Abstract
Background
Chemotherapy is essential to improve the prognosis of the patients with osteosarcoma, and the response to chemotherapy is an important prognostic factor. In this study, the impact of various radiological examinations on overall survival (OS) and event-free survival (EFS) was evaluated.
Method
Eighty-two patients with high-grade osteosarcoma were included in this study, and we evaluated the following factors for prognostic significance: age (≥40 years), gender (male), tumor location (truncal site), metastatic disease, histological response to chemotherapy, radiological response to chemotherapy assessed using X-ray, angiography, CT, MRI, 201Tl scintigraphy, and 99mTc-MIBI scintigraphy (99mTc-MIBI), and combined radiological score (CRS).
Results
Univariate analyses revealed that metastatic disease, histological response, 99mTc-MIBI, and CRS were significantly correlated with OS. Multivariate analyses showed that metastatic disease (OS: HR 35.9, P<0.001; EFS: HR 17.32, P<0.001) was an independent predictor of OS and EFS. Tumor location (HR 36.1, P = 0.003), histological response (HR 31.1, P = 0.036), and 99mTc-MIBI (HR 18.4, P = 0.038) were significant prognostic factors for OS. Moreover, CRS was a marginally significant predictor of OS and EFS.
Conclusion
The chemotherapeutic effects evaluated by 99mTc-MIBI and CRS could be considered as prognostic factors in osteosarcoma.
Introduction
Osteosarcoma is the most common primary malignant bone tumor and primarily affects adolescents. Multidisciplinary approaches, including chemotherapy and wide resection surgery, dramatically improve the treatment outcomes for patients with osteosarcoma; however, metastatic lesions and poor response to chemotherapy have been reported as important prognostic factors as well [1], [2]. The response to chemotherapy is commonly assessed by histological analysis, although it is difficult to evaluate the chemotherapeutic effects before tumor excision. On the other hand, we previously reported that Tc-99m-methoxyisobutyl-isonitrile scintigraphy (99mTc-MIBI) and combined radiological score (CRS) revealed significant correlation with histological response to chemotherapy [3], [4]. Radiological examination is useful to evaluate the chemotherapeutic effects before surgical treatment. At our institute, patients with osteosarcoma are examined using X-ray photography (X-ray), angiography, magnetic resonance imaging (MRI), Thallium-201 scintigraphy (201Tl), and 99mTc-MIBI before chemotherapy as well as after 3–5 courses of chemotherapy. Although there are many reports regarding the prognostic significance of histological response to chemotherapy, there are few reports that have assessed the prognostic value of radiological examinations in the treatment of osteosarcoma. Thus, we investigated the correlation between response to chemotherapy and clinical outcomes in patients with osteosarcoma.
Methods
Patients
Between May 1992 and July 2012, 137 patients with osteosarcoma were treated at Kanazawa University Hospital. The patients received X-ray, computed tomography (CT), MRI, and bone scintigraphy for the diagnosis and assessment of clinical staging. All the tumors were confirmed pathologically from the specimens obtained from biopsy and surgery. Among the 137 patients, 82 with clinical records, who were diagnosed as high-grade osteosarcoma and who received chemotherapy and surgical treatment, were included in the present study (Table 1). Patients with low-grade osteosarcoma and those who did not undergo surgery or receive chemotherapy were excluded from this study. This study was approved by the Institutional Review Board of the Kanazawa University Graduate School of Medical Science, Kanazawa, Japan. Written informed consent was obtained from all patients and/or their family. All the patients received 3–5 courses of preoperative chemotherapy at intervals of 2–3 weeks [5]. In each chemotherapy course, cisplatin (120 mg/m2) was continuously infused through a catheter for 1–2 h followed by 48 h of continuous doxorubicin infusion (30 mg/m2/day for 2 days) and 72 h of caffeine infusion (1.5 g/m2/day for 3 days). Before chemotherapy and after 3–5 courses of chemotherapy, the chemotherapeutic effects were evaluated using X-ray, angiography, MRI, 201Tl, and 99mTc-MIBI. Three to five courses of chemotherapy were administered, and subsequently, surgery was performed. All the tumors were surgically resected, and the chemotherapeutic effects were evaluated histologically. Further, 5 or 6 courses of additional chemotherapy were administered using ifosfamide (3 g/m2/day for 3 days), etoposide (60 mg/m2/day for 3 days), and caffeine (1.5 g/m2/day for 3 days). Follow-up evaluation to detect local recurrence and metastasis consisted of X-ray, CT, MRI, bone scan, and 201Tl scan.
