Abstract
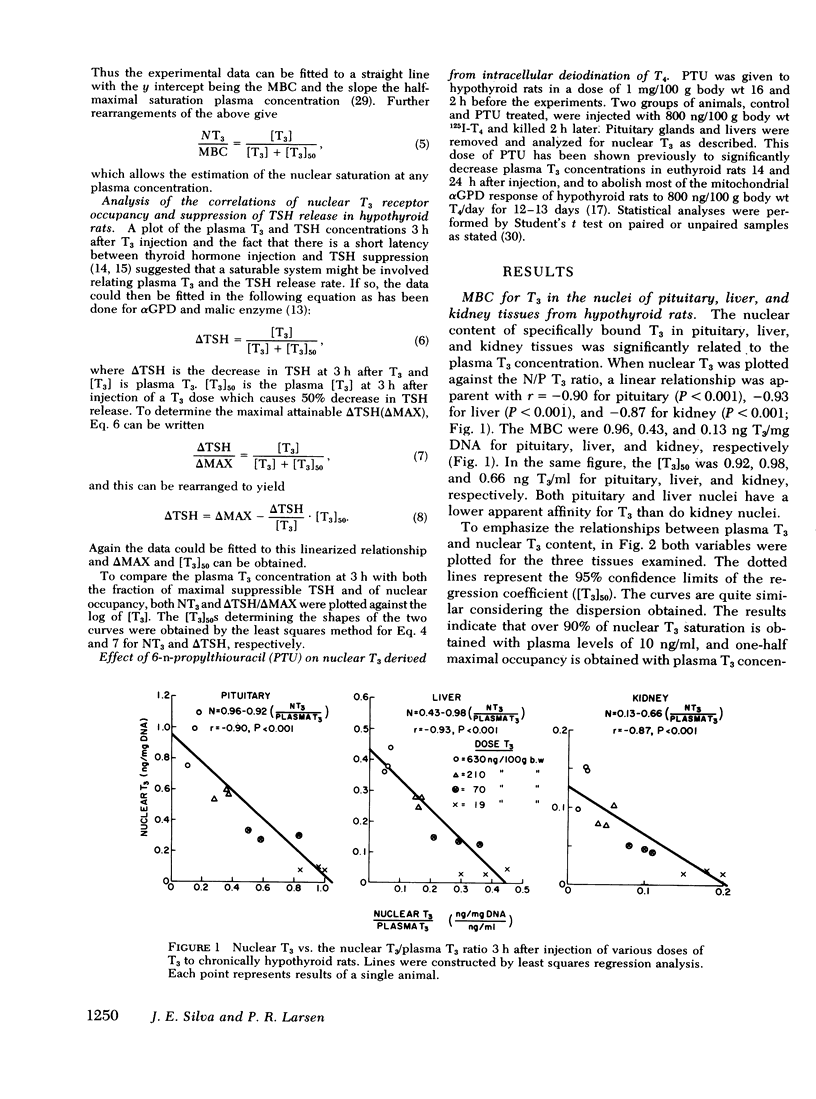
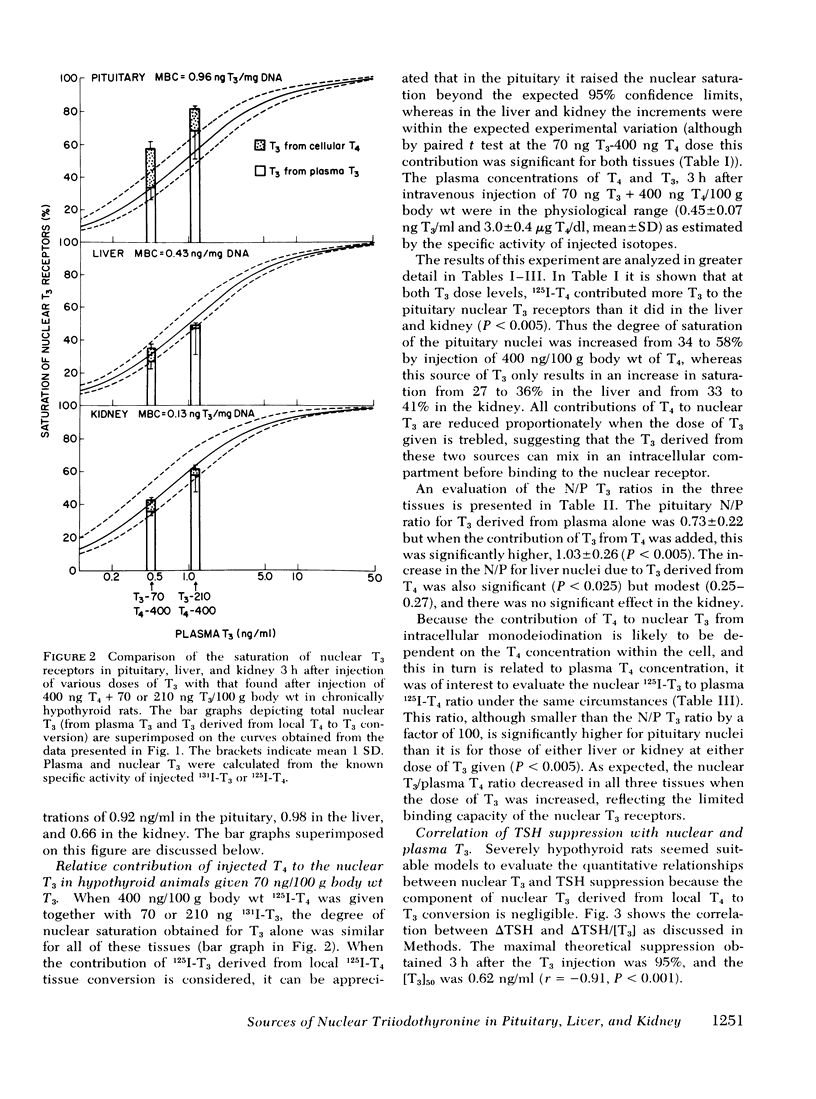
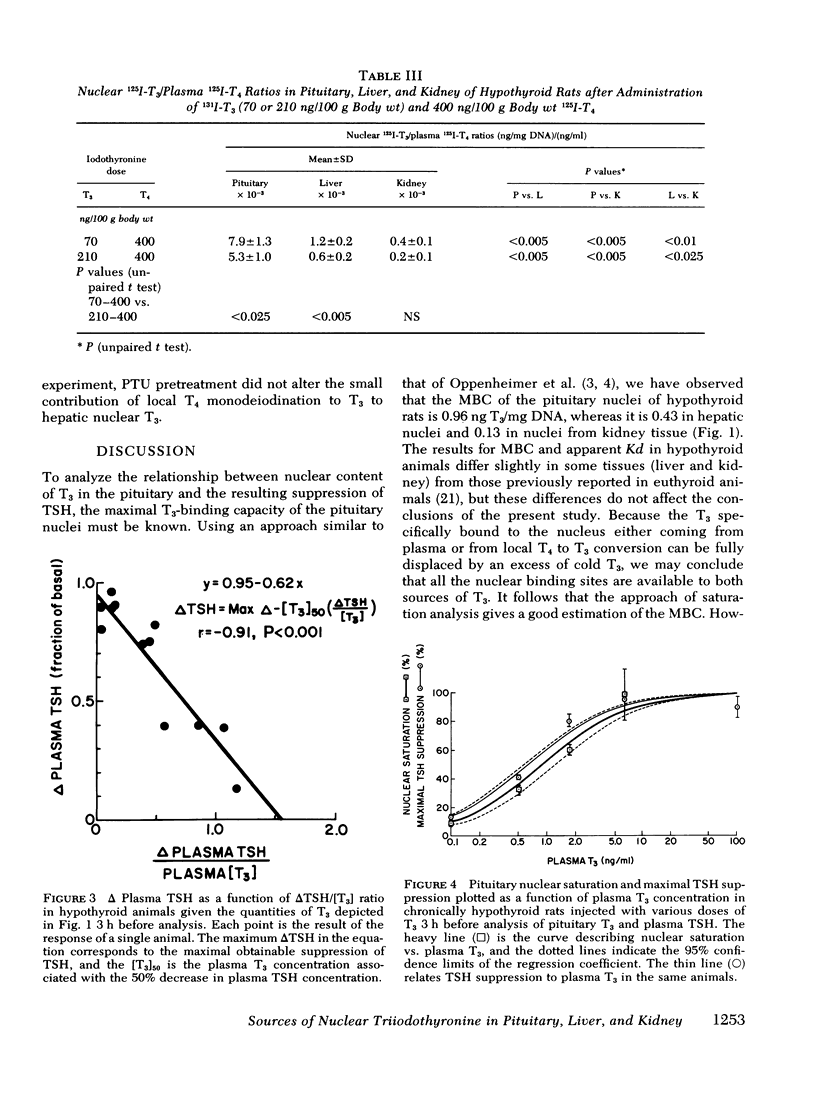
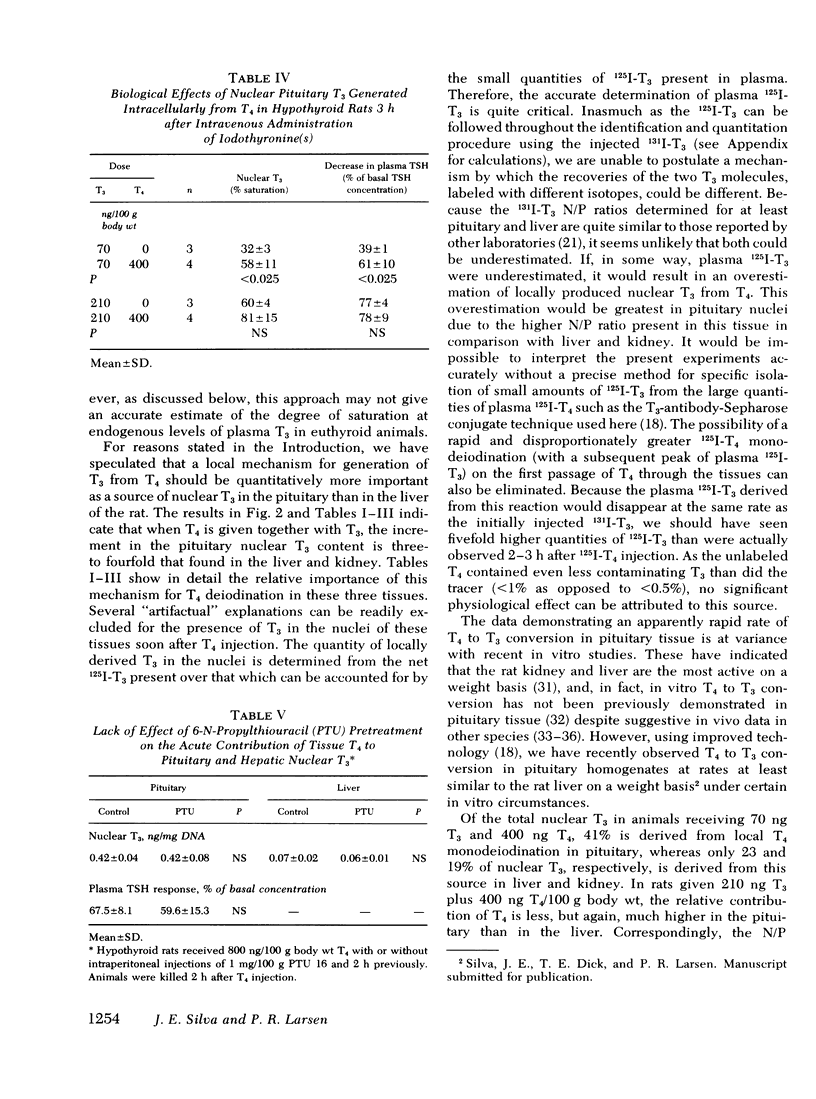
Injections of triiodothyronine (T3) and thyroxine (T4) into chronically hypothyroid rats were used to evaluate the contribution of intracellular T4 to T3 conversion to nuclear T3 in pituitary, liver, and kidney, and to correlate the occupancy of pituitary nuclear T3 receptors with inhibition of thyroid-stimulating hormone (TSH) release. Injection of a combination of 70 ng T3 and 400 ng T4/100 g body wt resulted in plasma T3 concentrations of 45±7 ng/dl (mean±SD) and 3.0±0.4 μg/dl T4 3 h later. At that plasma T3 level, the contribution of plasma T3 to the nuclear receptor sites resulted in saturation of 34±7% for pituitary, 27±5% for liver, and 33±2% for kidney. In addition to the T3 derived from plasma T3, there was additional T3 derived from intracellular monodeiodination of T4 in all three tissues that resulted in total nuclear occupancy (as percent saturation) of 58±11% (pituitary), 36±8% (liver), and 41±11% (kidney), respectively. The percent contribution of T3 derived from cellular T4 added 41% of the total nuclear T3 in the pituitary which was significantly higher than the contribution of this source in the liver (24%) or the kidney (19%). 3 h after intravenous injection of increasing doses of T3, the plasma T3 concentration correlated well with both the change in TSH and the nuclear occupancy, suggesting a linear relationship between the integrated nuclear occupancy by T3 and TSH release rate. The contribution of intrapituitary T4 to T3 conversion to nuclear T3 was accompanied by an appropriate decrease in TSH, supporting the biological relevance of nuclear T3. Pretreatment of the animals with 6-n-propylthiouracil before T4 injection decreased neither the nuclear T3 derived from intrapituitary T4 nor the subsequent decrease in TSH.
