Abstract
Background
Recent linkage between primary and secondary care data has provided valuable information for studying heath outcomes that may initially present in different health care settings. The aim of this study was therefore, twofold: to use linked primary and secondary care data to determine an optimum definition for estimating the incidence of first VTE in and around pregnancy; and secondly to conduct a systematic literature review of studies on perinatal VTE incidence with the purpose of comparing our estimates.
Methods
We used primary care data from the Clinical Practice Research Datalink (CPRD), which incorporates linkages to secondary care contained within Hospital Episode Statistics (HES) between 1997 and 2010 to estimate the incidence rate of VTE in the antepartum and postpartum period. We systematically searched the literature on the incidence of VTE during antepartum and postpartum periods and performed a meta-analysis to provide comparison.
Findings
Using combined CPRD and HES data and a restrictive VTE definition, the absolute rate during the antepartum period and first six weeks postpartum (early postpartum) were 99 (95%CI 85–116) and 468 (95%CI 391–561) per 100,000 person-years respectively. These were comparable to the pooled estimates from our meta-analysis (using studies after 2005) during the antepartum period (118/100,000 person-years) and early postpartum (424/100,000 person-years). When we used only secondary care data to identify VTE events, incidence was lower during the early postpartum period (308/100,000 person-years), whereas relying only on primary care data lead to lower incidence during the time around delivery, but higher rates during the postpartum period (558/100,000 person-years).
Conclusion
Using combined CPRD and HES data gives estimates of the risk of VTE in and around pregnancy that are comparable to the existing literature. It also provides more accurate estimation of the date of VTE diagnosis which will allow risk stratification during specific pregnancy and postpartum periods.
Introduction
Venous thromboembolism (VTE) is a serious complication of pregnancy, however due to the low incidence of pregnancy related VTEs and uncertainties over risk factors, prospective studies are unlikely to be done because of prohibitive costs. Therefore, to study its occurrence and risk factors, studies using routine data have been used as they provide sufficient power and population coverage to give robust and generalisable estimates [1], [2]. The recent linkage of electronic primary data in the Clinical Practice Research Datalink (CPRD) with the secondary care English Hospital Episode Statistics database (HES) may be useful in providing valuable information on maternal risk factors for VTE including hospitalisation, life style related factors and co-morbidities. However, there have been no studies done using these linked data to quantify the incidence of VTE in and around pregnancy. Furthermore no studies have assessed the disparities between the results from secondary and primary care data in a standalone fashion versus when used together. Additionally, defining VTE may be a concern as currently there have been no studies done to validate VTE in linked primary and secondary data. Whilst VTE has been previously validated in primary care data in non-pregnant women with a positive predictive value (PPV) of 84% [3], it is important to assess whether secondary care data add any further information on VTE events around pregnancy especially in the United Kingdom (UK) where almost all women deliver in hospital. For instance, the previous validation study does not give an indication of the negative predictive value and we cannot ignore the potential for the incidence of VTE recorded in primary care to be underestimated if certain VTE cases are solely recorded in secondary care. Further validation is also required to assess potential false positive diagnoses in the peripartum period.
One way of assessing clinical information captured on VTE during antepartum and postpartum periods is to compare estimates of incidence to previous studies of VTE incidence. However to-date, there has not been any formal data synthesis of these previous studies nor an attempt at providing pooled estimates of the incidence of VTE during the antepartum and postpartum period. The aim of this study was therefore, twofold: to use linked primary and secondary care data to determine an optimum definition for estimating the incidence of first VTE in and around pregnancy; and secondly to conduct a systematic literature review of studies on perinatal VTE incidence with the purpose of comparison to our estimates.
Methods
Databases and study population
Hospital Episode Statistics (HES)
The Hospital episode statistics dataset (HES) [4] contains details of all hospital admissions to National Health Service (NHS) hospitals in England. It contains demographic data along with information on discharge diagnoses and procedures which are coded using International Classification of Diseases (ICD) version 10 and Operation and Procedure Coding Supplement (OPCS) version 4 respectively. All diagnoses within the hospitalisation period (i.e. time they are admitted until the time they are discharged from hospital) are recorded within episodes (time period during which a patient is under a particular consultant). We also used HES maternity data and contains data on births across England and is the primary source of maternity statistics in England which have been validated [5].
Clinical Practice Research Datalink
The Clinical Practice Research Datalink (CPRD) [6] is a computerized primary health care database containing demographic, medical, prescription and lifestyle related information from anonymised patient records across the UK. The data are subjected to quality checks and only the data which is of high quality is used for research. The GPRD has been extensively validated for a wide range of chronic diagnoses and consistently found to be accurate [7], [8].
Linkage
The anonymised patient identifiers from CPRD and HES have been linked by a trusted third party using NHS number, date of birth and gender [9]. As HES only covers English hospitals, practices from Northern Island, Wales and Scotland were excluded. The primary care CPRD does not use a set sampling approach to for its linkage with secondary care HES data. It is based on practices that have consented to be linked to HES in CPRD. However all patients within a consented practice are included. Additionally our comparison of CPRD-HES linked data to Office for National Statistics (ONS) data showing the age distribution of the UK population has demonstrated similarities [10]. For this study, we used 51% of the CPRD practices that had linked HES data for their registered patients. We identified women of reproductive age (15–44 years old) between 1997 and 2010 registered within the CPRD-HES linked practices as this was the time for which HES linked data were available.
Defining venous thromboembolism
VTE diagnosis codes (including pulmonary embolism (PE) or deep vein thrombosis (DVT)) were extracted from women's primary care data using medical Read codes. From HES, all women with an ICD-10 code of venous thromboembolism including pulmonary embolism (I26.0, I26.9), deep vein thrombosis (I80.1–I80.9) and portal vein thrombosis (I82.0–I82.9) were extracted. ICD-10 codes of VTE specifically related to pregnancy or postpartum (O22.2, O22.3, O87.1, O87.0, O08.2 and O88.2) were also extracted. Information from either or both primary and secondary care data sources was used to define a VTE event and the first VTE diagnosis recorded in either data source was considered as the incident date. We assessed only the first recorded VTE during the study period and all subsequent VTEs were excluded. We then developed the following three VTE definitions;
Definition A
Our most stringent definition included only VTE diagnoses supported by prescription or evidence of anticoagulant therapy (with either warfarin or unfractionated heparin or low molecular weight heparin) within 90 days of the event or death within 30 days of the event. Given the restricted use of oral anticoagulants (e.g. warfarin) during the antepartum period due to its teratogenicity, the majority of cases during the antepartum period were confirmed based on heparin prescriptions.