Table 1. Characteristics of the study patients.
| Characteristic | No. |
| Follow up period (months) | 49.5 (6–190) |
| Age at diagnosis (y) | 22.1 (5–69) |
| <40 | 68 |
| ≥40 | 14 |
| Gender | |
| Male | 48 |
| Female | 34 |
| Primary tumor location | |
| Femur | 48 |
| Tibia | 21 |
| Humerus | 5 |
| Pelvis | 4 |
| Other | 4 |
| Pathologic subtype | |
| Osteoblastic | 58 |
| Chondroblastic | 16 |
| Fibroblastic | 4 |
| Other | 4 |
| Surgical approach | |
| Limb salvage | 78 |
| Amputation | 4 |
| Surgical stage | |
| IIA | 2 |
| IIB | 52 |
| IIIB | 28 |
| Huvos grade | |
| I (<50% necrosis) | 8 |
| II (50–89% necrosis) | 24 |
| III (≥90% necrosis) | 20 |
| IIIB (100% necrosis) | 30 |
Image Analyses
All the 82 patients were assessed using X-ray, 64 using angiography, 77 using MRI, 68 using 201Tl, and 57 using 99mTc-MIBI before chemotherapy and after 3–5 courses of chemotherapy. The images were assessed by radiologists without any knowledge of the histological response and clinical outcomes of these patients.
X-ray images were assessed on the basis of cortical recovery and continuity, and angiograms were assessed on the basis of changes in tumor vascularity (Table 2). On the basis of these results, patients were classified into the following groups: complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). CR and PR were classified as responders.
Table 2. Radiological evaluation method for chemotherapeutic effect.
| Definition of the responders | Point | |
| X-ray | Sclerotic change and/or cortical remodeling | 1 |
| Angiogram | Disappearance of pathologic vascularization | 1 |
| MRI | ΔEM ≥30% | 2 |
| 201Tl | ΔUR ≥30% | 2 |
| 99mTc-MIBI | ΔUR ≥30% | 2 |
| Combined radiological score | Total point ≥60% |
ΔEM: the percentage reduction in extraskeletal masses (EM).
ΔUR: the percentage reduction in uptake ratio (UR).
Combined radiological score (CRS) = Total of the radiological score/Full marks (%).
MR images were assessed on the basis of reduction of the maximum diameter of the extraskeletal mass (EM) [6]. The percentage reduction in EM (ΔEM) was calculated as follows:
Both 99mTc-MIBI and 201Tl images were assessed by the reduction in uptake ratio (UR) [3], [4]. For quantitative analysis of the images, a manual region of interest (ROI) was set on the tumor site, and a symmetrical ROI was set on the contralateral normal area as a background. UR in 99mTc-MIBI and 201Tl were calculated by dividing the count density of the lesion by that of the background ROI. For assessment of the chemotherapeutic effect, the percentage reduction in uptake ratio (ΔUR) was defined as follows:
On the basis of the images, patients who demonstrated ΔEM ≥30% in MRI, ΔUR ≥30% in 201Tl, and ΔUR ≥30% in 99mTc-MIBI were classified as responders [3], [4], [6].
We recently reported a combined radiological scoring system, which revealed a strong correlation with the histological response to chemotherapy (Table 2) [4]. For patients who were classified as good responders by X-ray or angiography, 1 extra point was added to the total number of points. For patients who were classified as good responders by MRI, 201Tl, or 99mTc-MIBI, 2 extra points were added to the total number of points. Combined radiological scores (CRS) were defined as follows:
For patients who could not be assessed using some of the imaging methods, the combined radiological score was calculated from the obtained images. Patients who demonstrated CRS ≥60% were classified as responders [4].
