Abstract
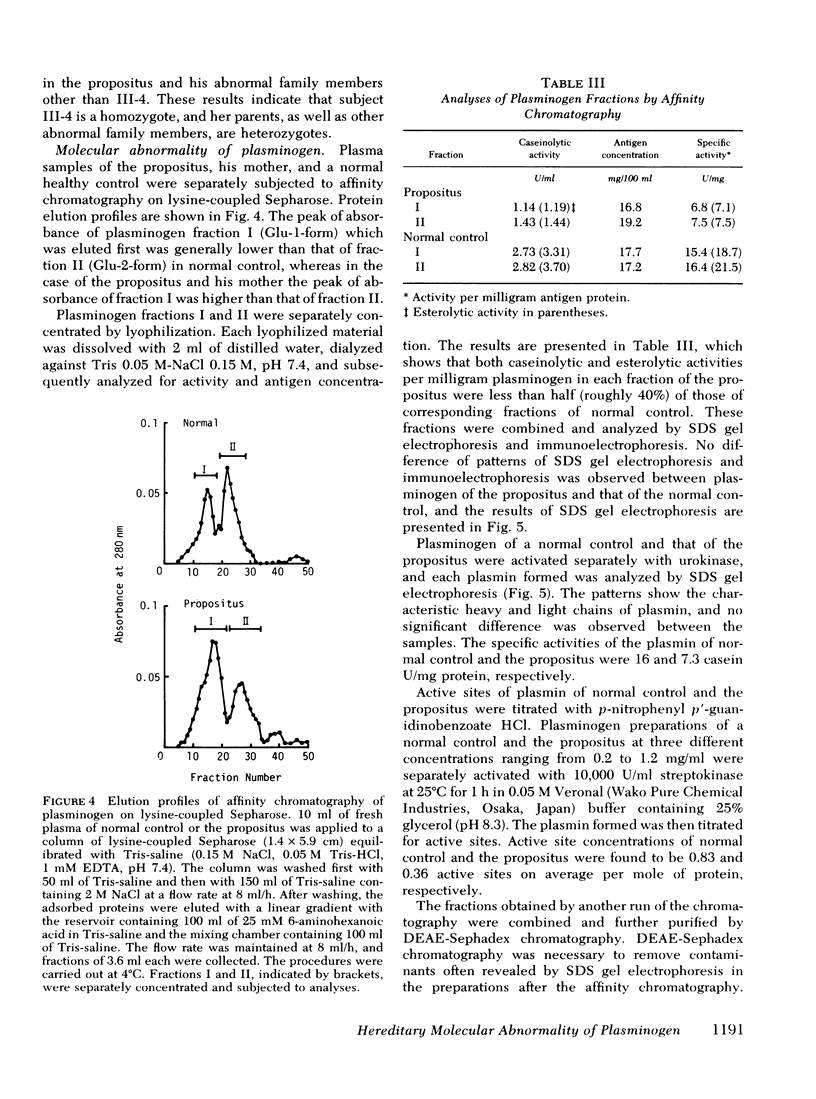
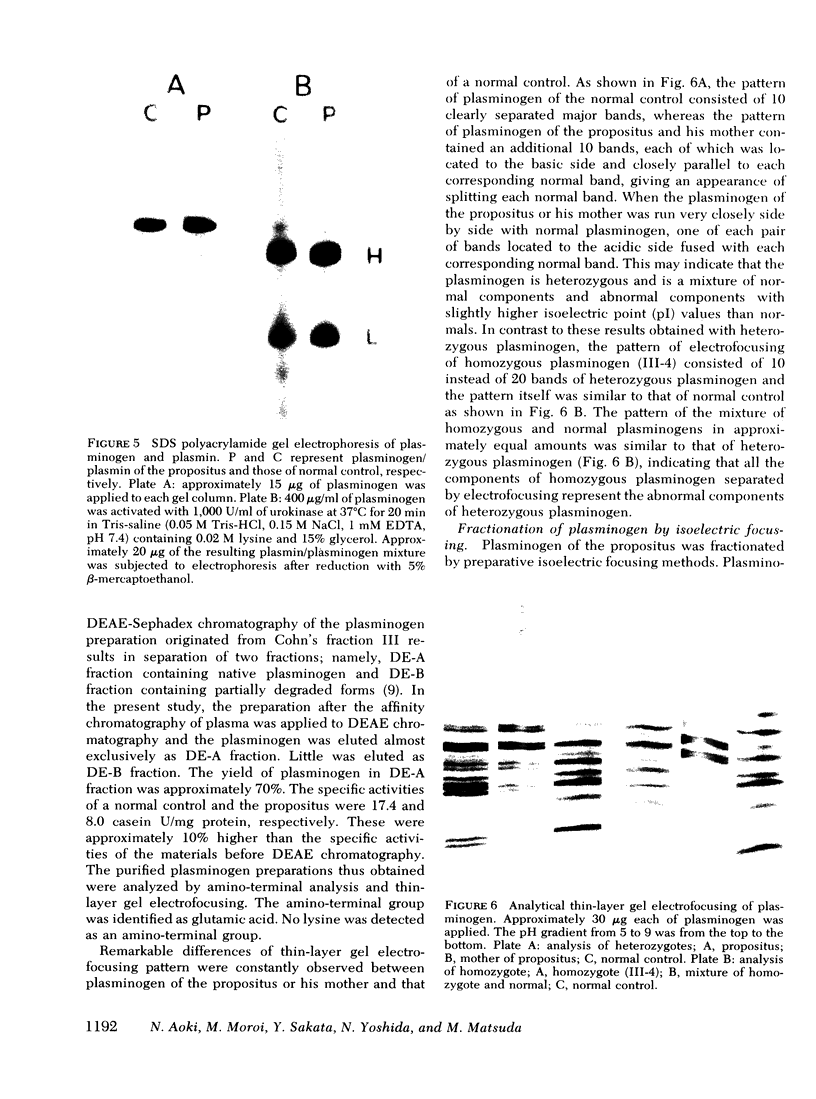
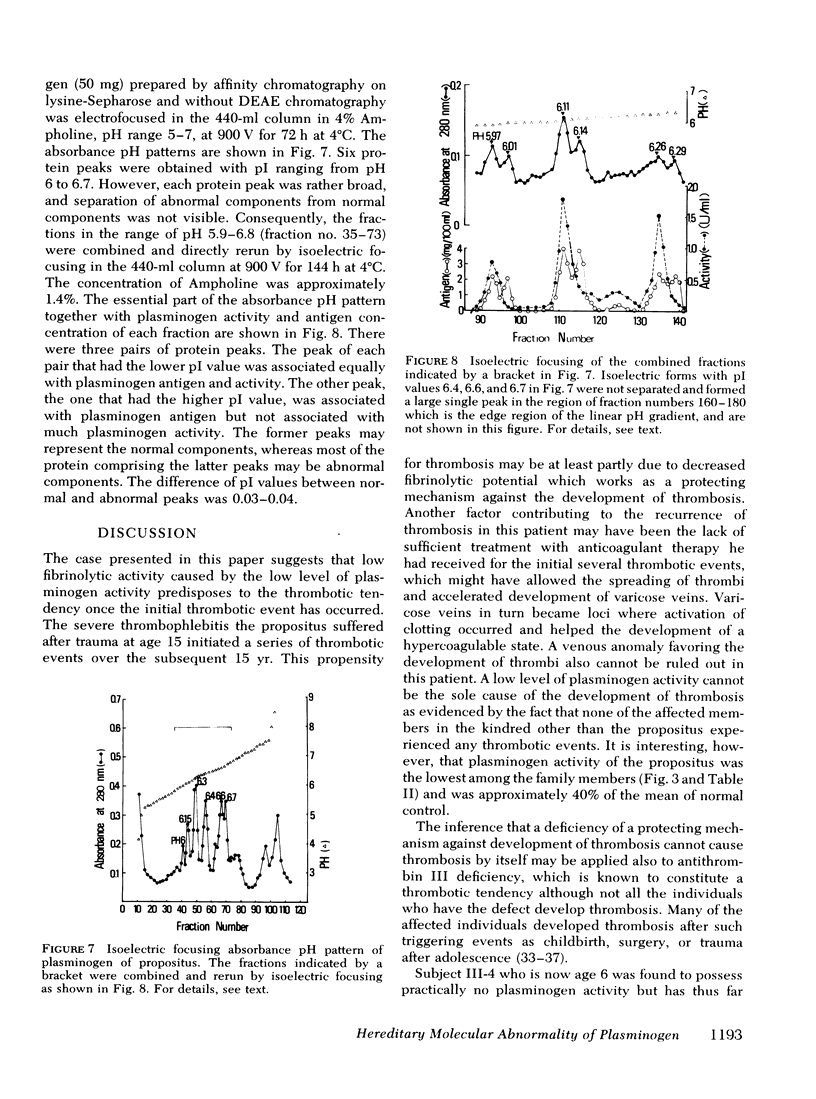
A patient who suffered a recurring thrombosis over the last 15 yr has been investigated. The only abnormality found in this patient was a significantly depressed level of plasminogen activity in plasma. In spite of the depressed plasminogen activity, the patient was found to have a normal level of plasminogen antigen concentration. It was calculated that the activity per milligram of plasminogen of the patient was approximately one-half the values of normal subjects. The same discrepancy between biological activity and antigen concentration was found in the other members of the kindred. A niece was found to have practically no plasminogen activity but possessed a normal concentration of plasminogen antigen. Both her parents were found to have approximately half the normal plasminogen activity and normal antigen levels. These studies suggested that the molecular abnormality was inherited as an autosomal characteristic, and the family members who had half the normal levels of activity with normal plasminogen antigen were heterozygotes whereas the one with practically no plasminogen activity was homozygote. Subsequent studies showed that the pattern of gel electrofocusing of purified plasminogen of the heterozygotes consisted of 10 normal bands and 10 additional abnormal bands, each of which had a slightly higher isoelectric point than each corresponding normal component. This indicates that plasminogen of the heterozygote is a mixture of normal and abnormal molecules in an approximately equal amount, which was substantiated by active site titration of purified plasminogen preparations obtained from the propositus and a normal individual. The gel electrofocusing pattern of the homozygote consisted of abnormal bands only. The defect is a hereditary abnormality of plasminogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. The mechanism of clot dissolution by plasmin. J Clin Invest. 