Definition B
This consisted cases where signs or symptoms of DVT (e.g. leg pain, calf pain), PE (e.g. chest pain, shortness of breath) or diagnostic tests for VTE (e.g. d-dimer, Ventilation-Perfusion (VQ) scan, Computed Tomography (CT)-scan, venography) had been recorded between 15 days before and 15 days after a first recorded diagnosis of VTE, but there was no evidence of anticoagulant therapy. Cases were also included if they had VTE diagnoses in both primary and secondary care up to 60 days apart, a cut-off based on the initial examination of the recording of VTE in both datasets and prior work we and others have published on identifying acute medical events in linked data [11], [12].
Definition C
This included all other diagnoses of VTE that did not fit the criteria for VTE definitions A or B. Specifically, all VTE diagnoses with no accompanying anticoagulant prescription, medical code indicating anticoagulant therapy, death, signs or symptoms of VTE, diagnostic tests for VTE. These cases were only recorded in one data source (HES or CPRD).
Defining pregnancy and associated time periods
Information on birth outcome (including live birth and stillbirth) was identified using the mother's delivery records in HES maternity data. For each pregnancy resulting in live or stillbirth, the date of delivery was extracted from the OPCS version 4 codes for delivery (e.g. emergency caesarean section, spontaneous vaginal delivery). The date of conception was defined by subtracting the length of gestation from the date of delivery. For those with no information on length of gestation (33%), 40 weeks was assigned. This corresponds to that of the majority of pregnant women in the National Health Service's (NHS) maternity statistics for England [13]. Women's follow-up time between age 15–44 years was divided into time associated with pregnancy (defined from the date of any conceptions she had during follow-up until 12 weeks postpartum) and “non-pregnant periods” (all other available follow-up time, which included all time for women who were never pregnant during the study period as previously described [14]). If the VTE event was recorded during the same hospital admission as the women's delivery (which accounted for 11% of all VTE events), there was thus potential for misclassifying the timing in relation to delivery. As 91% of deliveries occurred on the day women were admitted to hospital for delivery or on the day after and the median duration of hospital stay for delivery was only 2 days, the time associated with pregnancy was divided into the antepartum period (trimesters from the date of conception until 2 days before the date of delivery), time around delivery (1 day before until 2 days after delivery) and the postpartum period which was defined from 3 days after delivery until 12 weeks postpartum. The postpartum period was subdivided into individual weeks and also into early (first six weeks) and late (second six weeks) postpartum) period.
Statistical analyses
Cohort analysis in linked data
The absolute rates (AR) of VTE per 100,000 person-years and 95% Confidence Interval (CI) were calculated for the antepartum period, time around delivery, postpartum period and non-pregnant periods using VTE definitions A, B and C separately. This was done by dividing the total number of VTE events by person-years of follow-up. We then restricted the analysis to first VTEs identified only in primary care data and then VTEs only in secondary care data to compare these with the overall estimates using both sources.
Systematic review and meta-analysis of existing VTE incidence studies
For the purpose of comparing our rates to the existing literature, we systematically reviewed previous studies that have estimated VTE incidence among pregnant women or during the postpartum period. We searched MEDLINE and Embase for studies published between January 1960 and January 2013, combining a similar search strategy to that used in our previously published VTE systematic review [15] with an adapted version of the Cochrane Pregnancy Group search strategy to obtain pregnancy studies [16]. The strategy used for MEDLINE and Embase is summarised in Table S1 and S2 respectively. We included studies only if they had estimated the rate of VTE in pregnant and postpartum women in a manner that allowed us to extract the data for the purposes of meta-analysis. Studies' abstracts were independently reviewed for selection by two investigators (AAS and JW) with differences resolved by consensus.
For each study included in our meta-analysis, the natural logarithm of the incidence rate of VTE per 100,000 person-years was obtained along with the standard errors (1/√VTE events). For studies reporting rates of VTE per 100,000 pregnancies we converted this into person-years of antepartum time by multiplying the number of pregnancies by 0.75. For the meta-analysis, we only assessed the first six weeks after childbirth during the postpartum (although this was only the early postpartum period in our cohort study) as this was the definition of postpartum used in the majority of the included publications. These were then pooled separately for antepartum and postpartum periods assuming random effects using the generic inverse variance method. This method considers the inverse variance of the effect estimates i.e. 1/(standard error)2 as the weight given to each study, so a study with more VTE events was given greater weight than studies with fewer VTE events. A pooled estimate was also calculated for the third trimester of pregnancy.
Given that diagnosis modalities have improved over the years allowing for better ascertainment of VTE diagnosis in addition to the increasing prevalence of maternal risk factors for VTE (for example obesity), we performed a subgroup analysis by stratifying studies based on calendar year (before and after 2005). This cut-off was based on the initial examination of the forest plot on the incidence of VTE during pregnancy which showed a marked difference in the rate of VTE after 2005. We also stratified our analyses based on whether or not VTE cases were subjected to a degree of validation/confirmation, which varied from study to study, however, we accepted methods similar to our criteria. The methods used to validate/confirm VTE ranged from using a validated algorithm to confirm VTE diagnosis or a registry where VTE cases were previously validated to only including cases where VTE had been objectively confirmed by diagnostic tests. A diagnosis of pulmonary embolism may have been confirmed by pulmonary angiography, CT, Magnetic Resonance Imaging, VQ scan, or pathological confirmation of thrombus. A diagnosis of DVT may have been confirmed by Doppler ultrasound, duplex ultrasonography, venography, or pathological confirmation of thrombus.