Histological Evaluation of Chemotherapeutic Effects
Resected specimens were assessed according to histological response to chemotherapy. In patients with tumor-bearing bones that were reused as autografts, specimens of the resected bone were histologically assessed. Histological response to chemotherapy was assessed according to the degree of cellularity and necrosis in the largest slice of the resected tumor [7]. Grade IV (100% necrosis) and grade III (90%–99% necrosis) were considered as good responses to chemotherapy, whereas grade II (50%–89% necrosis) and grade I (0%–49% necrosis) were considered as poor responses to chemotherapy.
Statistical Analysis
The factors of patient age (<40 and ≥40 years), gender, tumor location (extremity or trunk), metastatic disease, histological response (<90% and ≥90% necrosis), and radiological response (X-ray, angiography, MRI, 201Tl, 99mTc-MIBI, and CRS) were subjected to univariate analyses. Overall survival (OS) and event-free survival (EFS) were calculated using the Kaplan–Meier method along with the log rank test. In addition, the Cox proportional hazards regression model was used for multivariate analyses to identify independent predictors of OS and EFS. Any factor with P<0.4 in the univariate analysis was included in the multivariate Cox proportional hazards model. OS was defined as the time from the initial diagnosis to death from any cause. On the other hand, EFS was defined as the time from the initial diagnosis to metastasis, local recurrence, or death from any cause. Statistical significance was defined as P<0.05. Marginal statistical significance was defined as 0.05≤P<0.10. Statistical analyses were performed using the EZR statistical software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Patients
A total of 82 patients with high-grade osteosarcoma, [48 male and 34 female; mean age, 22.1 (range, 5–69) years], who received chemotherapy and surgical treatment and could be reviewed through clinical records, were retrospectively enrolled in this study (Table 1). The mean follow-up period was 49.5 months (range, 6–190 months). At initial diagnosis, 28 patients (34.1%) demonstrated clinically detectable metastases, and 54 patients (65.9%) did not present any metastasis. Histologically, 58 patients were classified as osteoblastic type, 16 patients as chondroblastic type, 4 as fibrous type, and 4 as “other” type. The tumors were located in the femur in 48 cases, tibia in 21, humerus in 5, pelvis in 4, fibula in 2, clavicle in 1, and calcaneus in 1. On the basis of the Enneking’s surgical staging, 2 patients were classified as stage IIA, 52 as stage IIB, and 28 as stage IIIB [8].
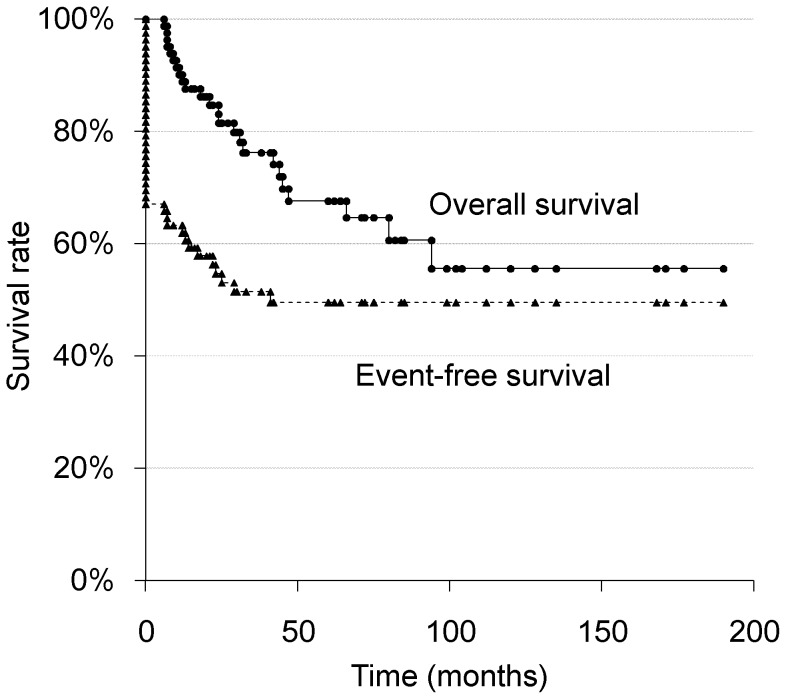
The 3-, 5-, 10-year OS and EFS rates of the 82 patients were 76.2, 67.6, and 55.6%, and 51.4, 49.6, and 49.6%, respectively (Fig. 1).
