Synopsis
Neurofibromatosis type I (NF1) is a common genetic disease that affects 1 in 3,500 individuals. The disease is completely penetrant but shows variable phenotypic expression in patients. NF1 is a large gene, and its pre-mRNA undergoes alternative splicing. The NF1 protein, neurofibromin, has a myriad of molecular functions, one of the best characterized being its function as a Ras-GAP. NF1 exon 23a is an alternative exon, which lies within the GAP-related domain of neurofibromin. This exon is predominantly included in most tissues, and it is skipped in central nervous system neurons. The isoform in which exon 23a is skipped has ten times higher Ras-GAP activity than the isoform in which exon 23a is included. Exon 23a inclusion is tightly regulated by at least three different families of RNA-binding proteins: CELF, Hu, and TIA-1/TIAR. The CELF and Hu proteins promote exon 23a skipping, while the TIA-1/TIAR proteins promote its inclusion. The widespread clinical variability that is observed among NF1 patients cannot be explained by NF1 mutations alone, and it is believed that modifier genes may have a role in the variability. We suggest that the regulation of alternative splicing may act as a modifier to contribute to the variable expression in NF1 patients.
Keywords: Neurofibromatosis type I (NF1), alternative splicing, genetic modifiers, CUG-BP and ETR-3 like factors (CELF), Hu proteins, TIA-1/TIAR proteins
Alternative Splicing
With the realization that the human genome contains far fewer genes than initially predicted, it is clear that complex molecular mechanisms must exist to increase the coding capacity of our genome. One of the major mechanisms is alternative splicing. It allows functionally distinct proteins to be generated from a single gene by selectively including or skipping particular exons in mature mRNA messages, which encode proteins. With recent technological advances, such as high-throughput sequencing, which allow large volumes of the genome to be interrogated, it is now accepted that alternative splicing is the general rule in humans with as many as 92–94% of human genes predicted to undergo this process [1, 2]. Alternative splicing is important in development, in establishing and maintaining tissue specificity, and in the development and progression of human diseases. Alternative splicing is a highly regulated process in which both cis-acting elements, parts of the RNA itself, and trans-acting factors, RNA-binding proteins, interact to influence the decision of whether a particular alternative exon will be included or skipped. Alternative splicing regulation and the factors involved in this process have been reviewed extensively by others [3–8].
Alternative splicing is important in the development and progression of human diseases. In fact, current estimates suggest that up to 60% of disease causing mutations affect splicing [9]. These mutations can affect cis-acting elements, or they can lead to the mis-regulation of trans-acting splicing factors. Mutations that affect splicing can lead to an alteration in the levels of correctly spliced transcripts, or they can disrupt the normal levels of protein isoforms created by alternative splicing. Several human diseases are known to be caused by mutations that lead to aberrant splicing, including myotonic dystrophy (DM), spinal muscular atrophy, and certain cancers [9–12]. Recently, it has been proposed that splicing may have a role as a genetic modifier of single-gene human diseases. Splicing can play this role by altering either the normal levels of correctly spliced RNA transcripts or the normal ratios of different isoforms that result from alternative splicing [13, 14].
One documented example of a human disease in which splicing acts as a genetic modifier is cystic fibrosis (CF). CF is a relatively common autosomal recessive genetic disorder in which the cystic fibrosis trans-membrane conductance regulator (CFTR) gene is mutated [15]. Thus far approximately 1,000 CF disease-causing mutations have been identified in the CFTR gene [16]. Many CF patients, even from the same family, show differences in the presentation or severity of the CF phenotype, which argues for a role for genetic modifiers in the disease severity. Splicing is believed to act as a genetic modifier of CF severity. Several characterized splicing mutations in the CFTR gene itself lead to the complete loss of correctly spliced transcripts, while other known CFTR mutations lead to an alteration in the levels of the correctly spliced products [15]. Cystic fibrosis patient studies have shown that lower levels of correctly spliced transcripts correlate with a more severe pulmonary system phenotype [15]. Interestingly, patients who carry the same mutations do not have the same levels of correctly spliced transcripts, and therefore modifier genes are implicated in contributing to CF disease severity [15].
Neurofibromatosis type I Disease (NF1)
NF1, also known as von Recklinghausen disease, is a prevalent human genetic disease that affects about 1 in 3,500 individuals without regard to ethnicity or sex [17, 18]. NF1 is the result of loss-of-function mutations to the NF1 gene, and the disease is inherited in an autosomal dominant fashion [19]. The disease is completely penetrant. Certain phenotypic characteristics are considered to be hallmarks of NF1 disease, as they occur in the majority of patients by the onset of adulthood. These phenotypic hallmarks include Lisch nodules of the eye, optic nerve gliomas, café au lait spots, auxiliary freckling, and the presence of benign neurofibromas [20, 21].
Neurofibromas are one of the defining characteristics of NF1 disease. Neurofibromas are benign tumors that involve Schwann cells, fibroblasts, perineurial cells, and mast cells, and they arise from nerve sheath cells [22]. Neurofibromas develop along peripheral nerves, and while they are benign they can cause serious complications in NF1 patients, such as spinal cord compression, other neurological problems, and cosmetic burden. Neurofibromas develop when both NF1 alleles are disrupted, and thus functional neurofibromin is not present. It is believed that NF1 tumor development follows Knudsen’s Two Hit Hypothesis, where one mutated allele is either inherited or is the result of a de novo mutation, and the second NF1 allele is then mutated or disrupted in somatic cells where tumors develop. Individuals with NF1 have an increased risk for developing malignant tumors.