The heterogeneity was assessed in terms of I. 2 All data management and statistical analysis was done using Stata MP11 (Stata Corp., College Station, Texas). This study was approved by the Independent Scientific Advisory Committee (ISAC) that governs use of the CPRD for research (reference number = 10_193R).
Results
Cohort analysis
Overall there were 1,117,691 women with follow-up data between the ages of 15 and 44 years experiencing 248,953 pregnancies resulting in live or stillbirths (Table 1). The median follow-up for each women was 3.2 years (IQR = 1.2–6.5).
Table 1. Basic characteristics of study population.
| Variables | N (%) |
| Total women in study | 1,117,691 |
| Median follow-up (Inter quartile range) in years | 3.2 (1.23–6.50) |
| Pregnancy outcome (total pregnancies N = 248,953) | |
| Live births | 247,436 (99.3) |
| Stillbirths | 1,517 (0.61) |
| Study follow-up time in person-years (total study period = 4,821,334) | |
| Not pregnant | 4,613,196 |
| Antepartum | 156,541 |
| Time around delivery | 2,381 |
| Postpartum | 49,216 |
Defining first VTE using linked primary and secondary care data
There were 3,507 cases of first VTE using both data sources. Around 51% of the VTE cases were categorised under VTE definition A of which 51% of diagnoses were recorded both in primary and secondary care data (Table 2). Twenty percent of all VTE cases were categorised under VTE definition B as they had supporting evidence including signs or symptoms or a diagnostic test documented 15 days before or after the date of VTE diagnosis but did not meet our criteria for VTE definition A. A total of 29% of all the VTEs were categorized as VTE definition C, i.e. diagnoses with no supporting evidence, the majority of which were in primary care data. When only using the primary care data to identify first VTE cases, a total of 2,923 cases were identified of which 58%, 19% and 23% where categorised under VTE definition A, B and C respectively (data not shown). Similarly 1,946 potential VTE cases were identified when only using secondary care data to identify first VTE of which 64%, 18% and 18% were categorized as VTE definition A, B and C respectively (data not shown).
Table 2. Percentages of VTE cases in the study population according to our three definitions.
| Cases of first VTE | N = 3,507 n (%) |
| VTE definition A 1 Total | 1,805 (51.0) |
| Diagnosis of VTE in HES and CPRD | 925 (51.2)* |
| Diagnosis in HES only | 168 (9.4)* |
| Diagnosis in CPRD only | 712 (39.4)* |
| VTE definition B 2 Total | 728 (20.6) |
| Diagnosis of VTE in HES and CPRD | 161 (22.1)* |
| Diagnosis of VTE in HES only with supporting evidence | 162 (22.2)* |
| Diagnosis of VTE in CPRD only with supporting evidence | 405 (55.6)* |
| VTE definition C 3 Total | 974 (27.6) |
| Only HES with no supporting evidence | 316 (32.4)* |
| Only CPRD with no supporting evidence | 658 (67.5)* |
Recorded VTE with supporting anticoagulant prescription, or medical code indicating anticoagulant therapy within 90 days of the event or death within 30 days of the event.
Recorded VTE codes with supporting sign or symptom of VTE within 15 days before or after the date of event.
Recorded VTE with no evidence of signs and symptoms or anticoagulant therapy.
Percentages based on different denominators (in bold).
Timing of VTE diagnosis (for cases diagnosed both in primary and secondary care)
Of the total 1,086 VTE cases documented in both primary and secondary care, 35% (n = 377) had the same date of diagnosis for VTE in both datasets. Of the total cases with a different date of diagnosis, 82% (n = 581) were first diagnosed in HES with a median delay of 7 days (IQR = 3–13) until it was recorded in patients' primary care records. For VTE cases first diagnosed in primary care (number of cases = 128) there was a median of 4 days difference (IQR = 1–18) in the recording between primary and secondary care date of VTE. This was broadly the same in the antepartum period, postpartum period and non-pregnant period.
Incidence of VTE in and around pregnancy
The rate of any VTE (VTE definition A, B or C) during the time not associated with pregnancy using both primary and secondary care data was 61 per 100,000 person-years (Table 3). This rate decreased by half when restricting to VTE definition A (32 per 100,000 person-years). When relying solely on primary care recording of first VTE, the rate using VTE definition A during antepartum, around delivery and postpartum periods was calculated to be 80, 461 and 324 per 100,000 person-years respectively (Table 4). Relying solely on secondary care data for the recording of first VTE, the calculated VTE rate during the time around delivery and postpartum was calculated to be 1799 and 180 per 100,000 person-years respectively.
Table 3. Rate of VTE per 100,000 person-years using different definitions of VTE in and around pregnancy (for pregnancies resulting in live or stillbirths).
| Time period | VTE definition A1, B2 or C3 | VTE definition A1 or B2 | VTE definition A1 | |||
| n | Rate* (95%CI) | n | Rate* (95%CI) | n | Rate* (95%CI) | |
| Not pregnant | 2,817 | 61 (58–63) | 2040 | 44 (42–46) | 1480 | 32 (30–33) |
| Antepartum | 377 | 240 (217–266) | 268 | 171 (151–192) | 156 | 99 (85–116) |
| Trimester 1 | 47 | 95 (71–127) | 41 | 83 (61–113) | 23 | 46 (31–70) |
| Trimester 2 | 76 | 148 (118–186) | 49 | 95 (74–126) | 30 | 58 (41–83) |
| Trimester 3 | 254 | 450 (398–509) | 178 | 315 (272–365) | 103 | 182 (150–221) |
| Around delivery | 76 | 3192 (423–546) | 38 | 1596 (1161–2193) | 34 | 1428 (1020–1998) |
| Postpartum | 237 | 481 (423–546) | 187 | 379 (329–438) | 135 | 274 (231–324) |
| Early postpartum | 200 | 801 (695–920) | 157 | 629 (537–735) | 177 | 468 (391–561) |
| Late postpartum | 37 | 151 (109–208) | 30 | 122 (95–175) | 18 | 73 (46–116) |
Rate per 100,000 person-years.
With women recorded as having a VTE event if they had a diagnosis in either database.
Recorded VTE with supporting anticoagulant prescription, or medical code indicating anticoagulant therapy within 90 days of the event or death within 30 days of the event.