Figure 1. Overall and event-free survival of all 82 patients.
Overall Survival (OS)
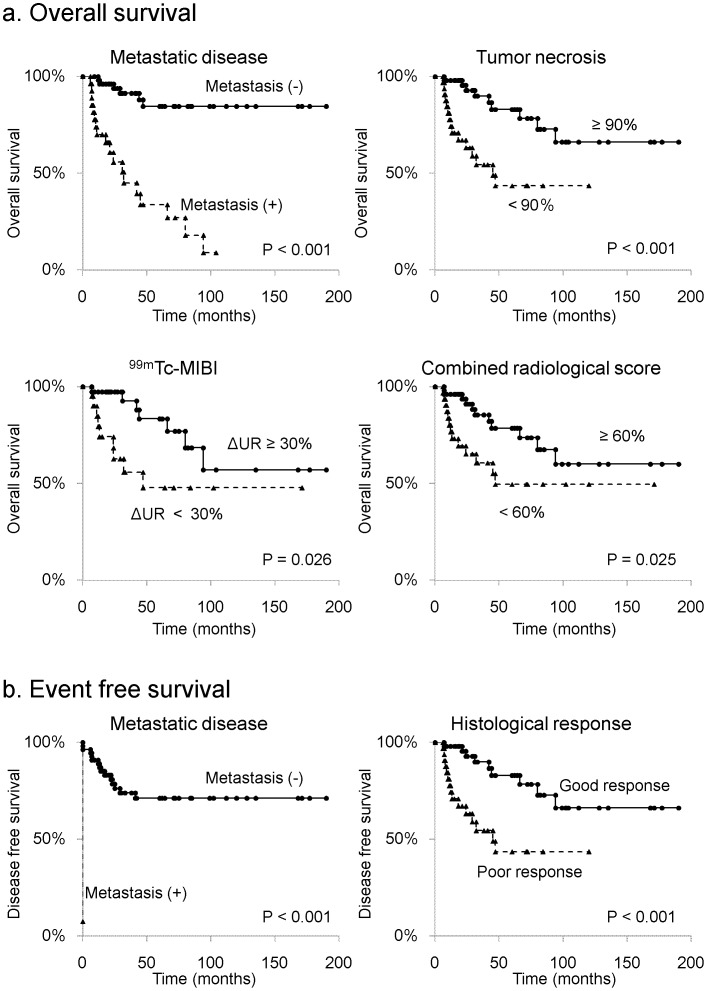
Kaplan–Meier analysis demonstrated that metastases at initial diagnosis (P<0.001), histological response (P<0.001), 99mTc-MIBI response (P = 0.026), and CRS (P = 0.025) were significantly associated with OS (Table 3). The 5-year OS rate of the patients with metastases at initial diagnosis was 33.8%, whereas the 5-year OS rate of the patients without metastasis was 84.6% (Fig. 2a). In addition, the 5-year OS rate of the patients with <90% necrosis was 43.6%, whereas the 5-year OS rate of the patients with ≥90% was 83.0% (Fig. 2a). Moreover, the 5-year OS rate of the patients who presented <30% ΔUR in 99mTc-MIBI was 47.8%, whereas the 5-year OS rate of the patients who presented ≥30%ΔUR in 99mTc-MIBI was 83.4% (Fig. 2a). Furthermore, the 5-year OS rate of the patients who revealed <60% CRS was 49.6%, whereas the 5-year OS rate of the patients revealed ≥60% CRS was 78.6% (Fig. 2a).