These results indicate that intracellular monodeiodination of T4 contributes substantially to the nuclear T3 in the pituitary of the hypothyroid rat, and suggest a linear inverse relationship between nuclear receptor occupancy by T3 in the pituitary and TSH release rate. The data further indicate that T4 to T3 monodeiodination is considerably more important as a source of nuclear T3 in the pituitary than in the liver and kidney. This provides a mechanism whereby the TSH secretion could respond promptly to a decrease in thyroid secretion (predominantly T4) before a decrease in plasma T3 would be expected to lead to significant metabolic hypothyroidism.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. M., Larsen P. R. Triiodothyronine and thyroxine in the serum and thyroid glands of iodine-deficient rats. J Clin Invest. 1973 Oct;52(10):2522–2531. doi: 10.1172/JCI107443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Bleich H. L., Boro E. S. Initiation of thyroid-hormone action. N Engl J Med. 1975 May 15;292(20):1063–1068. doi: 10.1056/NEJM197505152922006. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Hershman J. M., Hornabrook R. W. Serum thyroid hormone and thyrotropin levels in subjects from endemic goiter regions of New Guinea. J Clin Endocrinol Metab. 1975 Feb;40(2):326–333. doi: 10.1210/jcem-40-2-326. [DOI] [PubMed] [Google Scholar]