Recorded VTE codes with supporting sign or symptom of VTE within 15 days before or after the date of event.
Recorded VTE with no evidence of signs and symptoms or anticoagulant therapy.
Table 4. Incidence rate of VTE per 100,000 person-years in and around pregnancy (for pregnancies resulting in live or stillbirths) by data source.
| Time period | VTE definition A1 | |
| n | Rate* (95% CI) | |
| Using only primary care data to identify first VTE events | ||
| Not pregnant | 1387 | 30 (28–31) |
| Antepartum | 126 | 80 (67–95) |
| Trimester 1 | 22 | 44 (29–68) |
| Trimester 2 | 31 | 60 (42–86) |
| Trimester 3 | 73 | 129 (102–162) |
| Around delivery | 11 | 461 (255–833) |
| Postpartum | 160 | 324 (278–379) |
| Early postpartum | 138 | 552 (467–652) |
| Late postpartum | 22 | 898 (591–136) |
| Using only secondary care data to identify first VTE events | ||
| Not pregnant | 985 | 21 (19–22) |
| Antepartum | 123 | 78 (65–93) |
| Trimester 1 | 18 | 36 (23–58) |
| Trimester 2 | 25 | 48 (32–72) |
| Trimester 3 | 80 | 141 (113–176) |
| Around delivery | 43 | 1799 (1334–2426) |
| Postpartum | 89 | 180 (146–221) |
| Early postpartum | 76 | 303 (242–279) |
| Late postpartum | 13 | 52 (30–91) |
Rate per 100,000 person-years.
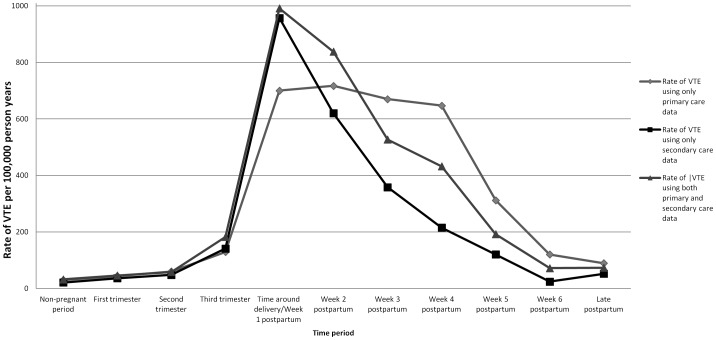
During the early postpartum period, the observed rate of VTE using both primary and secondary care data, peaked around the time of delivery and the first week of postpartum period (AR = 991 per 100,000 person years) after which the rates showed a graded decline throughout the remaining postpartum period (Figure 1). Compared to rates from the combined data sources, secondary care data showed a similar rate around delivery but much lower rates postpartum that decreased more rapidly following delivery. In contrast, primary care data showed lower rates around delivery but higher postpartum rates that remained consistently high until 4 weeks postpartum.