Table 3. Univariate analysis of overall survival.
| Charasteristic | 5-year OS | P | |
| Age | <40 years | 69.0 | 0.732 |
| ≥40 years | 63.3 | ||
| Gender | Male | 76.6 | 0.098 |
| Female | 52.3 | ||
| Location | Extremity | 70.3 | 0.312 |
| Trunk | 40.0 | ||
| Metastasis | Absent | 84.6 | <0.001** |
| Present | 33.8 | ||
| Histological response | necrosis<90% | 83.0 | <0.001** |
| necrosis≥90% | 43.6 | ||
| X-ray | Good | 70.9 | 0.691 |
| Poor | 58.4 | ||
| Angiography | Good | 74.7 | 0.051 |
| Poor | 39.1 | ||
| MRI | ΔEM≥30% | 76.7 | 0.113 |
| ΔEM<30% | 58.4 | ||
| 201Tl | ΔUR≥30% | 74.5 | 0.209 |
| ΔUR<30% | 63.6 | ||
| 99mTc-MIBI | ΔUR≥30% | 83.4 | 0.026* |
| ΔUR<30% | 47.8 | ||
| CRS | ≥60% | 78.6 | 0.025* |
| <60% | 49.6 | ||
P<0.05,
P<0.01.
Figure 2. Kaplan-Meier curves of overall survival and event-free survival for variables with prognostic significance in univariate analyses.
a. Overall survival. b. Event-free survival.
Multivariate analysis revealed that metastatic disease at initial diagnosis [hazard ratio (HR) = 35.9; P<0.001], truncal site (HR = 36.1; P = 0.003), histological response (HR = 31.1; P = 0.036), and 99mTc-MIBI response (HR = 18.4; P = 0.038) were significant independent predictive factors of OS (Table 4). CRS was a marginally significant predictive factor of OS (HR = 141.5; P = 0.059).
Table 4. Multivariate Cox models of overall survival for study patients.
| Charasteristic | HR | 95% CI | P |
| Male | 0.58 | 0.16–2.13 | 0.408 |
| Location (truncal site) | 36.05 | 3.46–375.20 | 0.003** |
| Metastatic disease | 35.88 | 5.77–223.30 | <0.001** |
| Histological response | 31.13 | 1.25–773.00 | 0.036* |
| Angiography | 2.36 | 0.52–10.62 | 0.264 |
| MRI | 2.13 | 0.26–17.18 | 0.479 |
| 201Tl | 2.35 | 0.22–25.32 | 0.482 |
| 99mTc-MIBI | 18.42 | 1.18–286.90 | 0.038* |
| CRS | 141.54 | 0.83–24102 | 0.059 |
P<0.05,
P<0.01.
Event-free Survival (EFS)
Kaplan–Meier analysis demonstrated that metastasis at initial diagnosis (P<0.001) and histological response (P = 0.040) were significantly associated with EFS (Table 5). The 5-year EFS of the patients with metastases was 0%, whereas the 5-year EFS rate of the patients without metastasis was 71.2% (Fig. 2b). In addition, the 5-year EFS of the patients with <90% necrosis was 33.7%, whereas the 5-year EFS rate of the patients with ≥90% necrosis was 60.7% (Fig. 2b).
Table 5. Univariate analysis of event-free survival.
| Charasteristic | 5-year EFS | P | |
| Age | <40 years | 49.1 | 0.517 |
| ≥40 years | 53.9 | ||
| Gender | Male | 52.6 | 0.350 |
| Female | 45.7 | ||
| Location | Extremity | 49.8 | 0.904 |
| Trunk | 44.4 | ||
| Metastasis | Absent | 71.2 | <0.001** |
| Present | 0 | ||
| Histological response | necrosis<90% | 60.7 | 0.040* |
| necrosis≥90% | 33.7 | ||
| X-ray | Good | 47.2 | 0.478 |
| Poor | 57.6 | ||
| Angiography | Good | 55.6 | 0.143 |
| Poor | 33.3 | ||
| MRI | ΔEM≥30% | 52.6 | 0.501 |
| ΔEM<30% | 44.7 | ||
| 201Tl | ΔUR≥30% | 54.9 | 0.565 |
| ΔUR<30% | 47.0 | ||
| 99mTc-MIBI | ΔUR≥30% | 55.8 | 0.385 |
| ΔUR<30% | 43.3 | ||
| CRS | ≥60% | 54.5 | 0.328 |
| <60% | 42.0 | ||
P<0.05,
P<0.01.
