Abstract
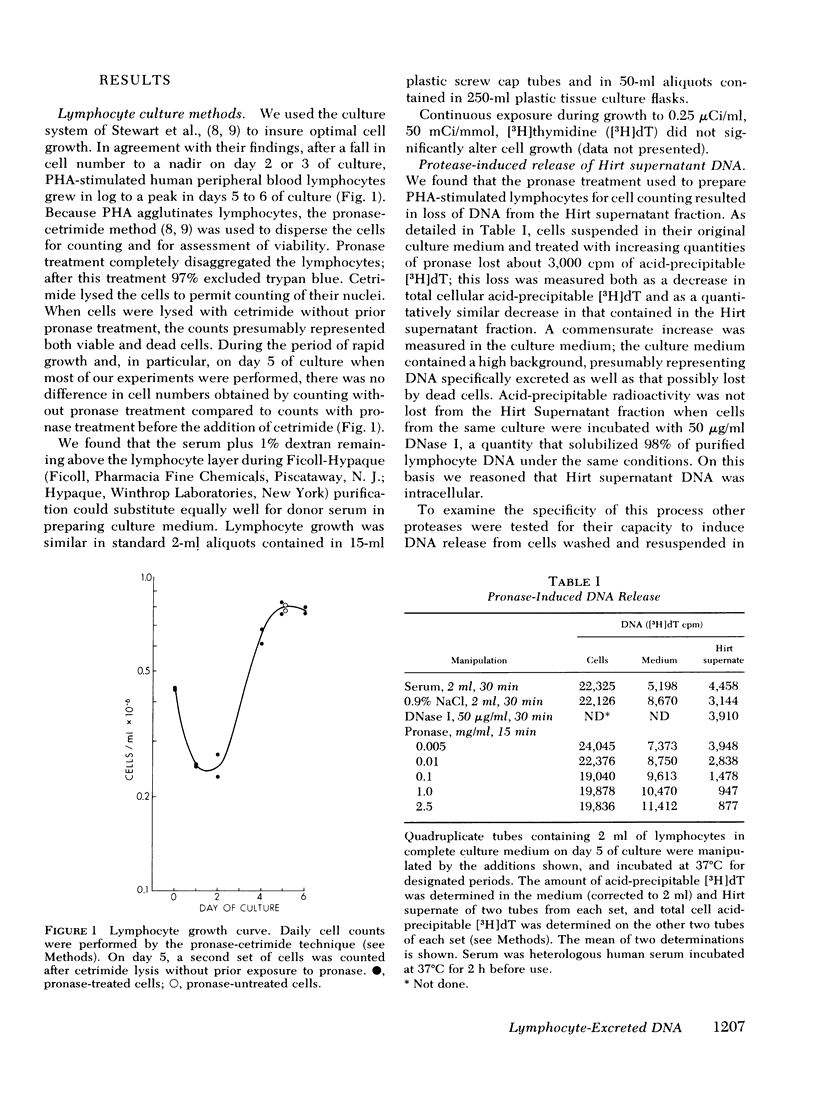
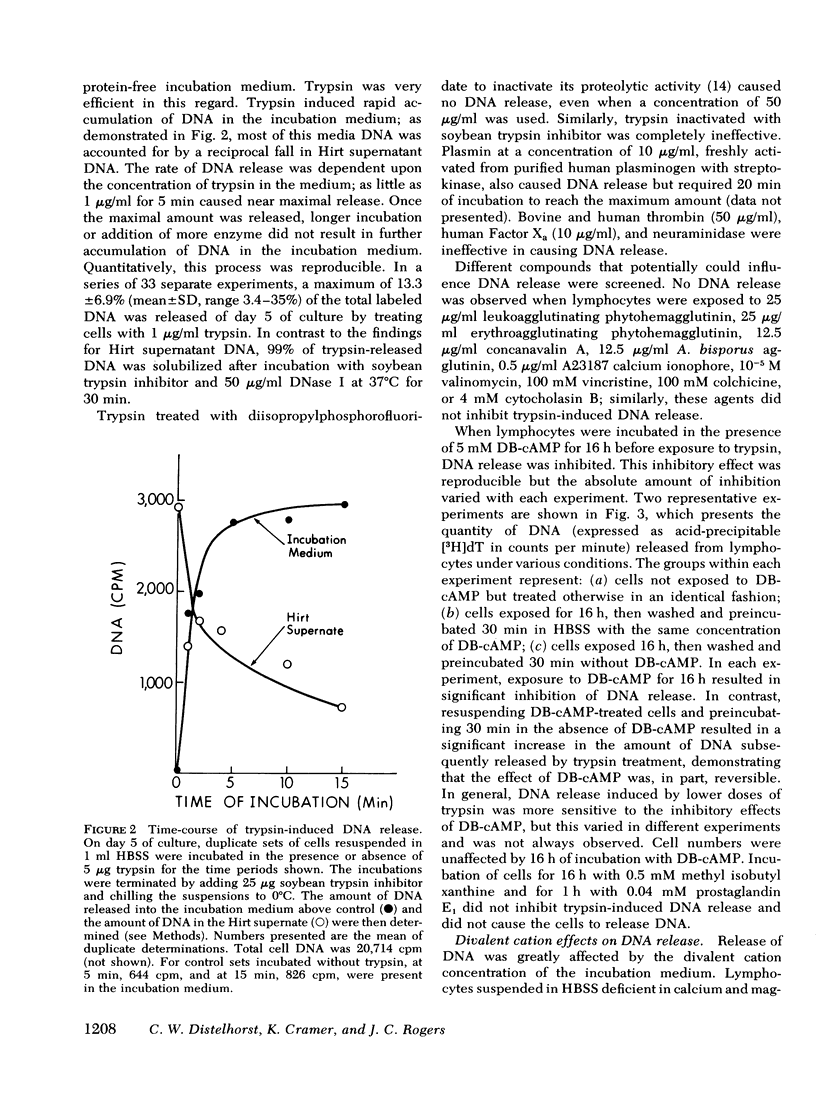
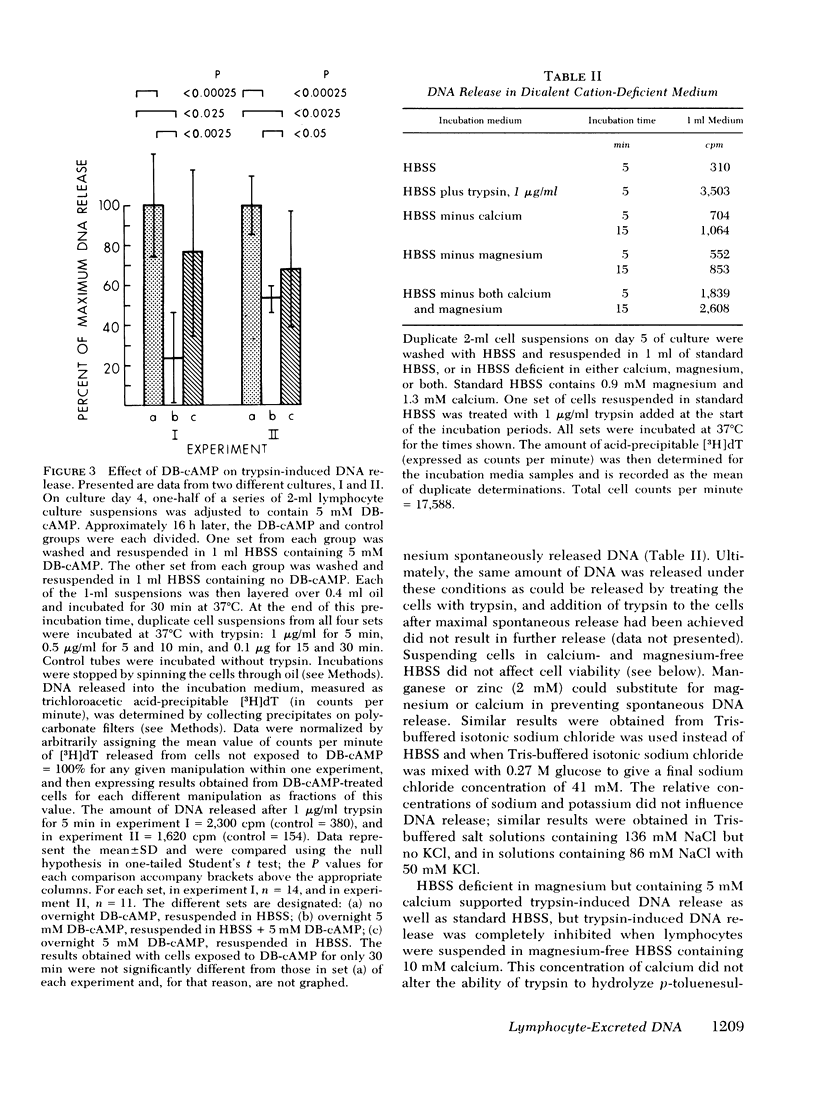
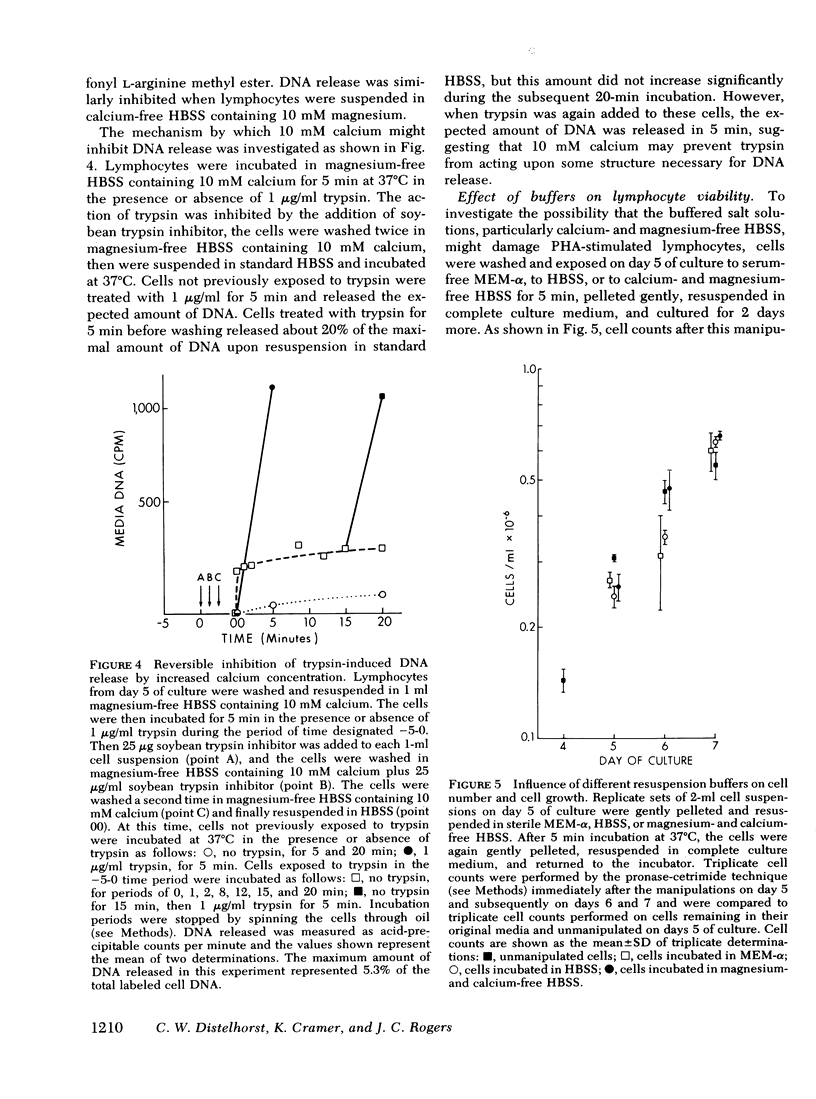
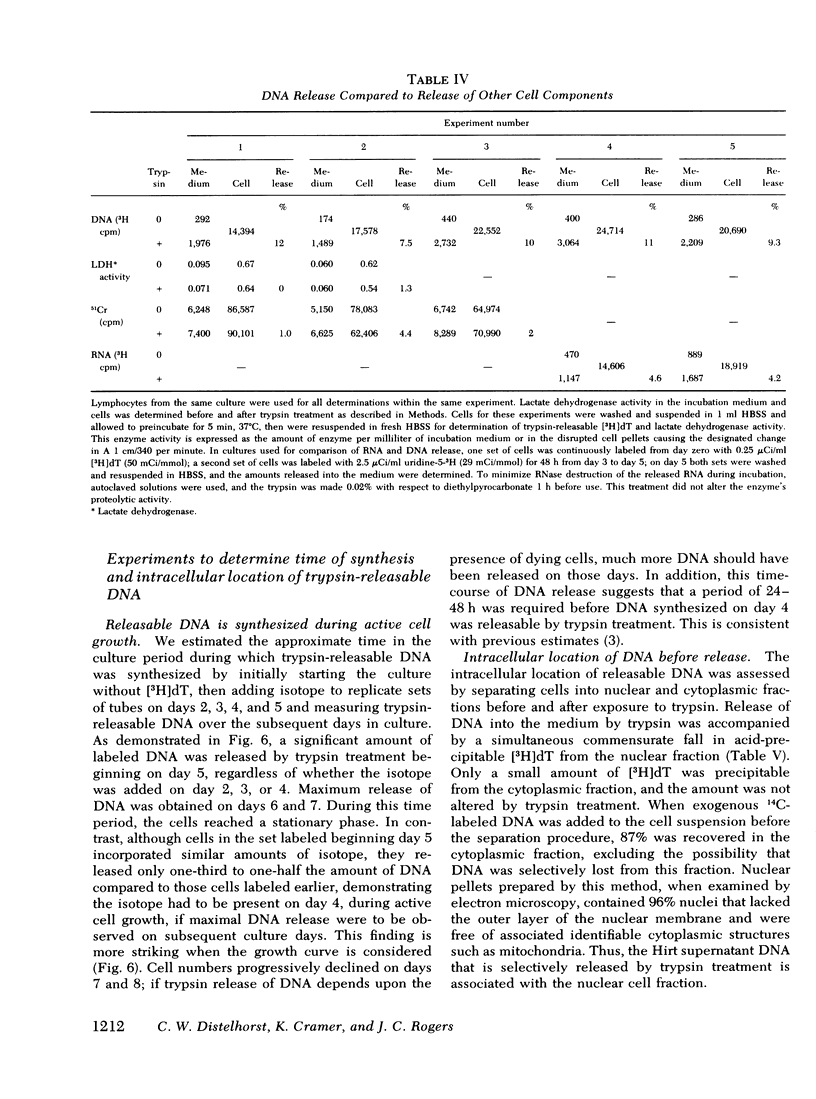
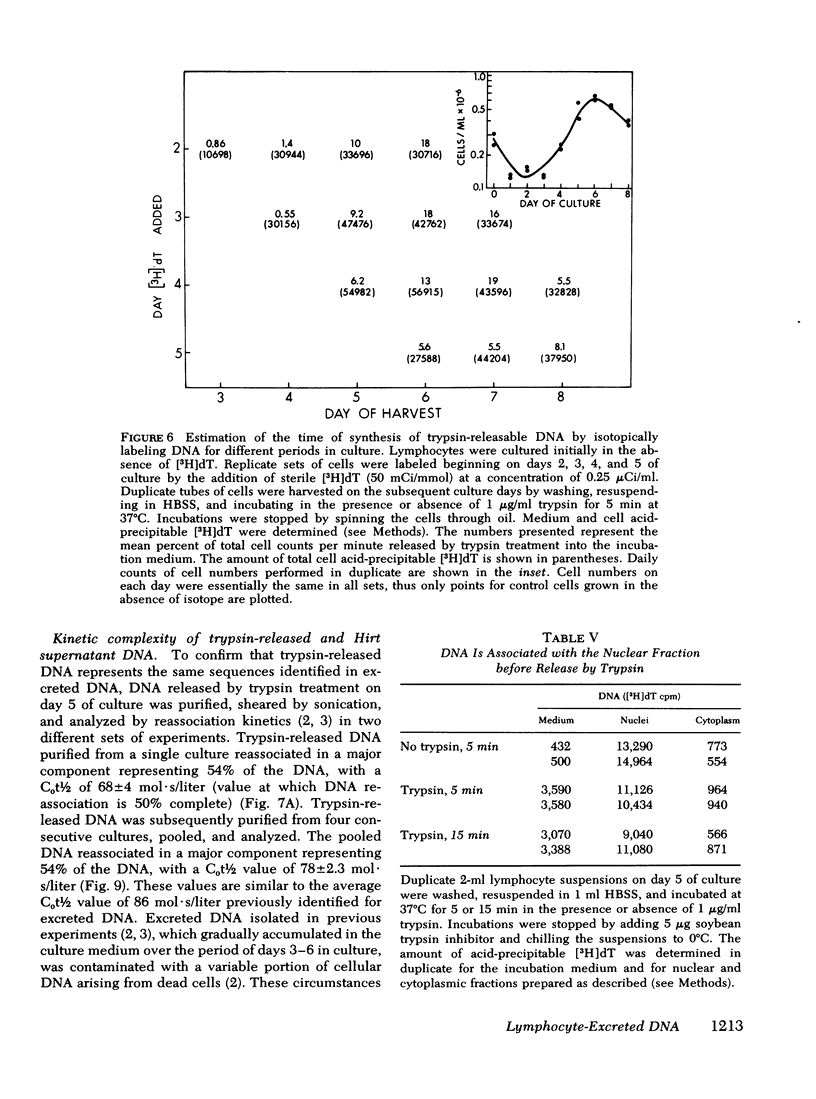
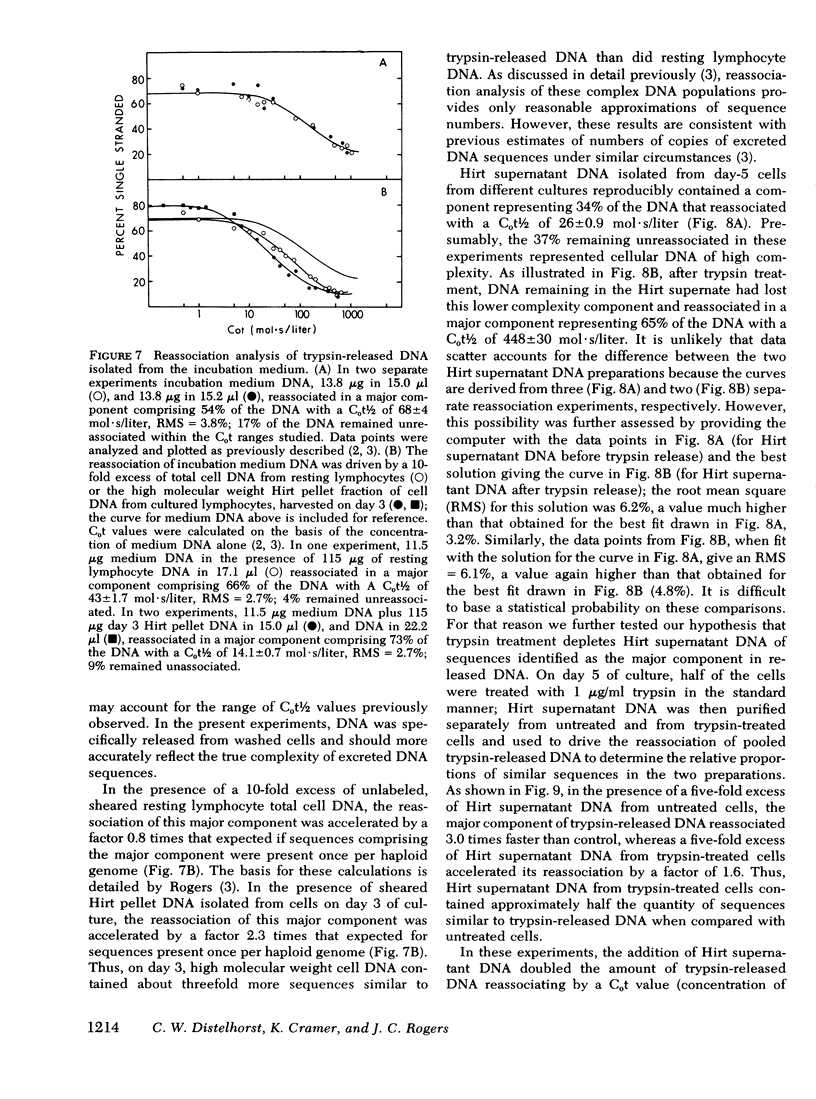
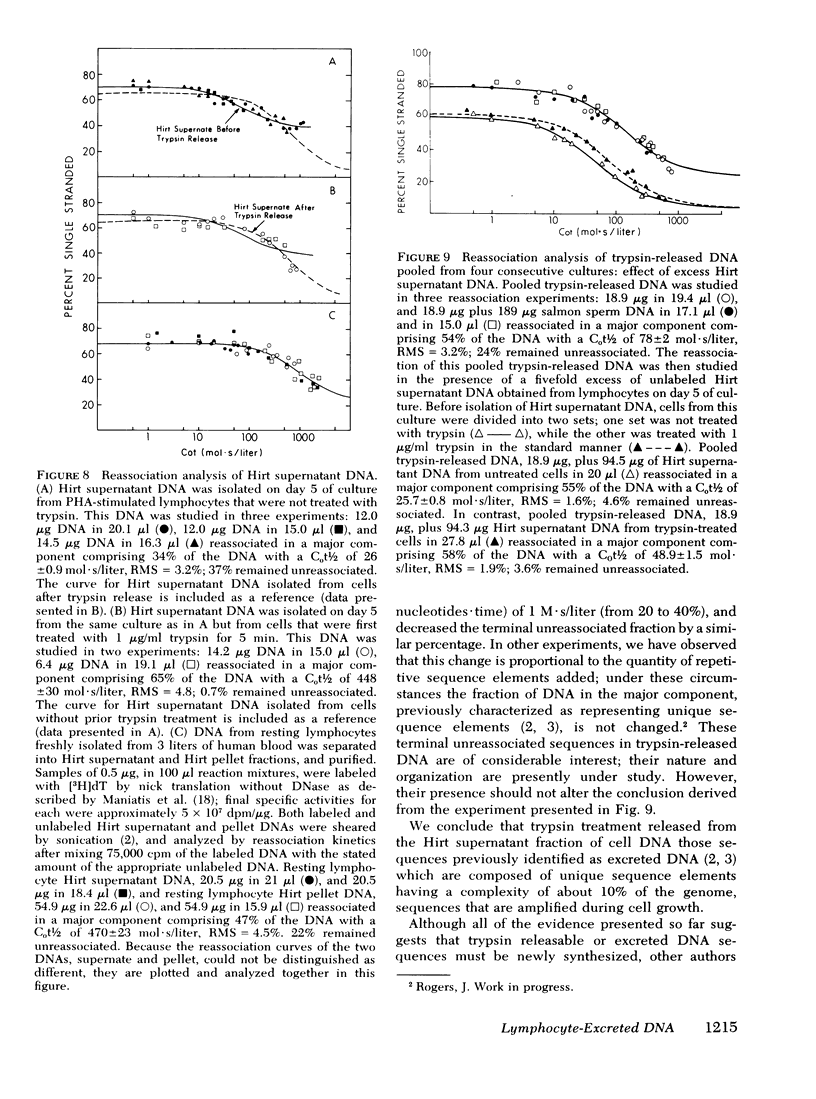
We studied the synthesis of excreted DNA sequences and their release from phytohemagglutinin-stimulated human peripheral blood lymphocytes under conditions permitting optimal cell growth. Cells were labeled by constant exposure to low specific activity [3H]thymidine. Excreted DNA sequences were synthesized during the period of logarithmic cell growth and moved slowly from the high molecular weight chromosomal DNA fraction