Figure 1. Rate of VTE in and around pregnancy and and non-pregnant periods using only VTE definition A.
Systematic review and meta-analysis of perinatal VTE incidence studies
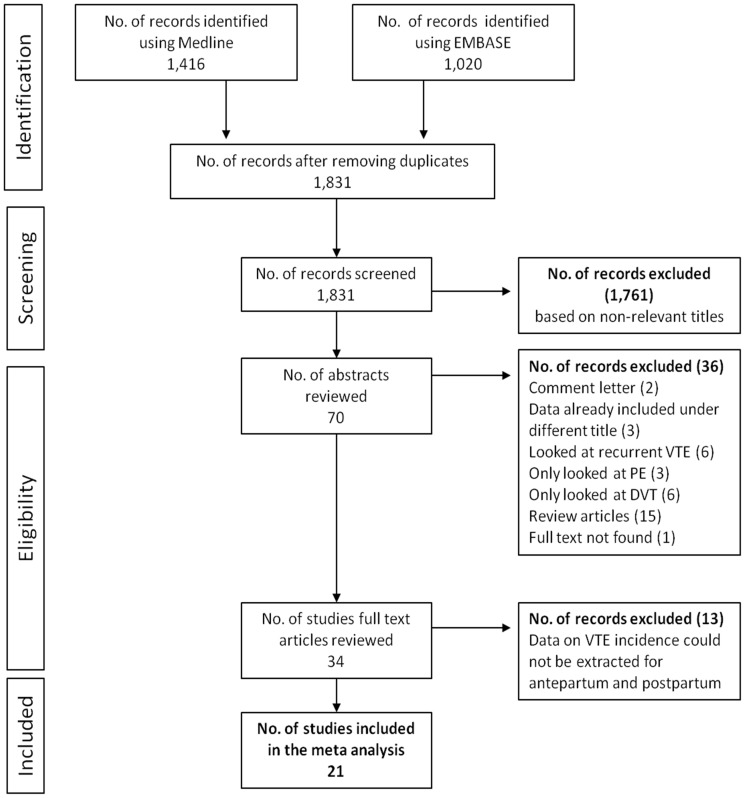
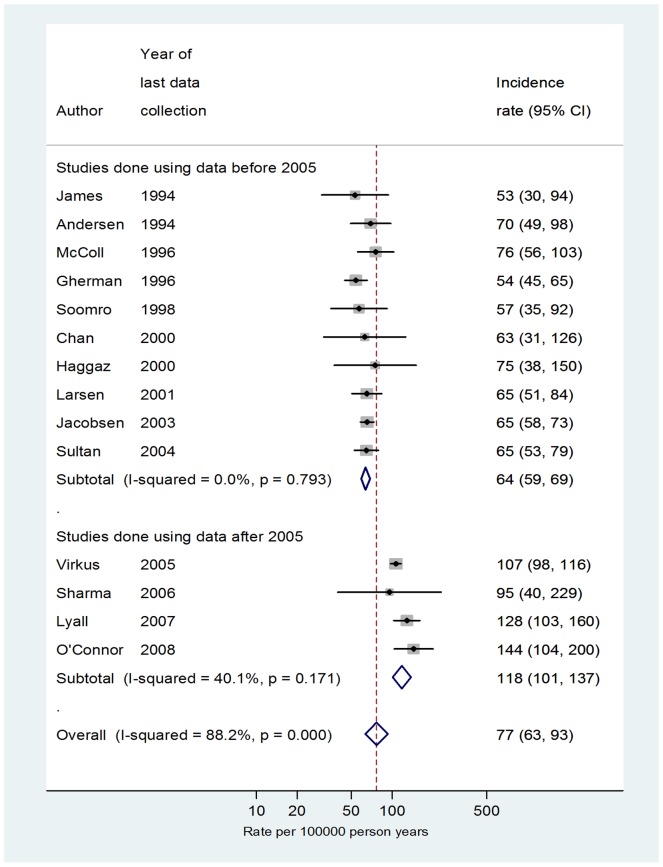
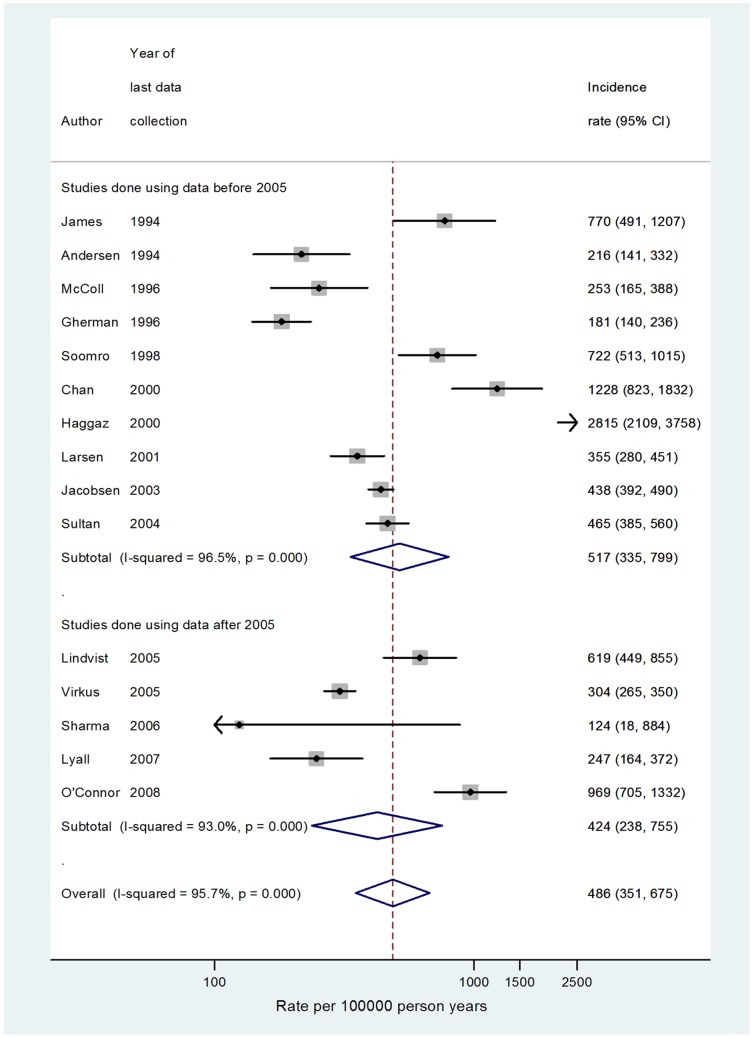
We identified 1,831 articles of which 34 had their full-texts reviewed and 21 were eventually included (Figure 2). The characteristics of the included studies are presented in Table 5. The incidence rate of VTE during the antepartum period from previous studies ranged from 37 per 100,000 person-years in the UK to 144 per 100,000 person-years in the U.S.A (data not shown). The pooled incidence rate of VTE during the antepartum period was 76 per 100,000 person-years (95% CI 65–90; heterogeneity I 2 = 97.6%). When restricting to studies where VTE cases were validated/confirmed (14 studies) we found a higher incidence of VTE after 2005 (AR = 118 per 100,000 person-years I 2 = 40%) compared to the rate before 2005 (AR = 64 per 100,000; I 2 = 0.0%) for the antepartum period. (Figure 3). The pooled absolute rate of VTE during the third trimester of pregnancy post 2005 (based on 389 VTE events; data not shown) was calculated to be 142 per 100,000 person-years (95% CI 93–158; I 2 = 70%) when a similar restriction was applied. Similarly the pooled absolute rate of VTE during the first six weeks postpartum (post 2005) was calculated to be 424 per 100,000 person-years (95%CI 238–755; I 2 = 96%; Figure 4).
Figure 2. PRISMA flow diagram for identification of VTE incidence studies during pregnancy, postpartum or both.
Table 5. Characteristics of the studies included by year of publication.
