 weeks gestation in the developing human eye (O’Brien et al. 2003). In agreement with this, we observed sporadic CRX-positive cells in the outer neuroblastic layer of human fetal retina at 10/11 weeks gestation. The great majority of the CRX-positive cells at 10/11 weeks gestation expressed both the proliferation marker MIB-1 and the bipolar progenitor/mature bipolar marker CHX10 (Liu et al. 1994). These data are consistent with CRX being first expressed in uncommitted proliferating cells and in cells committed to the bipolar lineage. In contrast to CRX, OTX2 was first observed in the photoreceptor lineage. It is only later in development, at 13 weeks gestation, that OTX2 was detected in bipolar cells in the most differentiated region of the retina, at the back of the eye. The fact that OTX2 is expressed before CRX in the photoreceptor lineage is in keeping with a role for OTX2 in the transcriptional regulation of CRX (Furukawa et al. 1997; Nishida et al. 2003); however, it is clear that factors other than OTX2 are involved in the transcription regulation of CRX in proliferating cells and bipolar cells. The CRX and OTX2 distribution patterns were reversed in the mature retina compared to the developing retina, with CRX highly expressed in photoreceptor cells and moderately expressed in bipolar cells, whereas OTX2 was highly expressed in bipolar cells and moderately expressed in photoreceptor cells. Similar distribution patterns for CRX and OTX2 have been reported in post-natal mouse eyes (Koike et al. 2007).
Widespread and elevated expression of CRX and OTX2 was observed in all retinoblastoma cell lines and tumors analysed. CRX-positive cells were abundant in histologically undifferentiated regions of the tumors as well as in large rosettes, Flexner-Wintersteiner rosettes and fleurettes, features typically associated with tumor differentiation. OTX2-positive cells had a similar distribution as CRX except that weaker staining was observed in some of the large rosettes and Flexner-Wintersteiner rosettes. These data are compatible with the cell-of-origin of retinoblastoma being a photoreceptor precursor, a bipolar cell precursor, or a progenitor cell with the potential to differentiate along the bipolar and/or photoreceptor lineages.
Unexpectedly, we observed CRX, OTX2 and CHX10 expression in the Müller glial cells of children with retinoblastoma, but not in the Müller glial cells of a normal 7 months gestation retina. Müller glia, a type of radial glial cells, span the entire width of the retina with their nuclei located near the middle of the inner nuclear layer. During brain development, radial glia form the fiber network along which neuronal cells migrate in order to reach their final destination. Radial glia can differentiate into both glial and neuronal cells, and demonstrate properties of neural progenitor/stem cells (Goldman 2003). Like brain radial glial cells, Müller glia are involved in retinal lamination and cell arrangement (Willbold et al. 2000). There is evidence suggesting that Müller glia also have retinal progenitor or stem cell properties. For example, neuronal gene markers are induced in Müller glia after injury to adult rodent retina (Ooto et al. 2004; Das et al. 2006). In the uninjured zebrafish retina, Müller glial cells proliferate at a low rate, and Müller glia-derived progenitors, which express CRX, remain competent to regenerate missing neurons (Bernardos et al. 2007). In keeping with a plastic role for Müller glial cells, Close et al. (2005) found that the presence of older mature retinal neurons in Müller glia cultures inhibits the proliferation of Müller glia cells. Treatment of these cultures with a Transforming growth factor beta inhibitor restores Müller glia cell proliferation, suggesting that production of Transforming growth factor beta by retinal neurons maintains the mitotic quiescence of Müller glia. Although our experiments are limited by the fact that we were only able to study the eyes of 2- to 4-year-old retinoblastoma patients and normal retinal tissue at 7 months gestation, it seems unlikely that neuronal markers would be naturally induced in the Müller glial cells of normal 2- to 4-year-old retinas. Thus, we propose that insult to the retina resulting from actively growing retinoblastoma tumors may affect Müller glia gene expression.
Evidence was presented a number of years ago indicating that newly emerging retinoblastoma tumors arise from the inner nuclear layer of the retina which is composed of amacrine, Müller glial, bipolar and horizontal cells (Gallie et al. 1999). The data presented here on an apparently emerging tumor nodule support this earlier report. Particularly compelling is the observation that the intensity of the CRX signal in tumor cells is virtually identical to that seen in the inner nuclear layer and therefore considerably lower than that found in photoreceptors. Therefore, our data are most compatible with the cell-of-origin of retinoblastoma being a bipolar precursor or a Müller glial-like cell. However, we cannot discount the possibilities that retinoblastoma arises from: (i) progenitor cells that retain differentiation capabilities, (ii) committed precursors that express CRX and OTX2 as a default pathway, or (iii) committed photoreceptors that express reduced levels of CRX as a consequence of cell transformation. Of note, Xu et al. (2009) have recently shown that human retinoblastoma cells specifically express markers of post-mitotic cone precursors (e.g. CRX, RXRγ, MDM2) but not markers of other retinal cell types, and suggest a cone photoreceptor lineage for retinoblastoma cells. We suggest that the key to identifying the cell-or-origin of retinoblastoma will be in understanding the transcriptional regulation of developmental genes that are abundantly expressed in retinoblastoma tumors such as CRX and OTX2.