Multivariate analysis revealed that only metastatic disease at initial diagnosis was a significant independent predictor of EFS (HR = 17.3; P<0.001; Table 6). Moreover, CRS was a marginally significant predictor of EFS (HR = 8.85; P = 0.079).
Table 6. Multivariate Cox models of event-free survival for study patients.
| Charasteristic | HR | 95% CI | P |
| Male | 0.769 | 0.31–1.92 | 0.573 |
| Metastatic disease | 17.32 | 3.89–77.20 | <0.001** |
| Histological response | 3.13 | 0.62–15.78 | 0.166 |
| Angiography | 1.91 | 0.68–5.38 | 0.221 |
| 99mTc-MIBI | 3.89 | 0.74–20.51 | 0.110 |
| CRS | 8.85 | 0.78–100.58 | 0.079 |
P<0.01.
Discussion
There are a large number of reports regarding prognostic factors for patients with osteosarcoma such as age, truncal location, gender, metastatic lesions at initial diagnosis, chemotherapeutic effects, tumor size, alkaline phosphatase (ALP), recurrence, and P-glycoprotein [1], [2], [9]–[15]. Among these factors, histological analysis of excised tumors remains the most accurate method to assess the response to chemotherapy, and the significance of this method in the prognosis of patients with osteosarcoma is well documented [1], [2]. The present study also indicated that tumor necrosis was significantly correlated with OS, which is in accordance with the findings of previous reports [1], [2].
In the treatment of osteosarcoma, various radiological examinations, including X-ray, MRI, 201Tl, 99mTc-MIBI, and positron emission tomography, are often performed before and after preoperative chemotherapy to assess extent of tumor invasiveness and cortical destruction and to plan the surgical treatment [3], [4], [6], [16]. Radiological examinations reflect the response to chemotherapy [3], [4], [6], and although X-ray is cheap and convenient, the results are often inaccurate. For patients who receive intra-arterial chemotherapy, angiography is also a convenient tool and depicts tumor vascularity, which reflects the chemotherapeutic effects. However, angiography is invasive for patients treated with intravenous chemotherapy, and the quantification of tumor blood flow using angiography is difficult. On the other hand, CT and MRI can be evaluated quantitatively and offer more accurate imaging of the local extent of osteosarcoma, which influences the surgical margins [6]. Reportedly, the changes in tumor size calculated using CT and MRI were significantly associated with the prognosis of the patients with osteosarcoma [6]. However, differentiation between residual tumors and fibrotic tissues using MRI is difficult, even with contrast enhancement [17]. In addition, the usefulness of 201Tl and 99mTc-MIBI to assess the chemotherapeutic effects has been reported, and each of these can be used for quantitative assessment [3], [4], [18]–[24]. The accumulation of 201Tl depends on sodium–potassium pump activity and cellular chloride co-transport system and is useful for assessment of tumor activity and differentiation between residual tumors and fibrotic tissue [25]. On the other hand, 99mTc-MIBI also accumulates in malignant tumors, and this accumulation depends on the mitochondrial function [26]–[28]. Assessment of the chemotherapeutic response by using only one radiological examination is limited in cases with no extraskeletal mass, few tumor vessels, and low accumulation of 201Tl and 99mTc-MIBI. Recently, we developed a combined radiological scoring system, which revealed strong correlation with the histological response to chemotherapy [4].