1959 Jul;38(7):1086–1095. doi: 10.1172/JCI103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Matsuda M., Tachiya K. The behavior of alpha2-plasmin inhibitor in fibrinolytic states. J Clin Invest. 1977 Aug;60(2):361–369. doi: 10.1172/JCI108784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford T. P., Weinstein M. C., Freiman D. G., Weinstein M. C. The role of the intrinsic fibrinolytic system in the prevention of stasis thrombosis in small veins. An electron microscopic study. Am J Pathol. 1968 Jun;52(6):1117–1127. [PMC free article] [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Barlow G. H., Summaria L., Robbins K. C. Molecular weight studies on human plasminogen and plasmin at the microgram level. J Biol Chem. 1969 Mar 10;244(5):1138–1141. [PubMed] [Google Scholar]
- Brakman P., Mohler E. R., Jr, Astrup T. A group of patients with impaired plasma fibrinolytic system and selective inhibition of tissue activator-induced fibrinolysis. Scand J Haematol. 1966;3(5):389–398. doi: 10.1111/j.1600-0609.1966.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Brockway W. J., Castellino F. J. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys. 1972 Jul;151(1):194–199. doi: 10.1016/0003-9861(72)90488-2. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Siefring G. E., Jr, Sodetz J. M., Bretthauer R. K. Amino terminal amino acid sequences and carbohydrate of the two major forms of rabbit plasminogen. Biochem Biophys Res Commun. 1973 Aug 6;53(3):845–851. doi: 10.1016/0006-291x(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Egeberg O. Inherited fibrinogen abnormality causing thrombophilia. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):176–187. [PubMed] [Google Scholar]