In summary, we report that CRX and OTX2 are differentially expressed in the developing human fetal retina, with CRX first appearing in proliferating cells and in cells committed to the bipolar lineage, and OTX2 first observed in photoreceptor cells. This pattern of expression is reversed in the mature retina, where CRX is most abundant in photoreceptors and OTX2 is primarily expressed in bipolar cells. In addition, both CRX and OTX2 are found in retinal pigmented epithelium, although OTX2 is considerably more abundant than CRX in these cells. Intriguingly, CRX and OTX2 expression is induced in the apparently normal Müller glial cells of patients with retinoblastoma. Analysis of retinoblastoma tumors and cell lines indicates that CRX and OTX2 are two of the most widely expressed differentiation markers reported in these tumors. In comparison, cone arrestin, a cone photoreceptor-specific marker, and arrestin1, a rod photoreceptor-specific marker, are found in a much smaller subset of tumor cells. We propose that retinoblastoma tumors originate from cells that have an inherent ability to express CRX and OTX2.
Supplementary Material
Acknowledgments
We thank Dr. Cheryl M. Craft, Mary D. Allen Laboratory for Vision Research, Doheny Eye Institute and Keck School of Medicine of the University of Southern California, for her kind gift of the anti-CRX15pep and cone arrestin antibodies, as well as manuscript suggestions. We also thank Dr. Brenda Gallie, Department of Medical Genetics, University of Toronto, for sharing her retinoblastoma cell lines with us, and Dr. Rod Bremner, Departments of Ophthalmology and Lab Medicine and Pathobiology, University of Toronto, for his gift of the sheep anti-CHX10 antibody. We are grateful to Faye Chambers for her help in collecting the paraffin-embedded retinoblastoma tumor tissue, to Stanley Poon for his help with the preparation of the CRX and OTX2 expression constructs and to Dr. Xuejun Sun and Gerry Barron for assistance with cell imaging. This work was supported by the Canadian Institutes of Health Research.
Abbreviations used
- CHX10
Ceh-10 homeodomain containing homolog
- CRX
cone-rod homeobox
- GS
glutamine synthetase
- GST
glutathione S-transferase
- IRBP
interphotoreceptor retinoid-binding protein
- OTX2
orthodenticle homeobox 2
- PDEB
phosphodiesterase β subunit
- PKCα
protein kinase C alpha
- RPE
retinal pigmented epithelium
- SDS
sodium dodecyl sulfate
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Experiments addressing the specificity of the anti-CRX and anti-OTX2 antibodies.
Figure S2. CRX and OTX2 expression in the peripheral region of normal human fetal retina at 13 weeks gestation.
Figure S3. Expression of CRX, OTX2 and PKCα in retinal pigmented epithelial cells.