Although NF1 shows complete penetrance, there is a high degree of variability in the presentation of the disease. Thus far, greater than 900 NF1 gene mutations have been identified and listed in the Human Gene Mutation Database, yet the mutations alone cannot account for the phenotypic variability that is seen in NF1 patients. A recent study of monozygotic twins with NF1 disease supports the notion that phenotypic differences exist not only in family members with similar mutations, but also in individuals who have inherited the same NF1 germ line mutations [23]. The fact that these twins do show discordant phenotypes for many of the NF1 clinical features suggests that somatic cell mutations affecting other genes could be responsible for the variability that is observed. Even though there are certain aspects of the NF1 phenotype that are common to most patients, there are some characteristics that vary in different individuals. Some of the variable characteristics of the NF1 phenotype are cardiovascular involvement, the development of plexiform neurofibromas and/or malignant peripheral nerve sheath tumors, learning disabilities, skeletal dysplasia, and optic pathway gliomas [20].
Learning disabilities, which vary among NF1 patients, are considered to be among the most difficult aspects of the disease for patients to manage. Current data suggest that as many as 30–65% of people with NF1 have specific learning deficits [18, 24–26]. In general, most NF1 patients with learning difficulties show visuospatial, language, and reading deficits, but global cognitive impairments are not common [20, 27, 28]. Although one study in monozygotic twins by Rieley and colleagues has shown a high degree of concordance for presentation of intelligence quotient (IQ) scores, speech disorders, and learning disabilities in twins with NF1, others have shown that phenotypic differences in learning exist in both human and animal subjects [23, 29, 30].
The widespread clinical variability that presents itself in NF1 patients leads to the hypothesis that other factors must be at play to modulate the NF1 disease phenotype. In fact, a recent family-based association study, by Sabbagh and colleagues, was utilized to investigate the normal NF1 allele and its potential role in phenotype variability [31]. The study examined 750 French NF1 patients from 275 different families by looking at phenotypic correlations between affected relatives for twelve different NF1 clinical features. The clinical features included the number and size of café au lait spots, the number of cutaneous, subcutaneous, and plexiform neurofibromas, the presence of Lisch nodules and blue-red macules, skin fold freckling, scoliosis, neoplasms, and learning disabilities [31]. The findings of Sabbagh et al, demonstrated that there is a strong genetic component for eleven of the twelve traits that they examined, and that there was no apparent influence from the identified NF1 mutation in each family [31]. Additional analysis of the normal NF1 allele showed that there was not much involvement of this allele in the differences observed in clinical presentation. Overall, these findings support a strong argument for the involvement of genetic modifiers, not linked to the NF1 locus, in NF1 disease variability [31].
NF1 Gene and Protein
Human neurofibromatosis type I (NF1) is a large gene, spanning about 350 kb of genomic DNA, located on chromosome 17q11.2. The full-length NF1 mRNA transcript contains sixty exons including several alternative exons (see below). The major protein product encoded by the NF1 gene, termed neurofibromin, consists of 2818 amino acids [32]. Neurofibromin is ubiquitously expressed with enrichment in neurons, Schwann cells, oligodendrocytes, astrocytes, leukocytes, and adrenal medulla, and it is highly conserved among species [33–39].
The neurofibromin protein is essential in mammals. Studies in mice in which NF1 protein is completely deleted result in embryonic lethality between embryonic days 12.5 and 13.5 due to cardiovascular defects, and they also exhibit endocardial cushion defects and a failure of the neural tube to close properly [40]. Importantly, NF1 and its downstream targets are highly conserved among different species, including mice and humans. Presumably due to its large size, the NF1 gene is one of the most highly mutated genes in the human genome. It has been suggested that many of the NF1 mutations affect pre-mRNA splicing [41].
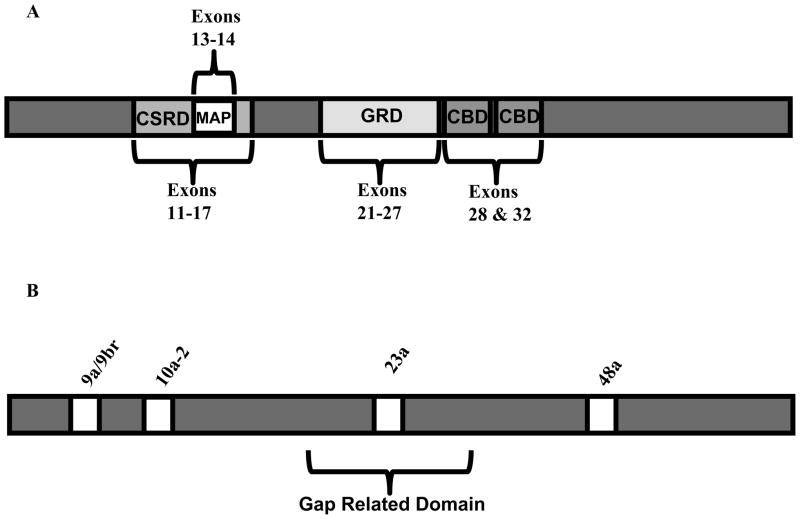
The large, cytoplasmically localized NF1 protein is multifunctional and is involved in diverse signaling cascades [42]. Several functional domains of the protein have been identified. One domain of importance is the SEC14 domain, which is a lipid-binding motif that shares homology with the yeast Sec14p protein [43]. NF1’s CSRD domain is enriched with the amino acids cysteine and serine, and it is encoded by exons 11–17 [42] (Figure 1A). This domain has been shown to increase the association of neurofibromin with actin upon its phosphorylation [42, 44–47]. Located within the CSRD domain is a microtubule-associated-protein (MAP) domain [42, 44, 45] (Figure 1A). Other domains of the NF1 protein are the caveolin-1 binding domains, which are encoded by exons 28 and 32 (Figure 1A). These domains are believed to be involved in the formation of a complex between neurofibromin and caveolin-1, and the complex is potentially involved in the regulation of several important signaling molecules including protein kinase C and p21ras [42, 48].
Figure 1.