| Author (Year of publication) | Country | Study period | Study design and methodology | Measures taken to confirm VTE |
| Sultan [14] (2012) | United Kingdom | 1987–2004 | Population based retrospective cohort. Cases were identified using primary care data which were validated | VTE cases were confirmed based on anticoagulant therapy |
| Vikrus [19] (2011) | Denmark | 1995–2005 | Population based retrospective cohort. Cases were identified from national registry using ICD-10 codes. | Registry was validated for VTE during pregnancy with PPV of more than 80% |
| O'Cornnor [29] (2011) | United States | 2003–2008 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Liu [30] (2009) | Canada | 1991–2006 | Cross sectional study. Cases were identified using hospital discharge database using ICD-10 codes. | No measures taken to confirm VTE |
| Lyall [31] (2008) | United Kingdom | 1999–2007 | Cross sectional study. Cases were identified from a single unit | VTE cases were objectively confirmed based on diagnostics tests1 |
| Jacobsen [25] (2008) | Norway | 1990–2003 | Cross sectional study. Cases were identified from patient and birth register. | VTE cases were validated for the subset of the data |
| Lindqvist [32] (2008) | Sweden | 1990–2005 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Sharma [27] (2008) | Australia | 1999–2006 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Larsen [33] (2007) | Denmark | 1980–2001 | Cross sectional study. Cases were identified from a single county. | VTE cases were objectively confirmed based on diagnostics tests1 |
| James [20] (2006) | United States | 2000–2001 | Cross-sectional study. Cases were identified from national inpatient sample which covers around 100 hospitals using ICD-10 codes. | VTE cases were objectively confirmed1. Included some probable and possible VTEs |
| Heit [34] (2005) | United States | 1966–1995 | Population based cohort. Potentially fertile women were prospectively followed in a single county. | VTE cases were objectively confirmed1. Included some probable and possible VTEs |
| Haggaz [35] (2003) | Sudan | 1999–2000 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Anderson [36] (2003) | Denmark | 1984–1994 | Cross sectional study. Cases were identified using inpatient registry. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Soomro [37] (2002) | Saudi Arabia | 1986–1998 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Chan [24] (2001) | Japan | 1998–2000 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Ros [1] (2001) | Sweden | 1987–1995 | Population based retrospective cohort. Cases were identified using inpatient registry using ICD-9 codes | No measures taken to confirm VTE |
| Simpon [21] (2001) | United Kingdom | 1988–1997 | Cross sectional study in which cases were identified using ICD codes from hospital database | No measures taken to confirm VTE |
| Gherman [26] (1999) | United States | 1978–1996 | Cross sectional study. Cases were identified from a single hospital. | VTE cases were objectively confirmed based on diagnostics tests1 |
| Lindqvist [38] (1999) | Sweden | 1990–1993 | Cross sectional study. Cases were identified from Swedish birth and patient registry | No measures taken to confirm VTE |
| McColl [39] (1997) | United Kingdom | 1985–1996 | Cross sectional study. Cases were identified using national health service data. | VTE cases were objectively confirmed based on diagnostics tests1 |
| James [40] (1996) | United States | 1989–1994 | Cross sectional study where cases were identified from medical records at a single hospital. | No measures taken to confirm VTE |
A diagnosis of pulmonary embolism may be confirmed by pulmonary angiography, CT, MRI, ventilation-perfusion scan, pathological confirmation of thrombus etc. whereas the diagnosis of DVT may be confirmed by Doppler ultrasound, duplex ultrasconography, venography, pathological confirmation of thrombus etc. each of which may vary from study to study.
Figure 3. Rate of VTE per 100,000 person years during the antepartum period only including studies where some degree of case validation/confirmation was used.
The data were stratified by year.
Figure 4. Rate of VTE per 100,000 person years during the postpartum (first six week after childbirth) period only including studies where some degree of case validation/confirmation was used.
The data were stratified by year.
Comparison of meta-analysis estimates to the cohort analysis
The estimates from the meta-analysis post-2005 are than lower those generated using the inclusive VTE definition (VTE definition A, B or C and VTE definition A or B). However using only cases categorised under VTE definition A (those confirmed based on prescriptions or death), the incidence rate of 99, 182 and 468 during the antepartum period, third trimester of antepartum and early postpartum period (first six weeks after birth) from our cohort analysis are in concordance with the pooled estimates from recent studies (118, 142 and 424 respectively).
Discussion
Main findings
In this study, we have shown that a highly specific definition of VTE (VTE definition A) using data from both primary and secondary care health care settings is required to accurately estimate the incidence of VTE in and around pregnancy. The estimates we have derived are comparable with the pooled incidence rates for antepartum and early postpartum periods from the existing literature. With the use of linked primary and secondary care data we were able to get a far more accurate date of diagnosis for VTE which resulted in more precise estimates of rates close to delivery. Whilst the VTE rate can be accurately estimated in the separate data sources in some circumstance (e.g. trimesters of antepartum), when relying solely on primary care data we found that the rate of VTE was much lower during the time around delivery but higher during the postpartum period compared to solely using secondary care data. This difference is likely because of a delay in hospitalised events being recorded in primary care. However when solely relying on secondary care data the rate of VTE was much lower during the postpartum and non-pregnant periods. We believe the best method for defining and quantifying the incidence of VTE in and around pregnancy is therefore to use both primary and secondary care data and apply our VTE definition A to identify cases, as it has previously been validated [3] and is externally comparable to other pregnancy studies.
Ascertainment of VTE events
In our study, we were able to analyse 1,117,689 women of childbearing age and over 248,000 pregnancies to determine how the incidence of VTE in and around pregnancy varies based on the VTE definition and the dataset used; such analysis has not been done before. Our VTE definition A mimics a previously validated VTE definition in primary care CPRD [3] data in a non-pregnant population with a reported positive predictive value of 84%. To this algorithm we added secondary care diagnosis data which has an overall accuracy of 91% [17]. One current drawback of UK secondary care data is the lack of information on hospital prescribed heparin and warfarin which may have lead to under ascertainment of cases using VTE definition A. However, we believe that the impact of this limitation should be minimal as pregnant women with a VTE diagnosis are expected to be on anticoagulation therapy throughout their pregnancies and this therapy period extends up to three months for postpartum women [18]. Therefore these prescriptions are likely to be captured in the primary care data. We must acknowledge that 33% of pregnancies had no information on the length on gestation. When we conducted a sensitivity analysis, however, and calculated the rate of VTE stratified by those with and without information on gestational age this showed no difference in our estimates of VTE within each trimester of pregnancy.
Date of VTE diagnosis
Another strength of linked data is the improvement in estimation of the date of diagnosis of VTE. The majority (84%) of the VTEs diagnosed in both primary and secondary care using the linked data had the diagnosis made in secondary care data first. Therefore if we were reliant on primary care data alone there would be a concern with the delay in recording of VTE from secondary to primary care. Our study demonstrated a median lag of 7 days in the recording of VTE events from secondary to primary care. This delay can restrict the ability to give precise incidence estimates in narrow windows of time such as around delivery and probably explains our low incidence rate during the time around delivery and prolonged high risk during the early weeks of postpartum when only relying on primary care data for VTE diagnosis. One potential limitation of the secondary care data is the reliance on episode start date as the date of VTE event. This creates problems in separating out antepartum versus postpartum VTE events around the time of delivery. An example of this problem is that reported in a cohort study of women delivering in hospital by Virkus et.al [19] who considered the date of admission to hospital as the date of diagnosis for VTE. They reported a high rate of VTE during the third trimester of the antepartum compared to previous studies in the meta-analysis post 2005. This may be explained by some postpartum VTE events occurring during the maternal admission having been classified as antepartum. A similar, yet not acknowledged, problem may be the case for other studies utilizing hospital discharge data [20], [21]. In our study, HES provides a better option in terms of date of episodes for each diagnosis within each hospital period. We think this rather than the date of hospital admission or discharge will more accurately estimate the true VTE diagnosis date (although not necessarily the actual biological onset of the VTE). Furthermore the division of pregnancy periods as antepartum, around delivery, and postpartum adequately addresses the concern of mis-classification of VTE events around childbirth.