Surgical margins can be reduced in patients whose radiological examinations indicate good response to chemotherapy. Intentional marginal excision has an outcome similar to that of wide excision in patients who show a complete response to chemotherapy [5]. On the other hand, a poor response to preoperative chemotherapy makes it difficult to reduce surgical margins and salvage the affected limbs. Although chemotherapeutic effects are important information to make the surgical treatment plan, it is impossible to assess chemotherapeutic effects before tumor excision. Therefore, preoperative radiological evaluation of the chemotherapeutic effects is extremely important and needs to be strongly correlated with the histological response and prognosis. We previously reported that 99mTc-MIBI and CRS were strongly correlated with the histological responses to chemotherapy [3], [4]. In the present study, 99mTc-MIBI revealed a significant association with OS in the patients with osteosarcoma. On the other hand, CRS revealed a marginally significant correlation with OS and EFS. Taken together, these findings suggest that 99mTc-MIBI and CRS could be used to assess the chemotherapeutic effects.
Conclusions
Tumor necrosis revealed significant correlation with OS as previously reported. In addition, 99mTc-MIBI response and CRS correlate with histologic response and are now confirmed as prognostic factors in patients with high-grade osteosarcoma receiving preoperative chemotherapy and surgical resection.
Funding Statement
The authors have no support or funding to report.
References
- 1. Davis AM, Bell RS, Goodwin PJ (1994) Prognostic factors in osteosarcoma: a critical review. J Clin Oncol 12: 423–431. [DOI] [PubMed] [Google Scholar]
- 2. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, et al. (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20: 776–790. [DOI] [PubMed] [Google Scholar]
- 3. Miwa S, Shirai T, Taki J, Sumiya H, Nishida H, et al. (2011) Use of 99mTc-MIBI scintigraphy in the Evaluation of the Response to Chemotherapy for Osteosarcoma: Comparison with 201Tl scintigraphy and angiography. Int J Clin Oncol 16: 373–378. [DOI] [PubMed] [Google Scholar]
- 4. Miwa S, Taki J, Yamamoto N, Shirai T, Nishida H, et al. (2012) A novel combined radiological method for evaluation of the response to chemotherapy for primary bone sarcoma. J Surg Oncol 106: 273–279. [DOI] [PubMed] [Google Scholar]
- 5. Tsuchiya H, Tomita K, Mori Y, Asada N, Yamamoto N (1999) Marginal excision for osteosarcoma with caffeine assisted chemotherapy. Clin Orhop Res 358: 27–35. [PubMed] [Google Scholar]
- 6. Ueda T, Naka N, Araki N, Ishii T, Tsuchiya H, et al. (2008) Validation of radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas: Japanese Orthopaedic Association Committee on Musculoskeletal Tumors Cooperative Study. J Orhop Sci 13: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, et al. (1982) Preoperative chemotherapy for osteogenic osteosarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 49: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 8. Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153: 106–120. [PubMed] [Google Scholar]
- 9. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, et al. (2006) Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 106: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 10. Kawai A, Healey JH, Boland PJ, Lin PP, Huvos AG, et al. (1998) Prognostic factors for patients with sarcomas of the pelvic bones. Cancer 82: 851–859. [PubMed] [Google Scholar]
- 11. Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH (1999) Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol 70: 109–115. [DOI] [PubMed] [Google Scholar]
- 12. Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, et al. (2005) Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res 11: 2561–2567. [DOI] [PubMed] [Google Scholar]