Schematic representations of neurofibromin protein domains and alternative exons. (A) Neurofibromin protein with important domains and the exons which encode them highlighted. Protein domains are shown as grey or white boxes. The MAP domain is found within the CSRD. (B) Neurofibromin protein with naturally occurring alternative exons shown as white boxes. Exon 23a is found within the GAP related domain.
The best-characterized functional domain of neurofibromin is the Ras GTPase-activating protein related domain (GRD), which is encoded by exons 21 to 27 (Figure 1). The Ras-GTPase domain shares similarity with the catalytic domain of the yeast IRA-1 and IRA-2 proteins as well as the mammalian GTPase activating protein, and accounts for approximately 10% of the entire protein [42]. Several groups have demonstrated that the GRD of neurofibromin has Ras-GAP activity in vitro and in vivo [49–52]. The NF1 GRD imparts tumor suppressor function to the NF1 protein since it exerts negative control on the oncogene Ras, resulting in decreased cellular proliferation and growth. GAPs, like neurofibromin, function by accelerating the conversion of active GTP-bound Ras to inactive GDP-bound Ras. Aberrant activation of Ras is associated with many human malignancies [53].
NF1 Alternative Splicing
The NF1 pre-mRNA undergoes alternative splicing. Although some alternative splicing events are the results of mutations, the focus of this review will be on naturally occurring splice variants.
Several alternative exons that do not alter the reading frame of NF1 have been identified in humans and rodents, including 9a/9br, 10a-2, 23a, and 48a (Figure 1B) [54–58]. Alternative exon 9a/9br adds ten amino acids to the transcript, and its inclusion seems to be restricted to the central nervous system [37, 54, 59]. The inclusion of 9a/9br does not appear to affect the function of the GRD. 10a-2 is an alternative exon that is inserted between exons 10a and 10b, and it adds fifteen amino acids to the transcript, which in turn adds a trans-membrane segment that is absent from the isoform lacking this exon [55]. Although its function has not been studied extensively, the 10a-2 alternative exon is believed to serve a housekeeping function at the intracellular membrane [55]. Alternative exon 48a adds eighteen amino acids to the transcript. The expression of NF1 exon 48a is highest in both fetal and adult cardiac and skeletal muscle tissues [58]. Although its function is still under investigation, it is believed that exon 48a might have a role in the development and differentiation of heart and skeletal muscles [60, 61]. Additionally, the inclusion of exon 48a does not appear to affect the GRD. The main focus of this review will be on NF1 exon 23a, so it will be more thoroughly discussed later in the text.
There are other naturally occurring NF1 splice forms which alter the reading frame. Previous work identified alternative exons 29 and 30 in humans by RT-PCR [62]. Three different NF1 isoforms are generated by either omitting one or both of these alternative exons in the mature transcript. RT-PCR analysis of a panel of both fetal and adult human tissues, including liver, heart, and brain, showed that at least one of the isoforms is detectable in every tissue assayed [62]. Each of these NF1 isoforms introduces a premature stop codon, and it is believed that they result in truncated proteins that end just downstream of the GRD of neurofibromin. Interestingly, these alternative isoforms were not identified in mouse tissues, presumably due to differences in alternative processing. Thus far, the functions of these alternative exons have not been determined.
Exon 23b is a rodent-specific alternative exon, which when included causes a frame shift that inserts a premature stop codon [63]. The introduction of the stop codon causes the truncated protein to lack much of its GRD. The Nf1 isoform that includes exon 23b but lacks exon 23a is enriched in the testes of both rats and mice [60, 63].
NF1 Exon 23a and its Biological Importance
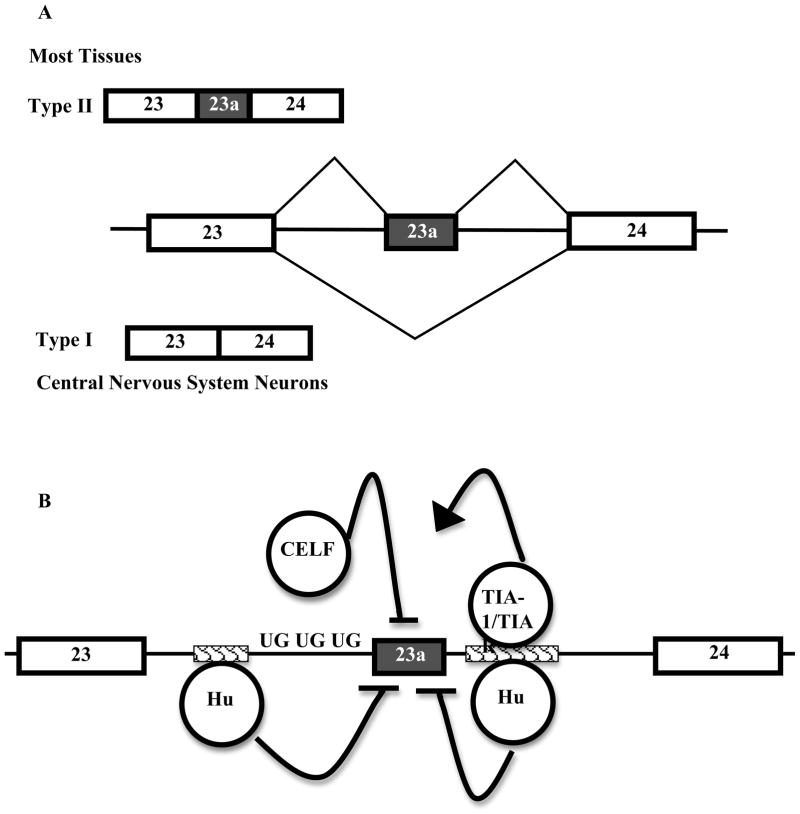
The first two NF1 isoforms that were identified, types I and II, have the alternative exon 23a skipped and included, respectfully. NF1 exon 23a is an alternative exon that lies within the GRD of neurofibromin. Exon 23a is considered to be one of the most interesting NF1 alternative exons, due to both its location within the GRD and the observation that the two protein isoforms differ in their abilities to control Ras signaling. The inclusion of exon 23a inserts 21 amino acids between constitutive exons 23 and 24. Exon 23a is differentially spliced in a tissue-specific manner (Figure 2A). Exon 23a is predominantly included in most tissues and predominantly skipped in central nervous system neurons. Although the NF1 isoform that includes exon 23a still functions as a Ras GAP, in vitro studies have shown that it is about tens times weaker in regulating Ras signaling than the isoform in which exon 23a is skipped. Exon 23a adds basic amino acids, which significantly change the structure of the NF1 GAP-related domain, and thus weaken its ability to regulate Ras [57, 64].