- DeGroot L. J., Strausser J. L. Binding of T3 in rat liver nuclei. Endocrinology. 1974 Jul;95(1):74–83. doi: 10.1210/endo-95-1-74. [DOI] [PubMed] [Google Scholar]
- DeGroot L. J., Torresani J. Triiodothyronine binding to isolated liver cell nuclei. Endocrinology. 1975 Feb;96(2):357–359. doi: 10.1210/endo-96-2-357. [DOI] [PubMed] [Google Scholar]
- Escobar del Rey F., Garcia M. D., Bernal J., Morreale de Escobar G. Concomitant decrease of the effects of thyroxin on TRH-induced TSH release, and of the pituitary content of triiodothyronine in animals on propylthiouracil. Endocrinology. 1974 Sep;95(3):916–921. doi: 10.1210/endo-95-3-916. [DOI] [PubMed] [Google Scholar]
- FORD D. H., GROSS J. The metabolism of 1131-labelled thyroid hormones in the hypophysis and brain of the rabbit. Endocrinology. 1958 Apr;62(4):416–436. doi: 10.1210/endo-62-4-416. [DOI] [PubMed] [Google Scholar]
- GRINBERG R., VOLPERT E. M., WERNER S. C. In vivo deiodination of labeled L-thyroxine to L-3,5,3'-triiodothyronine in mouse and human pituitaries. J Clin Endocrinol Metab. 1963 Feb;23:140–142. doi: 10.1210/jcem-23-2-140. [DOI] [PubMed] [Google Scholar]
- Geffner D. L., Azukizawa M., Hershman J. M. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975 Feb;55(2):224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Technical aspects of the estimation of triiodothyronine in human serum: evidence of conversion of thyroxine to triiodothyronine during assay. Metabolism. 1971 Jun;20(6):609–624. doi: 10.1016/0026-0495(71)90009-6. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Triiodothyronine: review of recent studies of its physiology and pathophysiology in man. Metabolism. 1972 Nov;21(11):1073–1092. doi: 10.1016/0026-0495(72)90038-8. [DOI] [PubMed] [Google Scholar]