Figure S4. Co-staining of OTX2 and MIB-1 in retinoblastoma tumors.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Akita J, Haruta M, et al. Iris-derived cells from adult rodents and primates adopt photoreceptor-specific phenotypes. Invest Ophthalmol Vis Sci. 2005;46:3411–3419. doi: 10.1167/iovs.04-1112. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb LC, Holt JK, Tarttelin EE, Hodges MD, Gregory-Evans K, Rutherford A, Lucas RJ, Sowden JC, Gregory-Evans CY. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum Mol Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Borst DE, Peoples JW, Bruno J, Edwards CL, Si JS, Nickerson JM. A major cis activator of the IRBP gene contains CRX-binding and Ret-1/PCE-I elements. Mol Vis. 1997;3:15. [PubMed] [Google Scholar]
- Boatright JH, Borst DE, Stodulkova E, Nickerson JM. Endogenous CRX expression and IRBP promoter activity in retinoblastoma cells. Brain Res. 2001;916:136–142. doi: 10.1016/s0006-8993(01)02884-0. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015– 3026. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- Craft CM, Whitmore DH, Wiechmann AF. Cone arrestin identified by targeting expression of a functional family. J Biol Chem. 1994;269:4613–4619. [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Freund CL, Gregory-Evans CY, Furukawa T, et al. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Fujimaki T, Huang ZY, Kitagawa H, Sakuma H, Murakami A, Kanai A, McLaren MJ, Inana G. Truncation and mutagenesis analysis of the human X-arrestin gene promoter. Gene. 2004;339:139–147. doi: 10.1016/j.gene.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gallie BL, Campbell C, Devlin H, Duckett A, Squire JA. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 1999;59:1731s–1735s. [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Haruta M, Kosaka M, Kanegae Y, Saito I, Inoue T, Kageyama R, Nishida A, Honda Y, Takahashi M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4:1163–1164. doi: 10.1038/nn762. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takezawa S, Hara K, Yu RT, Umesono Y, Agata K, Taniwaki M, Yasuda K, Umesono K. Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci USA. 1999;96:4814–4819. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Nishida A, Ueno S, et al. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol Cell Biol. 2007;27:8318–8329. doi: 10.1128/MCB.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka J, Suzuki A, Morii E, Nomura S. Differential localization and expression of alpha and beta isoenzymes of protein kinase C in the rat retina. J Neurosci Res. 1998;54:655–663. doi: 10.1002/(SICI)1097-4547(19981201)54:5<655::AID-JNR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Li A, Zhu X, Brown B, Craft CM. Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2003;44:996–1007. doi: 10.1167/iovs.02-0434. [DOI] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel c murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen Y, Rest JS, Raymond PA, Zack DJ. Isolation and characterization of a zebrafish homologue of the cone rod homeobox gene. Invest Ophthalmol Vis Sci. 2001;42:481–487. [PubMed] [Google Scholar]
- MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Raviola E, Cepko CL. Synaptogenesis and outer segment formation are perturbed in the neural retina of Crx mutant mice. BMC Neurosci. 2005;6:5. doi: 10.1186/1471-2202-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Nork TM, Schwartz TL, Doshi HM, Millecchia LL. Retinoblastoma. Cell of origin Arch Ophthalmol. 1995;113:791–802. doi: 10.1001/archopht.1995.01100060117046. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Schulte D, Hendrickson AE. Expression of photoreceptor-associated molecules during human fetal eye development. Mol Vis. 2003;9:401–409. [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell SW, Zhu X, Wang X, Craft CM. Deciphering the contribution of known cis-elements in the mouse cone arrestin gene to its cone-specific expression. Invest Ophthalmol Vis Sci. 2004;45:3877–3884. doi: 10.1167/iovs.04-0663. [DOI] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:1187. doi: 10.1111/j.1460-9568.1991.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Robanus-Maandag E, Dekker M, van der Valk M, Carrozza ML, Jeanny JC, Dannenberg JH, Berns A, te Riele H. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 1998;12:1599–1609. doi: 10.1101/gad.12.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Wang QL, Wu W, Cook J, Coats C, Xu S, Chen S, Zack DJ, Sieving PA. Leber congenital amaurosis caused by a homozygous mutation (R90W) in the homeodomain of the retinal transcription factor CRX: direct evidence for the involvement of CRX in the development of photoreceptor function. Hum Mol Genet. 1999;8:299–305. doi: 10.1093/hmg/8.2.299. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Munakata S, Ishida Y, Nakajima T, Sugano I, Nagao K, Minoda K, Kondo Y. Photoreceptor differentiation of retinoblastoma: an electron microscopic study of 29 retinoblastomas. Pathol Int. 1994;44:837–843. doi: 10.1111/j.1440-1827.1994.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Ts’o MO, Fine BS, Zimmerman LE. The nature of retinoblastoma. II Photoreceptor differentiation: an electron microscopic study. Am J Ophthalmol. 1970;69:350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Willbold E, Rothermel A, Tomlinson S, Layer PG. Muller glia cells reorganize reaggregating chicken retinal cells into correctly laminated in vitro retinae. Glia. 2000;29:45–57. [PubMed] [Google Scholar]
- Wyatt A, Bakrania P, Bunyan DJ, et al. Novel heterozygous OTX2 mutations and whole gene deletions in anophthalmia, microphthalmia and coloboma. Hum Mutat. 2008;29:E278–E283. doi: 10.1002/humu.20869. [DOI] [PubMed] [Google Scholar]
- Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D, Liu A, Jhanwar SC, Abramson DH, Cobrinik D. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specificMDM2signaling. Cell. 2009;137:1018–1031. doi: 10.1016/j.cell.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Craft CM. Modulation of CRX transactivation activity by phosducin isoforms. Mol Cell Biol. 2000;20:5216–5226. doi: 10.1128/mcb.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.