Figure 2.
Schematics of NF1 exon 23a endogenous splicing patterns and exon 23 regulation. (A) Exon 23a is included in most tissues and skipped in the central nervous system. Constitutive exons 23 and 24 are shown as white boxes and alternative exon 23a is shown as a grey box. Introns are shown as straight black lines. (B) Exon 23a splicing regulation. Constitutive exons 23 and 24 are shown as white boxes and alternative exon 23a is shown as a grey box. Introns are shown as straight black lines. Proteins that act as splicing factors are shown as circles. Hu proteins promote exon 23a skipping by binding to intronic splicing silencer elements located upstream and downstream of exon 23a, given by a textured rectangle. TIA-1 and TIAR proteins promote exon 23a inclusion by compete with Hu proteins for binding of the downstream sequence element. CELF proteins promote skipping of exon 23a by binding UG-rich elements upstream.
In the early 2000s, Costa and coworkers sought to better understand the biological importance of NF1 exon 23a, so they generated a mouse model in which exon 23a is deleted in all tissues [65]. Unlike the mouse model in which NF1 is completely deleted, the NF1 23a−/23a− mice are viable and develop normally. While these mice do not exhibit a tumor phenotype, they do show differences in spatial learning and memory when tested by the Morris water maze and another hippocampal-dependent assay [65]. These findings suggest that it is necessary to have a balance between the two NF1 isoforms that either contain or skip exon 23a.
Regulation of NF1 Exon 23a Inclusion
Much research effort has put effort into elucidating the regulatory mechanisms that govern NF1 exon 23a inclusion. It is common for alternative exons to be under tight regulation by a combination of different protein factors. The inclusion of NF1 exon 23a is tightly regulated both developmentally and in specific tissues, and it has been determined that this alternative splicing event is under complex control. Thus far two families of proteins that act as negative regulators of this system by promoting exon 23a skipping and another family of proteins, which promotes inclusion have been identified. There are at least two families of proteins, Hu proteins and the CUG-BP1 and ETR-3 like factors (CELF), which promote exon 23a skipping [66, 67] (Figure 2B). Both of these protein families are highly expressed in the brain, where exon 23a is predominantly skipped [66, 68–71]. TIA-1 and TIAR proteins are positive regulators, which promote NF1 exon 23a inclusion [67].
There are four members in the Hu protein family, HuR, HuB, HuC, and HuD. HuR is expressed ubiquitously, while HuB, HuC, and HuD are brain-specific family members [71]. Hu proteins are RNA-binding proteins with diverse molecular functions, including roles in mRNA stability, cellular stress response, and alternative splicing [72]. Hu proteins bind to AU or U-rich sequences on RNA. Currently, there are four documented Hu protein-mediated alternative splicing events, which include Fas exon 6, HuD exon 6, the Ikaros isoforms, and NF1 exon 23a [73–77]. Hu proteins exert their effects as negative regulators by binding to AU-rich sequences, which function as intronic splicing silencers (ISS), upstream and downstream of NF1 exon 23a. In vitro analyses have shown that at the downstream site, Hu proteins block the binding of the critical splicing machinery components, U1 and U6 to the 5′ splice site [67]. Hu proteins also inhibit the binding of critical splicing components upstream of this regulated exon. Zhu and colleagues have shown that Hu proteins decrease the binding of the splicing factor, U2AF65 at the 3′ splice site of the upstream intron [67].
There are six CELF protein family members: CUG-BP1, ETR-3/CUG-BP2, CELF4, CELF4, CELF5, and CELF6. CELF family members 3–6 are considered to be brain-specific, while CUG-BP1 and ETR-3 are widespread with enrichment in the brain [68–70]. The CELF proteins are well-established regulators of alternative splicing [69, 70, 78]. CELF proteins bind to UG-rich cis-sequences on RNA [78, 79]. The CELF proteins are closely related to the Hu proteins, as all of the proteins share the common structure of three RNA recognition motifs and a hinge domain. In our laboratory, we have shown that the CELF proteins promote exon 23a skipping by binding to the UG-rich elements upstream of NF1 exon 23a. Although the specific cis-elements responsible for CELF protein mediated splicing regulation have not been elucidated, in vitro binding assays suggest that the binding sites are located upstream of exon 23a [66]. While all of these family members promote exon 23a skipping, CELF6 is a weaker regulator [66], presumably due to differences in its amino acid composition and/or cellular localization, which have been characterized by others [70]. In vitro UV Crosslinking/Immunoprecipitation experiments show that recombinant CUG-BP1 competes with endogenous U2AF65, a critical component of the splicing machinery, in HeLa nuclear extract [66].
TIA-1 and TIAR proteins have been shown to promote exon 23a inclusion. Hu proteins and TIA-1 and TIAR proteins engage in a competition for binding of a U-rich sequence downstream of NF1 exon 23a, and the competition is decided depending on which factors are present at higher levels in a particular tissue. In vitro studies using nuclear extracts have shown that TIA-1 and TIAR promote exon 23a inclusion, by binding to the downstream U-rich site and increasing the binding of the U1 and U6 snRNPs at the 3′ splice site [67].