- MacLeod K. N., Baxter J. D. DNA binding of thyroid hormone receptors. Biochem Biophys Res Commun. 1975 Feb 3;62(3):577–583. doi: 10.1016/0006-291x(75)90437-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I., Koerner D., Dillmann W. H. Nuclear receptors and the initiation of thyroid hormone action. Recent Prog Horm Res. 1976;32:529–565. doi: 10.1016/b978-0-12-571132-6.50029-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Nuclear binding capacity appears to limit the hepatic response to L-triiodothyronine (T3). Endocr Res Commun. 1975;2(4-5):309–325. doi: 10.1080/07435807509089004. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Surks M. I., Schwartz H. L. The metabolic significance of exchangeable cellular thyroxine. Recent Prog Horm Res. 1969;25:381–422. doi: 10.1016/b978-0-12-571125-8.50011-9. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Pharoah P. O., Hornabrook R. W., Hetzel B. S. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab. 1973 Nov;37(5):783–789. doi: 10.1210/jcem-37-5-783. [DOI] [PubMed] [Google Scholar]
- Saberi M., Sterling F. H., Utiger R. D. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J Clin Invest. 1975 Feb;55(2):218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J. Thyroid hormone action: in vitro demonstration of putative receptors in isolated nuclei and soluble nuclear extracts. Science. 1974 Jun 14;184(4142):1188–1191. doi: 10.1126/science.184.4142.1188. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadlow A. R., Surks M. I., Schwartz H. L., Oppenheimer J. H. Specific triiodothyronine binding sites in the anterior pituitary of the rat. Science. 1972 Jun 16;176(4040):1252–1254. doi: 10.1126/science.176.4040.1252. [DOI] [PubMed] [Google Scholar]