into the low molecular weight cell DNA fraction (Hirt supernate) from which they could be specifically released by treating the cells briefly with small amounts of various proteases; 1 microgram/ml trypsin for 5 min was optimal. On day 5 of culture, 13.3 +/- 6.9% of the total cellular acid-precipitable [3H]thymidine was released by this treatment. Trypsin-induced release was partially and reversibly inhibited by incubating the cells for 16 h with 5 mM dibutyryl-cyclic AMP. Cells incubated in the absence of divalent cations spontaneously released this Hirt supernatant DNA; after maximal release had occurred under these circumstances, additional trypsin treatment caused no further release of DNA. Trypsin-induced DNA release could be completely and reversibly inhibited by incubating the cells in the presence of 10 mM calcium. Trypsin-released DNA was isolated and analyzed by reassociation kinetics. A major component, representing 54% of the DNA, reassociated with a C0t1/2 of 68 mol.s/liter (the value at which DNA association is 50% complete). The reassociation of this DNA was studied in the presence of an excess of DNA isolated from stimulated lymphocytes on day 3 in culture, and in the presence of an excess of resting lymphocyte DNA. The high molecular weight fraction of day-3 cell DNA contained three times more copies of the trypsin-released DNA major component as compared to resting lymphocyte DNA. Hirt supernatant DNA isolated from day-5 stimulated lymphocytes reassociated in an intermediate component