Meta-analysis
Our meta-analyses showed high levels of heterogeneity occurring among the individual studies for both antepartum and postpartum pooled estimates. In descriptive epidemiological studies where a statistic is estimated among a single group (such as pregnant women in this review), the potential for heterogeneity is far greater than for analytic or comparative studies (i.e., when two groups are compared to calculate a measure of effect such as an odds or risk ratio). This is because incidence rates are very sensitive to the choice of study population, outcome definition and dataset used meaning at least some heterogeneity will be inevitable; other published meta-analyses of this type also report very high levels of heterogeneity [15], [22], [23]. The heterogeneity in our data during the antepartum period was partially explained by calendar year and whether VTE cases were subjected to a certain degree of validation/confirmation or not. For instance, when we restricted our analysis only to studies where VTE cases were validated/confirmed and stratified them by calendar year, our I2 value was less than 50%. For the postpartum, incidence rates during the first six weeks post-delivery were largely inconsistent even after restricting to studies with validated/confirmed VTE and stratifying the estimates by calendar year. This wide variation in the reported rates can probably be explained by the various countries' health care systems and their thromboprophylaxis practices after childbirth. The UK Royal College of Obstetricians and Gynaecologists recommendation on VTE risk assessment and thromboprophylaxis post-caesarean section dates back to 1993, which may have been adapted by different countries at different points in time. For instance a study from China [24] reported the rate of VTE to be 1228 per 100,000 person-years where there was no concept of thromboprophylaxis prior to the year 2000 versus studies from Norway [25] and UK [14] with lower reported rates (AR around 400 per 100,000 person-years). This is something we were not able to account of in our meta-analysis for postpartum VTE. Most of the previous literature on this subject has relied on secondary care data which will inevitably miss many non-fatal VTE events diagnosed and managed exclusively in primary care, particularly during the postpartum period [1], [19], [26], [27].
Although we did no external validation of our VTE definitions among women included in our cohort study, based on our most inclusive definition (i.e. including events categorised under VTE definition A, B or C) our calculated rates of VTE during antepartum and postpartum periods were considerably higher than the pooled incidence rate of previous studies. This suggests the inclusion of many false positive events, limiting the validity of such an inclusive VTE definition. The same issue occurred, although to a lesser extent, when including VTE events classified under VTE definition A or B where we included cases with clinical signs and symptoms of VTE or evidence of diagnostic tests in addition to anticoagulant therapy. This may be due to the fact that leg swelling and calf pain are common in the third trimester of pregnancy in women without DVT which can lead to potential misclassification. Additionally, D-dimer levels increase [28] in pregnancy, with gestational hypertension, and in preterm labour leading to false positive events which may add to that misclassification. In contrast the absolute rates of VTE using VTE definition A for antepartum and postpartum periods of 99 and 468 per 100,000 person-years respectively are broadly in concordance with pooled estimates from previous studies using similar methodology where VTE cases were validated/confirmed.
Conclusions
Our results have important implications for the way in which VTE is studied in pregnancy using routinely available electronic health care records, data which are crucial for assessing outcomes that are severe and rare and thus rely on evidence from large population-based sources. Firstly, we have quantified the incidence of VTE in and around pregnancy using a variety of VTE definitions. This demonstrated that the absolute rate of VTE greatly varies based on the VTE definition used, with our VTE definition A providing the most comparable estimates of the absolute rates to previous work. We have also demonstrated that there are some important limitations in using solely primary care or secondary care data in terms of the date and ascertainment of VTE diagnosis which need to be considered when interpreting studies that do this. We have shown in our study that using both primary and secondary care data not only provide better estimation of the date of VTE diagnosis, but also enable researchers to comprehensively identify VTE cases diagnosed and recorded both in primary or secondary care. Furthermore, the use of both primary and secondary care data combined may provide better ascertainment of maternal risk factors for VTE, information on hospitalization, primary care prescriptions, information on life style related factors and co-morbidities. This vital information could be used to better understand the occurrence and risk factors of VTE in and around pregnancy for future research
Supporting Information
Search strategy used for Medline database.
(DOCX)
Search strategy used for Embase database.
(DOCX)
Funding Statement
The authors have no funding or support to report.
References
- 1. Ros SH, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S (2001) Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 12: 456–460. [DOI] [PubMed] [Google Scholar]
- 2. Virkus RA, Lokkegaard ECL, Bergholt T, Mogensen U, Langhoff-Roos J, et al. (2011) Venous thromboembolism in pregnant and puerperal women in Denmark 1995–2005. A national cohort study. Thrombosis and Haemostasis 106: 304–309. [DOI] [PubMed] [Google Scholar]
- 3. Lawrenson R, Todd JC, Leydon GM, Williams TJ, Farmer RD (2000) Validation of the diagnosis of venous thromboembolism in general practice database studies. British Journal of Clinical Pharmacology 49: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hospital Episode Statistics.
- 5. Dattani N, Datta-Nemdharry P, Macfarlane A (2012) Linking maternity data for England 2007: methods and data quality. Health Statistics Quarterly 53. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Practice Research Database.
- 7. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. British Journal of Clinical Pharmacology 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jick H, Jick SS, Derby LE (1991) Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ: British Medical Journal 302: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eaton SC, Williams TJ, Puri S, VanStaa T (2008) The feasibility of linking the English Hospital Episode Statistics to the GPRD. Pharmacoepidemiol Drug Saf 17: S214. [Google Scholar]
- 10.Crooks C (2013) Epidemiology of upper gastrointestinal bleeding studying its causes and outcomes using case control studies and surivival analyses. Ph.D. Thesis. The University of Nottingham.