A Role for Alternative Splicing in NF1 Phenotypic Variability?
As discussed above, the phenotypic variability seen in NF1 patients cannot be explained solely by NF1 gene mutations. Many researchers have hinted at the idea of genetic modifiers for this disease, particularly since Nf1+/− mice from different genetic backgrounds show differences in phenotype severity with regards to the learning and behavioral aspects of the phenotype, as well as the differences in the susceptibility to form astrocytomas [81, 82]. An intriguing hypothesis is that changes in the levels of protein isoforms generated via pre-mRNA alternative splicing could act as a genetic modifier in NF1 as it does in other diseases like cystic fibrosis.
While it is unlikely that the NF1 patient tumor phenotype, which results from the loss of both copies of NF1, is strongly affected by the action of alternative splicing regulation as a genetic modifier, such a mechanism could become important for aspects of the NF1 phenotype that are due to the haploinsufficiency of neurofibromin. Two interesting examples of aspects of the NF1 phenotype which are thought to be due to neurofibromin haploinsufficiency are vascular disease and cognitive impairment.
An important variable aspect of the NF1 phenotype, which has come under closer scrutiny in recent studies, is vascular disease. It has been suggested that vascular disease is a major cause of mortality in young NF1 patients, but even so very few patients are routinely screened for this aspect of the disease [83, 84]. One group has reported that juvenile NF1 patients who underwent brain MRIs showed a number of cerebrovascular system abnormalities including narrowing of vessels and aneurysms [85]. Much has been learned about vasculopathy using Nf1+/− mice as models of Nf1 haploinsufficiency. These heterozygous mice have an increased risk of developing tumors, and they exhibit specific learning deficits [30, 86]. One study by Lasater and colleagues focused on neointima formation in Nf1+/− and wild-type controls with mechanical carotid artery injuries. Neointimas are newly formed or thickened inner linings of blood vessels, which commonly occur after tissue injury. The study showed that there was increased neointima formation in response to the injuries in the heterozygous mice compared with the wild-type controls [87]. More recently, the same group has determined that heterozygous inactivation of Nf1 in mouse bone marrow-derived cells (BMDCs) is necessary and sufficient to bring about neointima formation [88]. In addition to being prone to neointima formation upon arterial injury, there is evidence of vascular inflammation in Nf1+/− mice. Interestingly, peripheral blood samples from human NF1 patients also show evidence of chronic inflammation with increased levels of circulating monocyte cells compared to unaffected controls [88]. It is established that vascular smooth muscle cells that lack neurofibromin proliferate and migrate more readily than control cells in response to platelet-derived growth factor, and this is believed to be due to the hyperactivation of the Ras-Erk pathway.
The learning disability phenotype for NF1 patients has profound implications for the management of the disease. The NF1+/− mouse features learning disabilities, which appropriately mimic the human learning and behavior phenotypes, suggesting that these aspects of the phenotype are due to neurofibromin haploinsufficiency. The mouse model fails at tasks that parallel attention deficits and visual-spatial deficits in NF1 patients [28]. Another model of the learning disabilities phenotype is the NF1 exon 23a −/− mouse. The mouse model shows that it is important to have the right balance of the two isoforms (type I and type II) during development, since this animal lacks exon 23a and has a learning phenotype.
It is possible that alterations of alternative splicing could have a role as a genetic modifier of the severity of vascular disease and learning in NF1 patients. Specifically the regulation of NF1 exon 23a could play an important role. Like other alternative exons, exon 23a is characterized by the presence of weak consensus sequences, surrounding the exon, that are not readily recognized by the splicing machinery, and as mentioned above, there are many regulatory factors involved in the regulation of exon 23a inclusion or skipping. Although they have not yet been identified, it is possible that mutations that affect the cis-acting elements around exon 23a could result in the failure of important trans-acting factors to bind, and this could in turn lead to an altered ratio of the two isoforms. Conversely, it is also possible that mutations could exist in the genes that encode the regulatory factors that bind to the NF1 cis-acting elements. These effects could increase inclusion of this exon to a level that would allow hyperactivation of the Ras-Erk pathway in specific cells involved in the formation of neointima in the case of the vascular aspects of the disease. Likewise, the mutations could cause an imbalance in the distribution of the type I and type II isoforms and lead to phenotypic variance in learning in patients.
With these concepts in mind, it is crucial to identify any additional cis-acting and trans-acting factors that regulate NF1 exon 23a inclusion. It is also of interest to further mechanistically dissect and understand the biological importance of all of the naturally occurring NF1 splice variants. An intriguing approach to better understand the biological importance of these splice variants is to use genetic techniques to generate mouse models in which the ratio of protein isoforms is altered. Manipulation of the alternative transcript levels in different tissues will most likely provide new insights into why these alternative exons are so tightly regulated in vivo.
Acknowledgments
We would like to thank Melissa Hinman for critical reading of this manuscript. We also thank the other Lou laboratory members for helpful discussions.
Funding
Hua Lou was supported by a National Institutes of Health grant [NS-049103] and a Department of Defense grant [NF060083]. Victoria Barron was supported by a developmental biology training grant from the National Institutes of Health [T32HD00710432] and a pre-doctoral fellowship from the American Heart Association [0815373D].