- Isacson S., Nilsson I. M. Defective fibrinolysis in blood and vein walls in recurrent "idiopathic" venous thrombosis. Acta Chir Scand. 1972;138(4):313–319. [PubMed] [Google Scholar]
- Johnson A. J., Kline D. L., Alkjaersig N. Assay methods and standard preparations for plasmin, plasminogen and urokinase in purified systems, 1967-1968. Thromb Diath Haemorrh. 1969 Apr 30;21(2):259–272. [PubMed] [Google Scholar]
- Kato N., Morimatsu M., Tanaka K., Horie A. Effects of trans-4-aminomethylcyclohexane carboxylic acid as an antifibrinolytic agent on arterial wall and experimental atherosclerotic lesions in rabbits. Thromb Diath Haemorrh. 1970 Oct 31;24(1):85–99. [PubMed] [Google Scholar]
- MONKHOUSE F. C. THE INFLUENCE OF INORGANIC SALTS ON PLASMA ANTITHROMBIN ACTIVITY. Thromb Diath Haemorrh. 1963 Jul 15;143:387–394. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marciniak E., Farley C. H., DeSimone P. A. Familial thrombosis due to antithrombin 3 deficiency. Blood. 1974 Feb;43(2):219–231. [PubMed] [Google Scholar]
- McKay D. G., Müller-Berghaus G. Therapeutic implications of disseminated intravascular coagulation. Am J Cardiol. 1967 Sep;20(3):392–410. doi: 10.1016/0002-9149(67)90063-x. [DOI] [PubMed] [Google Scholar]
- Merskey C., Kleiner G. J., Johnson A. J. Quantitative estimation of split products of fibrinogen in human serum, relation to diagnosis and treatment. Blood. 1966 Jul;28(1):1–18. [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- NAEYE R. L. Thrombotic disorders with increased levels of antiplasmin and antiplasminogen. N Engl J Med. 1961 Nov 2;265:867–871. doi: 10.1056/NEJM196111022651801. [DOI] [PubMed] [Google Scholar]
- NILSSON I. M., KROOK H., STERNBY N. H., SODERBERG E., SODERSTROM N. Severe thrombotic disease in a young man with bone marrow and skeletal changes and with a high content of an inhibitor in the fibrinolytic system. Acta Med Scand. 1961 Mar;169:323–337. doi: 10.1111/j.0954-6820.1961.tb07838.x. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., MENZIE C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951 Feb;37(2):316–320. [PubMed] [Google Scholar]