- 11. Wright FL, Green J, Canoy D, Cairns BJ, Balkwill A, et al. (2012) Vascular disease in women: comparison of diagnoses in hospital episode statistics and general practice records in England. BMC medical research methodology 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crooks C, Card TR, West J (2012) Defining upper gastrointestinal bleeding from linked primary and secondary care data and the effect on occurrence and 28 day mortality. BMC health service research 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health and social care information center (2011) NHS Maternity Statistics - England, 2010–2011.
- 14. Sultan AA, West J, Tata LJ, Fleming KM, Nelson-Piercy C, et al. (2012) Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. British Journal of Haematology 156: 366–373. [DOI] [PubMed] [Google Scholar]
- 15. Horsted F, West J, Grainge MJ (2012) Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis. PLoS Medicine 9: e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Pregnancy and Childbirth Group Search methods for identifying trial reports for the Cochrane Pregnancy and Childbirth Group's Trials Register,.
- 17. Campbell SE, Campbell MK, Grimshaw JM, Walker AE (2001) A systematic review of discharge coding accuracy. Journal of Public Health 23: 205–211. [DOI] [PubMed] [Google Scholar]
- 18.Royal College of Obstetricians and Gynaecologist (2010) The acute management of thrombosis and embolism during pregnancy and puerperium. Green-top guideline No. 37b London: RCOG Press.
- 19. Virkus RA, Løkkegaard ECL, Bergholt T, Mogensen U, Langhoff-Roos J, et al. (2011) Venous thromboembolism in pregnant and puerperal women in Denmark 1995–2005. Thrombosis and Haemostasis 106: 304–309. [DOI] [PubMed] [Google Scholar]
- 20. James AH, Jamison MG, Brancazio LR, Myers ER, Jamison MG, et al. (2006) Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. American Journal of Obstetrics & Gynecology 194: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 21. Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD (2001) Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG: An International Journal of Obstetrics & Gynaecology 108: 56–60. [DOI] [PubMed] [Google Scholar]
- 22. Eaden J, Abrams K, Mayberry J (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas T, Abrams K, Robinson R, Mayberry J (2007) Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Alimentary Pharmacology and Therapeutics 25: 657–668. [DOI] [PubMed] [Google Scholar]
- 24. Chan LY, Tam WH, Lau TK (2001) Venous thromboembolism in pregnant Chinese women. Obstetrics & Gynecology 98: 471–475. [DOI] [PubMed] [Google Scholar]
- 25. Jacobsen AF, Skjeldestad FE, Sandset PM (2008) Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium–a register-based case-control study. American Journal of Obstetrics & Gynecology 198: 233.e231–237. [DOI] [PubMed] [Google Scholar]
- 26. Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, et al. (1999) Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstetrics & Gynecology 94: 730–734. [DOI] [PubMed] [Google Scholar]
- 27. Sharma S, Monga D (2008) Venous thromboembolism during pregnancy and the post-partum period: incidence and risk factors in a large Victorian health service. Australian & New Zealand Journal of Obstetrics & Gynaecology 48: 44–49. [DOI] [PubMed] [Google Scholar]
- 28. Drife J (2003) Thromboembolism. British Medical Bulletin 67: 177–190. [DOI] [PubMed] [Google Scholar]
- 29. O'Connor DJ, Scher LA, Gargiulo NJ 3rd, Jang J, Suggs WD, et al. (2011) Incidence and characteristics of venous thromboembolic disease during pregnancy and the postnatal period: a contemporary series. Annals of Vascular Surgery 25: 9–14. [DOI] [PubMed] [Google Scholar]
- 30. Liu S, Rouleau J, Joseph KS, Sauve R, Liston RM, et al. (2009) Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstétrique et gynécologie du Canada : JOGC 31: 611–620. [DOI] [PubMed] [Google Scholar]
- 31. Lyall H, Myers B (2008) Incidence and management of 82 cases of pregnancy-associated venous thromboembolism occurring at a single centre–comparison with Voke et al. British Journal of Haematology 142: 309–311 author reply 311–302. [DOI] [PubMed] [Google Scholar]
- 32. Lindqvist PG, Torsson J, Almqvist A, Bjorgell O (2008) Postpartum thromboembolism: severe events might be preventable using a new risk score model. Vascular Health & Risk Management 4: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsen TB, Sorensen HT, Gislum M, Johnsen SP (2007) Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: a population-based nested case-control study. Thrombosis Research 120: 505–509. [DOI] [PubMed] [Google Scholar]
- 34. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, et al. (2005) Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Annals of Internal Medicine 143: 697–706. [DOI] [PubMed] [Google Scholar]
- 35. Haggaz AARD, Mirghani OA, Adam I (2003) Venous thromboembolism in pregnancy and the puerperium in Sudanese women. International Journal of Gynaecology & Obstetrics 83: 309–310. [DOI] [PubMed] [Google Scholar]
- 36. Anderson FA Jr, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107: 17. [DOI] [PubMed] [Google Scholar]
- 37. Soomro RM, Bucur IJ, Noorani S (2002) Cumulative incidence of venous thromboembolism during pregnancy and puerperium: a hospital-based study. Angiology 53: 429–434. [DOI] [PubMed] [Google Scholar]
- 38. Lindqvist P, Dahlblack B, Marsal K (1999) Thrombotic risk during pregnancy: a population study. Obstetrics & Gynecology 94: 595–599. [DOI] [PubMed] [Google Scholar]
- 39. McColl MD, Ramsay JE, Tait RC, Walker ID, McCall F, et al. (1997) Risk factors for pregnancy associated venous thromboembolism. Thrombosis & Haemostasis 78: 1183–1188. [PubMed] [Google Scholar]
- 40. James K, Lohr J, Deshmukh R, Cranley J (1996) Venous thrombotic complications of pregnancy. Cardiovascular Surgery 4: 777–782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy used for Medline database.
(DOCX)
Search strategy used for Embase database.
(DOCX)