Abbreviations
- BMDCs
bone marrow-derived cells
- CELF
CUG-BP (Cytosine-Uridine-Guanine-Binding Protein) and ETR-3 (ELAV (Embryonic Lethal Abnormal Vision)-Type RNA-Binding Protein) Like Factor
- CF
cystic fibrosis
- CFTR
cystic fibrosis trans-membrane conductance regulator
- CSRD
cysteine and serine rich domain
- DM
myotonic dystrophy
- GDP
guanosine diphosphate
- GRD
GTPase-activating protein related domain
- GTP
guanosine triphosphate
- IQ
intelligence quotient
- IRA-1
inhibitory regulatory protein 1
- IRA-2
inhibitory regulatory protein 2
- MAP
microtubule-associated-protein
- mRNA
messenger ribonucleic acid
- NF1
Neurofibromatosis type I
- RNA
ribonucleic acid
- TIA-1
T-cell intracellular antigen 1
- TIAR
T-cell intracellular antigen 1 related protein
- U2AF65
U2 auxiliary factor large subunit
References
- 1.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 2.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 4.Black DL, Grabowski PJ. Alternative pre-mRNA splicing and neuronal function. Prog Mol Subcell Biol. 2003;31:187–216. doi: 10.1007/978-3-662-09728-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui J. Regulation of mammalian pre-mRNA splicing. Sci China C Life Sci. 2009;52:253–260. doi: 10.1007/s11427-009-0037-0. [DOI] [PubMed] [Google Scholar]
- 7.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8:819–831. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 9.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Blanco MA, Baraniak AP, Lasda EL. Alternative splicing in disease and therapy. Nat Biotechnol. 2004;22:535–546. doi: 10.1038/nbt964. [DOI] [PubMed] [Google Scholar]
- 11.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward AJ, Cooper TA. The pathobiology of splicing. J Pathol. 2009;220:152–163. doi: 10.1002/path.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissim-Rafinia M, Kerem B. The splicing machinery is a genetic modifier of disease severity. Trends Genet. 2005;21:480–483. doi: 10.1016/j.tig.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Nissim-Rafinia M, Kerem B. Splicing regulation as a potential genetic modifier. Trends Genet. 2002;18:123–127. doi: 10.1016/s0168-9525(01)02619-1. [DOI] [PubMed] [Google Scholar]
- 15.Nissim-Rafinia M, Kerem B. Splicing modulation as a modifier of the CFTR function. Prog Mol Subcell Biol. 2006;44:233–254. doi: 10.1007/978-3-540-34449-0_10. [DOI] [PubMed] [Google Scholar]
- 16.Salvatore F, Scudiero O, Castaldo G. Genotype-phenotype correlation in cystic fibrosis: the role of modifier genes. Am J Med Genet. 2002;111:88–95. doi: 10.1002/ajmg.10461. [DOI] [PubMed] [Google Scholar]
- 17.Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A:327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 18.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6. [PubMed] [Google Scholar]
- 19.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- 20.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–133. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin. 2002;20:841–865. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 22.Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89:31–37. doi: 10.1002/(sici)1096-8628(19990326)89:1<31::aid-ajmg7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Rieley MB, Stevenson DA, Viskochil DH, Tinkle BT, Martin LJ, Schorry EK. Variable expression of neurofibromatosis 1 in monozygotic twins. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.33851. [DOI] [PubMed] [Google Scholar]
- 24.Ward BA, Gutmann DH. Neurofibromatosis 1: from lab bench to clinic. Pediatr Neurol. 2005;32:221–228. doi: 10.1016/j.pediatrneurol.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.North K, Joy P, Yuille D, Cocks N, Hutchins P. Cognitive function and academic performance in children with neurofibromatosis type 1. Dev Med Child Neurol. 1995;37:427–436. doi: 10.1111/j.1469-8749.1995.tb12026.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosser TL, Packer RJ. Neurocognitive dysfunction in children with neurofibromatosis type 1. Curr Neurol Neurosci Rep. 2003;3:129–136. doi: 10.1007/s11910-003-0064-3. [DOI] [PubMed] [Google Scholar]
- 27.Kayl AE, Moore BD., 3rd Behavioral phenotype of neurofibromatosis, type 1. Ment Retard Dev Disabil Res Rev. 2000;6:117–124. doi: 10.1002/1098-2779(2000)6:2<117::AID-MRDD5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Shilyansky C, Lee YS, Silva AJ. Molecular and cellular mechanisms of learning disabilities: a focus on NF1. Annu Rev Neurosci. 2010;33:221–243. doi: 10.1146/annurev-neuro-060909-153215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa RM, Silva AJ. Molecular and cellular mechanisms underlying the cognitive deficits associated with neurofibromatosis 1. J Child Neurol. 2002;17:622–626. doi: 10.1177/088307380201700813. discussion 627–629, 646–651. [DOI] [PubMed] [Google Scholar]
- 30.Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 31.Sabbagh A, Pasmant E, Laurendeau I, Parfait B, Barbarot S, Guillot B, Combemale P, Ferkal S, Vidaud M, Aubourg P, Vidaud D, Wolkenstein P. Unravelling the genetic basis of variable clinical expression in neurofibromatosis 1. Hum Mol Genet. 2009;18:2768–2778. doi: 10.1093/hmg/ddp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchuk DA, Saulino AM, Tavakkol R, Swaroop M, Wallace MR, Andersen LB, Mitchell AL, Gutmann DH, Boguski M, Collins FS. cDNA cloning of the type 1 neurofibromatosis gene: complete sequence of the NF1 gene product. Genomics. 1991;11:931–940. doi: 10.1016/0888-7543(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 33.Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A. 1991;88:9658–9662. doi: 10.1073/pnas.88.21.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeClue JE, Cohen BD, Lowy DR. Identification and characterization of the neurofibromatosis type 1 protein product. Proc Natl Acad Sci U S A. 1991;88:9914–9918. doi: 10.1073/pnas.88.22.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daston MM, Ratner N. Neurofibromin, a predominantly neuronal GTPase activating protein in the adult, is ubiquitously expressed during development. Dev Dyn. 1992;195:216–226. doi: 10.1002/aja.1001950307. [DOI] [PubMed] [Google Scholar]