- 13. Magnan H, Chou AJ, Chou JF, Yeung HW, Healey JH, et al. (2010) Noninvasive imaging with thallium-201 scintigraphy may not correlate with survival in patients with osteosarcoma. Cancer 116: 4147–4151. [DOI] [PubMed] [Google Scholar]
- 14. Min D, Lin F, Shen Z, Zheng S, Tan L, et al. (2013) Analysis of prognostic factors in 333 Chinese patients with high-grade osteosarcoma treated by multidisciplinary combined therapy. Asia Pac J Clin Oncol 9: 71–79. [DOI] [PubMed] [Google Scholar]
- 15. Nishida Y, Isu K, Ueda T, Nishimoto Y, Tsuchiya H, et al. (2009) Osteosarcoma in the elderly over 60 years: a multicenter study by the Japanese Musculoskeletal Oncology Group. J Surg Oncol 100: 48–54. [DOI] [PubMed] [Google Scholar]
- 16. Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, et al. (2009) Prediction model of chemotherapy resonse in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med 9: 1435–1440. [DOI] [PubMed] [Google Scholar]
- 17. van der Woude HJ, Bloem JL, Verstraete KL, Taminiau AH, Nooy MA, et al. (1995) Osteosarcoma and Ewing’s sarcoma after neoadjuvant chemotherapy; value of dynamic MR imaging in detecting viable tumor before surgery. AJR Am J Roentgenol 165: 593–598. [DOI] [PubMed] [Google Scholar]
- 18. Kunisada T, Ozaki T, Kawai A, Sugihara S, Taguchi K, et al. (1999) Imaging assessment of the response of osteosarcoma patients to preoperative chemotherapy. angiography compared with thallium-201 scintigraphy. Cancer 86: 949–956. [DOI] [PubMed] [Google Scholar]
- 19. Taki J, Inaki A, Wakabayashi H, Sumiya H, Tsuchiya H, et al. (2010) Early prediction of histopathological tumor response to preoperative chemotherapy by Tc-99m MIBI imaging in bone and soft tissue sarcomas. Clin Nucl Med 35: 154–159. [DOI] [PubMed] [Google Scholar]
- 20. Taki J, Sumiya H, Tsuchiya H, Tomita K, Nonomura A, et al. (1997) Evaluating benign and malignant bone and soft-tissue lesions with technetium-99m-MIBI scintigraphy. J Nucl Med 38: 501–506. [PubMed] [Google Scholar]
- 21. Taki J, Higuchi T, Sumiya H, Tsuchiya H, Minato H, et al. (2008) Prediction of final tumor response to preoperative chemotherapy by Tc-99m MIBI imaging at the middle of chemotherapy in malignant bone and soft tissue tumors: comparison with Tl-201 imaging. J Orthop Res 26: 411–418. [DOI] [PubMed] [Google Scholar]
- 22. Rosen G, Loren GJ, Brien EW, Ramana L, Waxman A, et al. (1993) Serial thallium-201 scintigraphy in osteosarcoma. Correlation with tumor necrosis after after preoperative chemotherapy. Clin Orthop Rel Res 293: 302–306. [PubMed] [Google Scholar]
- 23. Lin J, Leung W, Ho SK, Ho KC, Kumta SM, et al. (1995) Quantitative evaluation of thallium-201 uptake in predicting chemotherapeutic response of osteosarcoma. Eur J Nucl Med 22: 553–555. [DOI] [PubMed] [Google Scholar]
- 24. Söderlund V, Larsson SA, Bauer HCF, Brosjö O, Larsson O, et al. (1997) Use of 99mTc-MIBI scintigraphy in the evaluation of the response of osteosarcoma to chemotherapy. Eur J Nucl Med 24: 511–515. [DOI] [PubMed] [Google Scholar]
- 25. Kostakoglu L, Panicek DM, Divgi CR, Botet J, Healey J, et al. (1995) Correlation of the findings of thallium-201 chloride scans with those of other imaging modalities and histology following therapy in patients with bone and soft tissue sarcomas. Eur J Nucl Med 22: 1232–1237. [DOI] [PubMed] [Google Scholar]
- 26. Beanlands RS, Dawood F, Wen WH, McLaughlin PR, Butany J, et al. (1990) Are the kinetics of technetium-99m methoxyisobutyl isonitrile affected by cell metabolism and viability?. Circulation 82: 1802–1814. [DOI] [PubMed] [Google Scholar]
- 27. Carvalho PA, Chiu ML, Kronauge JF, Kawamura M, Jones AG, et al. (1992) Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med 33: 1516–1522. [PubMed] [Google Scholar]
- 28. Piwnica-Worms D, Kronauge JF, Chiu ML (1990) Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium in cultured chick myocardial cells; mitochondrial and plasma membrane potential dependence. Circulation 82: 1826–1838. [DOI] [PubMed] [Google Scholar]