- REMMERT L. F., COHEN P. P. Partial purification and properties of a proteolytic enzyme of human serum. J Biol Chem. 1949 Nov;181(1):431–448. [PubMed] [Google Scholar]
- Sas G., Blaskó G., Bánhegyi D., Jákó J., Pálos L. A. Abnormal antithrombin III (antithrombin III "Budapest") as a cause of a familial thrombophilia. Thromb Diath Haemorrh. 1974 Sep 30;32(1):105–115. [PubMed] [Google Scholar]
- Summaria L., Arzadon L., Bernabe P., Robins K. C. Characterization of the NH 2 -terminal glutamic acid and NH 2 -terminal lysine forms of human plasminogen isolated by affinity chromatography and isoelectric focusing methods. J Biol Chem. 1973 May 10;248(9):2984–2991. [PubMed] [Google Scholar]
- Summaria L., Spitz F., Arzadon L., Boreisha I. G., Robbins K. C. Isolation and characterization of the affinity chromatography forms of human Glu- and Lys-plasminogens and plasmins. J Biol Chem. 1976 Jun 25;251(12):3693–3699. [PubMed] [Google Scholar]
- VON KAULLA K. N., SCHULTZ R. L. Methods for the evaluation of human fibrinolysis; studies with two combined technics. Am J Clin Pathol. 1958 Feb;29(2):104–112. doi: 10.1093/ajcp/29.2.104. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. II. Separation and partial characterization of different molecular forms of human plasminogen. Biochim Biophys Acta. 1972 Jan 26;257(1):122–134. doi: 10.1016/0005-2795(72)90261-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van der Meer J., Stoepman-van Dalen E. A., Jansen J. M. Antithrombin-3 deficiency in a Dutch family. J Clin Pathol. 1973 Jul;26(7):532–538. doi: 10.1136/jcp.26.7.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kaulla E., von Kaulla K. N. Deficiency of antithrombin 3 activity associated with hereditary thrombosis tendency. J Med. 1972;3(6):349–358. [PubMed] [Google Scholar]