- 36.Gutmann DH. Tumor suppressor genes as negative growth regulators in development and differentiation. Int J Dev Biol. 1995;39:895–908. [PubMed] [Google Scholar]
- 37.Gutmann DH, Zhang Y, Hirbe A. Developmental regulation of a neuron-specific neurofibromatosis 1 isoform. Ann Neurol. 1999;46:777–782. doi: 10.1002/1531-8249(199911)46:5<777::aid-ana15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Bernards A, Snijders AJ, Hannigan GE, Murthy AE, Gusella JF. Mouse neurofibromatosis type 1 cDNA sequence reveals high degree of conservation of both coding and non-coding mRNA segments. Hum Mol Genet. 1993;2:645–650. doi: 10.1093/hmg/2.6.645. [DOI] [PubMed] [Google Scholar]
- 39.The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 40.Lakkis MM, Golden JA, O’Shea KS, Epstein JA. Neurofibromin deficiency in mice causes exencephaly and is a modifier for Splotch neural tube defects. Dev Biol. 1999;212:80–92. doi: 10.1006/dbio.1999.9327. [DOI] [PubMed] [Google Scholar]
- 41.Ars E, Serra E, Garcia J, Kruyer H, Gaona A, Lazaro C, Estivill X. Mutations affecting mRNA splicing are the most common molecular defects in patients with neurofibromatosis type 1. Hum Mol Genet. 2000;9:237–247. doi: 10.1093/hmg/9.2.237. [DOI] [PubMed] [Google Scholar]
- 42.Larizza L, Gervasini C, Natacci F, Riva P. Developmental abnormalities and cancer predisposition in neurofibromatosis type 1. Curr Mol Med. 2009;9:634–653. doi: 10.2174/156652409788488801. [DOI] [PubMed] [Google Scholar]
- 43.Welti S, Fraterman S, D’Angelo I, Wilm M, Scheffzek K. The sec14 homology module of neurofibromin binds cellular glycerophospholipids: mass spectrometry and structure of a lipid complex. J Mol Biol. 2007;366:551–562. doi: 10.1016/j.jmb.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 44.Fahsold R, Hoffmeyer S, Mischung C, Gille C, Ehlers C, Kucukceylan N, Abdel-Nour M, Gewies A, Peters H, Kaufmann D, Buske A, Tinschert S, Nurnberg P. Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am J Hum Genet. 2000;66:790–818. doi: 10.1086/302809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory PE, Gutmann DH, Mitchell A, Park S, Boguski M, Jacks T, Wood DL, Jove R, Collins FS. Neurofibromatosis type 1 gene product (neurofibromin) associates with microtubules. Somat Cell Mol Genet. 1993;19:265–274. doi: 10.1007/BF01233074. [DOI] [PubMed] [Google Scholar]
- 46.Izawa I, Tamaki N, Saya H. Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett. 1996;382:53–59. doi: 10.1016/0014-5793(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 47.Mangoura D, Sun Y, Li C, Singh D, Gutmann DH, Flores A, Ahmed M, Vallianatos G. Phosphorylation of neurofibromin by PKC is a possible molecular switch in EGF receptor signaling in neural cells. Oncogene. 2006;25:735–745. doi: 10.1038/sj.onc.1209113. [DOI] [PubMed] [Google Scholar]
- 48.Boyanapalli M, Lahoud OB, Messiaen L, Kim B, Anderle de Sylor MS, Duckett SJ, Somara S, Mikol DD. Neurofibromin binds to caveolin-1 and regulates ras, FAK, and Akt. Biochem Biophys Res Commun. 2006;340:1200–1208. doi: 10.1016/j.bbrc.2005.12.129. [DOI] [PubMed] [Google Scholar]
- 49.Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63:851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- 50.Xu GF, Lin B, Tanaka K, Dunn D, Wood D, Gesteland R, White R, Weiss R, Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63:835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- 51.Xu GF, O’Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 52.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O’Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 53.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 54.Danglot G, Regnier V, Fauvet D, Vassal G, Kujas M, Bernheim A. Neurofibromatosis 1 (NF1) mRNAs expressed in the central nervous system are differentially spliced in the 5′ part of the gene. Hum Mol Genet. 1995;4:915–920. doi: 10.1093/hmg/4.5.915. [DOI] [PubMed] [Google Scholar]
- 55.Kaufmann D, Muller R, Kenner O, Leistner W, Hein C, Vogel W, Bartelt B. The N-terminal splice product NF1-10a-2 of the NF1 gene codes for a transmembrane segment. Biochem Biophys Res Commun. 2002;294:496–503. doi: 10.1016/S0006-291X(02)00501-6. [DOI] [PubMed] [Google Scholar]
- 56.Gutman DH, Andersen LB, Cole JL, Swaroop M, Collins FS. An alternatively-spliced mRNA in the carboxy terminus of the neurofibromatosis type 1 (NF1) gene is expressed in muscle. Hum Mol Genet. 1993;2:989–992. doi: 10.1093/hmg/2.7.989. [DOI] [PubMed] [Google Scholar]
- 57.Andersen LB, Ballester R, Marchuk DA, Chang E, Gutmann DH, Saulino AM, Camonis J, Wigler M, Collins FS. A conserved alternative splice in the von Recklinghausen neurofibromatosis (NF1) gene produces two neurofibromin isoforms, both of which have GTPase-activating protein activity. Mol Cell Biol. 1993;13:487–495. doi: 10.1128/mcb.13.1.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutmann DH, Cole JL, Collins FS. Modulation of neurofibromatosis type 1 gene expression during in vitro myoblast differentiation. J Neurosci Res. 1994;37:398–405. doi: 10.1002/jnr.490370312. [DOI] [PubMed] [Google Scholar]