representing 34% of the DNA with a Cot1/2 of mol.s/liter; after cells were treated with trypsin, this component could no longer be identified in the Hirt supernatant fraction, presumably because it had been released into the incubation medium. These data describe a quantitatively reproducible system with which synthesis and release of excreted DNA sequences can be studied.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker P., Stroun M., Maurice P. A. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res. 1975 Sep;35(9):2375–2382. [PubMed] [Google Scholar]
- Berger S. L., Cooper H. L. Very short-lived and stable mRNAs from resting human lymphocytes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3873–3877. doi: 10.1073/pnas.72.10.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt D. H., MacDermott R. P., Speckart S. F., Nash G. S. Excretion of DNA by purified human lymphocyte subpopulations. J Immunol. 1977 Apr;118(4):1495–1498. [PubMed] [Google Scholar]
- Harris G., Olsen I. Cell division and deoxyribonucleic acid (DNA) synthesis in cultures of stimulated lymphocytes. Immunology. 1976 Aug;31(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Meinke W., Goldstein D. A. Membrane-associated DNA in the cytoplasm of diploid human lymphocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1212–1216. doi: 10.1073/pnas.68.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Reassociation and dissociation of cytoplasmic membrane-associated DNA. J Mol Biol. 1974 Jul 15;86(4):757–773. doi: 10.1016/0022-2836(74)90352-0. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presant C. A., Kornfeld S. Characterization of the cell surface receptor for the Agaricus bisporus hemagglutinin. J Biol Chem. 1972 Nov 10;247(21):6937–6945. [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Characterization of DNA excreted from phytohemagglutinin-stimulated lymphocytes. J Exp Med. 1976 May 1;143(5):1249–1264. doi: 10.1084/jem.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Identification of an intracellular precursor to DNA excreted by human lymphocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3211–3215. doi: 10.1073/pnas.73.9.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston I., Smith R. W., Buell D. N., Huang E. S., Pagano J. S. Autologous human B and T lymphoblastoid cell lines. Nature. 1974 Oct 25;251(5477):745–746. doi: 10.1038/251745a0. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Cramer S. F., Steward P. G. The response of human peripheral blood lymphocytes to phytohemagglutinin: determination of cell numbers. Cell Immunol. 1975 Apr;16(2):237–250. doi: 10.1016/0008-8749(75)90115-x. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Ingram M. A method for counting phytohemagglutinin-stimulated lymphocytes. Blood. 1967 Apr;29(4 Suppl):628–639. [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Pharmacologic modulation of inflammatory mediator release by rat mast cells. Am J Pathol. 1976 Nov;85(2):437–464. [PMC free article] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]