- Shaw W., Smith J., Spierto F. W., Agnese S. T. Linearization of data for saturation-type competitive protein binding assay and radioimmunoassay. Clin Chim Acta. 1977 Apr 1;76(1):15–24. doi: 10.1016/0009-8981(77)90114-0. [DOI] [PubMed] [Google Scholar]
- Silva E. Disposal rates of thyroxine and triiodothyronine in iodine-deficient rats. Endocrinology. 1972 Dec;91(6):1430–1435. doi: 10.1210/endo-91-6-1430. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- TATA J. R. Inhibition of the biological action of thyroid hormones by actinomycin D and puromycin. Nature. 1963 Mar 23;197:1167–1168. doi: 10.1038/1971167a0. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: stimulation of growth hormone and inhibition of prolactin secretion in cultured GH1 cells. Biochem Biophys Res Commun. 1974 Jul 10;59(1):420–428. doi: 10.1016/s0006-291x(74)80223-8. [DOI] [PubMed] [Google Scholar]
- VOLPERT E. M., GRINBERG R., WERNER S. C. Studies with mouse pituitary thyrotropic tumors. IV. Presence of 3,5,3'-triiodothyronine in certain mouse pituitary tumors after the injection of labeled L-thyroxine. Endocrinology. 1962 Sep;71:361–368. doi: 10.1210/endo-71-3-361. [DOI] [PubMed] [Google Scholar]
- WERNER S. C., VOLPERT E. M., GRINBERG R. Difference in metabolism of labelled thyroxine between thyrotropic and adrenotropic mouse pituitary tumours. Nature. 1961 Dec 23;192:1193–1194. doi: 10.1038/1921193b0. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. Immunoassay studies of thyrotropin in rat pituitary glands and serum. Endocrinology. 1967 Jul;81(1):145–151. doi: 10.1210/endo-81-1-145. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]