- 59.Geist RT, Gutmann DH. Expression of a developmentally-regulated neuron-specific isoform of the neurofibromatosis 1 (NF1) gene. Neurosci Lett. 1996;211:85–88. doi: 10.1016/0304-3940(96)12730-0. [DOI] [PubMed] [Google Scholar]
- 60.Skuse GR, Cappione AJ. RNA processing and clinical variability in neurofibromatosis type I (NF1) Hum Mol Genet. 1997;6:1707–1712. doi: 10.1093/hmg/6.10.1707. [DOI] [PubMed] [Google Scholar]
- 61.Gutmann DH, Andersen LB, Cole JL, Swaroop M, Collins FS. An alternatively-spliced mRNA in the carboxy terminus of the neurofibromatosis type 1 (NF1) gene is expressed in muscle. Hum Mol Genet. 1993;2:989–992. doi: 10.1093/hmg/2.7.989. [DOI] [PubMed] [Google Scholar]
- 62.Park VM, Kenwright KA, Sturtevant DB, Pivnick EK. Alternative splicing of exons 29 and 30 in the neurofibromatosis type 1 gene. Hum Genet. 1998;103:382–385. doi: 10.1007/s004390050837. [DOI] [PubMed] [Google Scholar]
- 63.Mantani A, Wakasugi S, Yokota Y, Abe K, Ushio Y, Yamamura K. A novel isoform of the neurofibromatosis type-1 mRNA and a switch of isoforms during murine cell differentiation and proliferation. Gene. 1994;148:245–251. doi: 10.1016/0378-1119(94)90695-5. [DOI] [PubMed] [Google Scholar]
- 64.Uchida T, Matozaki T, Suzuki T, Matsuda K, Wada K, Nakano O, Konda Y, Nishisaki H, Nagao M, Sakamoto C, et al. Expression of two types of neurofibromatosis type 1 gene transcripts in gastric cancers and comparison of GAP activities. Biochem Biophys Res Commun. 1992;187:332–339. doi: 10.1016/s0006-291x(05)81497-4. [DOI] [PubMed] [Google Scholar]
- 65.Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- 66.Barron VA, Zhu H, Hinman MN, Ladd AN, Lou H. The neurofibromatosis type I pre-mRNA is a novel target of CELF protein-mediated splicing regulation. Nucleic Acids Res. 2010;38:253–264. doi: 10.1093/nar/gkp766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu H, Hinman MN, Hasman RA, Mehta P, Lou H. Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol Cell Biol. 2008;28:1240–1251. doi: 10.1128/MCB.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chapple JP, Anthony K, Martin TR, Dev A, Cooper TA, Gallo JM. Expression, localization and tau exon 10 splicing activity of the brain RNA-binding protein TNRC4. Hum Mol Genet. 2007;16:2760–2769. doi: 10.1093/hmg/ddm233. [DOI] [PubMed] [Google Scholar]
- 69.Ladd AN, Charlet N, Cooper TA. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol. 2001;21:1285–1296. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladd AN, Nguyen NH, Malhotra K, Cooper TA. CELF6, a member of the CELF family of RNA-binding proteins, regulates muscle-specific splicing enhancer-dependent alternative splicing. J Biol Chem. 2004;279:17756–17764. doi: 10.1074/jbc.M310687200. [DOI] [PubMed] [Google Scholar]
- 71.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izquierdo JM. Heterogeneous ribonucleoprotein C displays a repressor activity mediated by T-cell intracellular antigen-1-related/like protein to modulate Fas exon 6 splicing through a mechanism involving Hu antigen R. Nucleic Acids Res. 2010;38:8001–8014. doi: 10.1093/nar/gkq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Izquierdo JM. Cell-specific regulation of Fas exon 6 splicing mediated by Hu antigen R. Biochem Biophys Res Commun. 2010;402:324–328. doi: 10.1016/j.bbrc.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 75.Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem. 2008;283:19077–19084. doi: 10.1074/jbc.M800017200. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Molfenter J, Zhu H, Lou H. Promotion of exon 6 inclusion in HuD pre-mRNA by Hu protein family members. Nucleic Acids Res. 2010;38:3760–3770. doi: 10.1093/nar/gkq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bellavia D, Mecarozzi M, Campese AF, Grazioli P, Talora C, Frati L, Gulino A, Screpanti I. Notch3 and the Notch3-upregulated RNA-binding protein HuD regulate Ikaros alternative splicing. EMBO J. 2007;26:1670–1680. doi: 10.1038/sj.emboj.7601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barreau C, Paillard L, Mereau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie. 2006;88:515–525. doi: 10.1016/j.biochi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Faustino NA, Cooper TA. Identification of putative new splicing targets for ETR-3 using sequences identified by systematic evolution of ligands by exponential enrichment. Mol Cell Biol. 2005;25:879–887. doi: 10.1128/MCB.25.3.879-887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barron VA, Zhu H, Hinman MN, Ladd AN, Lou H. The neurofibromatosis type I pre-mRNA is a novel target of CELF protein-mediated splicing regulation. Nucleic Acids Res. 38:253–264. doi: 10.1093/nar/gkp766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hawes JJ, Tuskan RG, Reilly KM. Nf1 expression is dependent on strain background: implications for tumor suppressor haploinsufficiency studies. Neurogenetics. 2007;8:121–130. doi: 10.1007/s10048-006-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 83.Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE, McManus B, Korf BR. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;64:553–555. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 86.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 87.Lasater EA, Bessler WK, Mead LE, Horn WE, Clapp DW, Conway SJ, Ingram DA, Li F. Nf1+/− mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–2344. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lasater EA, Li F, Bessler WK, Estes ML, Vemula S, Hingtgen CM, Dinauer MC, Kapur R, Conway SJ, Ingram DA., Jr Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. J Clin Invest. 2010;120:859–870. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]